Fulvestrant plus palbociclib versus fulvestrant plus placebo for

Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor positive, HER-2 negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicenter, double-blind, phase-3 randomised controlled study Massimo Cristofanilli, M. D. , F. A. C. P. Professor of Medicine Associate Director of Translational Research and Precision Medicine Robert H Comprehensive Lurie Cancer Center, Northwestern University Chicago, USA
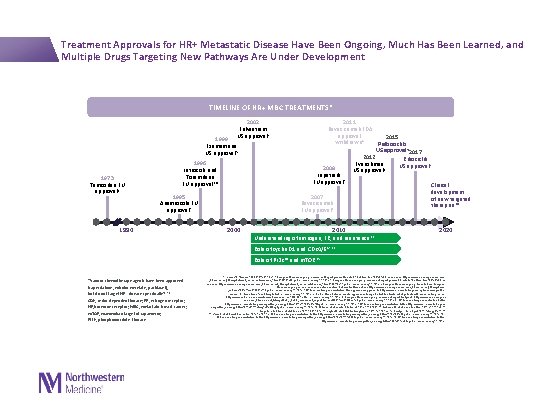
Treatment Approvals for HR+ Metastatic Disease Have Been Ongoing, Much Has Been Learned, and Multiple Drugs Targeting New Pathways Are Under Development TIMELINE OF HR+ MBC TREATMENTS* 2002 Fulvestrant US approval 5 1999 Exemestane US approval 5 1973 Tamoxifen EU approval 1 1996 Letrozole and Toremifene EU approval 3, 4 1995 Anastrozole EU approval 2 1990 2000 2011 Bevacizumab FDA approval 2015 withdrawn 8 Palbociclib US approval 5 2017 2012 Ribociclib Everolimus US approval 5 2008 US approval 9 Lapatinib EU approval 7 Clinical development 2007 of new targeted Bevacizumab therapies 10 EU approval 6 2010 Understanding of estrogen, ER, and resistance 11 2020 Role of cyclin D 1 and CDK 4/612, 13 Role of PI 3 K 14 and m. TOR 15 *Various chemotherapy agents have been approved (capecitabine, eribulin mesylate, paclitaxel), but do not target HR+ disease specifically 16– 18 CDK, cyclin-dependent kinase; ER, estrogen receptor; HR, hormone receptor; MBC, metastatic breast cancer; m. TOR, mammalian target of rapamycin; PI 3 K, phosphoinositide 3 -kinase 1. Jordan VC. Steroids. 2007; 72(13): 829 -842. 2. European Medicines Agency. Assessment Report pursuant to Article 30 of Directive 2001/83/EC, as amended. http: //www. ema. europa. eu/docs/en _GB/document_library/Referrals_document/Arimidex_30/WC 500109487. pdf. Accessed January 29, 2016. 3. European Medicines Agency. Assessment report pursuant to Article 30 of Directive 2001/83/EC, as amended. http: //www. ema. europa. eu/docs/en_GB/document_library/Referrals_document/Femara_30/WC 500128493. pdf. Accessed January 29, 2016. 4. European Medicines Agency. Press Release. European Medicines Agency recommends new contraindication for Fareston (toremifene). http: //www. ema. europa. eu/docs/en_GB/document_library/Press _release/2009/11/WC 500014597. pdf. Accessed January 29, 2016. 5. US Food and Drug Administration. FDA-approved drug products. http: //www. accessdata. fda. gov/scripts/cder/drugsatfda /index. cfm? fuseaction= Search. Drug. Details. Accessed January 29, 2016. 6. Roche. Media Release. Avastin approved in Europe for first-line treatment of patients with advanced lung cancer. http: //www. roche. com/media/store/releases/med-cor-2007 -08 -24. htm. Accessed January 29, 2016. 7. European Medicines Agency. Assessment report for tyverb. http: //www. ema. europa. eu /docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000795/WC 500044960. pdf. Accessed January 29, 2016. 8. US Food and Drug Administration. Letter. http: //www. accessdata. fda. gov/drugsatfda_docs/appletter/2011/125085 s 0241 ltr. pdf. Accessed January 29, 2016. 9. US Food and Drug Administration. Letter. http: //www. accessdata. fda. gov/ drugsatfda_docs/appletter/2012/022334 Orig 1 s 016 ltr. Repl. pdf. Accessed January 29, 2016. 10. Milani A, et al. World J Clin Oncol. 2014; 5: 990 -1001. 11. Osborne CK, et al. Annu Rev Med. 2011; 62: 233 -247. 12. Lange CA, et al. Endocr Relat Cancer. 2011; 18: C 19 -C 24. 13. Asghar U, et al. Nat Rev Drug Discov. 2015; 14: 130 -146. 14. Baselga J. Oncologist. 2011; 16(suppl 1): 12 -19. 15. Vicier C, et al. Breast Cancer Res. 2014; 16: 203. 16. US Food and Drug Administration. Letter. http: //www. accessdata. fda. gov/drugsatfda_docs/appletter/1998/20896 ltr. pdf. Accessed January 29, 2016. 17. US Food and Drug Administration. Letter. http: //www. accessdata. fda. gov/drugsatfda_docs/appletter/2010/201532 s 000 ltr. pdf. Accessed January 29, 2016. 18. US Food and Drug Administration. Letter http: //www. accessdata. fda. gov/drugsatfda_docs/appletter/2005/021660 ltr. pdf. Accessed January 29, 2016.

Clinical Cancer Advancement 2017 12/7/2020 3
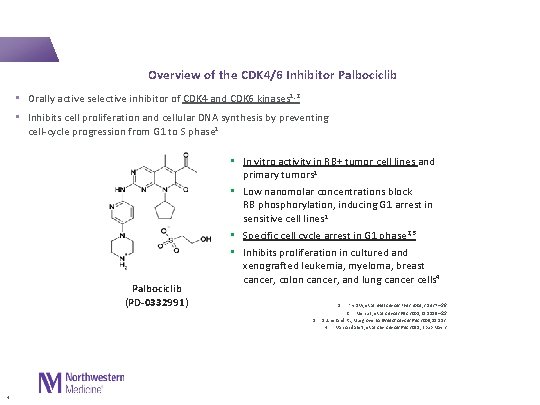
Overview of the CDK 4/6 Inhibitor Palbociclib • Orally active selective inhibitor of CDK 4 and CDK 6 kinases 1, 2 • Inhibits cell proliferation and cellular DNA synthesis by preventing cell-cycle progression from G 1 to S phase 1 Palbociclib (PD-0332991) • In vitro activity in RB+ tumor cell lines and primary tumors 1 • Low nanomolar concentrations block RB phosphorylation, inducing G 1 arrest in sensitive cell lines 1 • Specific cell cycle arrest in G 1 phase 2, 3 • Inhibits proliferation in cultured and xenografted leukemia, myeloma, breast cancer, colon cancer, and lung cancer cells 4 1. Fry DW, et al. Mol Cancer Ther 2004; 3: 1427 – 38 Menu E, et al. Cancer Res 2008; 68: 5519 – 23 Sutherland RL, Musgrove EA. Breast Cancer Res 2009; 11: 112 4. Van Arsdale T, et al. Clin Cancer Res 2015; Epub May 2 2. 3. 4
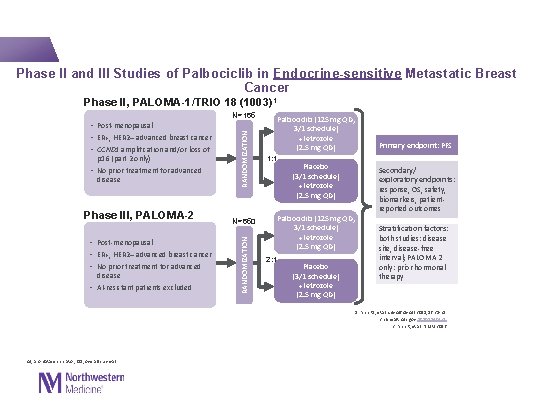
Phase II and III Studies of Palbociclib in Endocrine-sensitive Metastatic Breast Cancer Phase II, PALOMA-1/TRIO 18 (1003)1 Phase III, PALOMA-2 (1008)2, 3 • Post-menopausal • ER+, HER 2– advanced breast cancer • No prior treatment for advanced disease • AI-resistant patients excluded N=650 RANDOMIZATION p 16 (part 2 only) • No prior treatment for advanced disease RANDOMIZATION N=165 • Post-menopausal • ER+, HER 2– advanced breast cancer • CCND 1 amplification and/or loss of Palbociclib (125 mg QD, 3/1 schedule) + letrozole (2. 5 mg QD) 1: 1 Placebo (3/1 schedule) + letrozole (2. 5 mg QD) Palbociclib (125 mg QD, 3/1 schedule) + letrozole (2. 5 mg QD) 2: 1 Placebo (3/1 schedule) + letrozole (2. 5 mg QD) Primary endpoint: PFS Secondary/ exploratory endpoints: response, OS, safety, biomarkers, patientreported outcomes Stratification factors: both studies: disease site, disease-free interval; PALOMA 2 only: prior hormonal therapy 1. Finn RS, et al. Lancet Oncol 2015; 16: 25 -35 2. clinicaltrials. gov NCT 01740427 3. Finn R, et al. NEJM 2016 AI, aromatase inhibitor; OS, overall survival
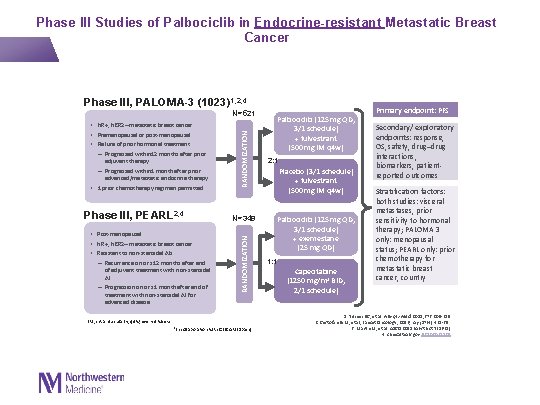
Phase III, PALOMA-3 (1023)1, 2, 4 adjuvant therapy – Progressed within 1 month after prior advanced/metastatic endocrine therapy • 1 prior chemotherapy regimen permitted Phase III, PEARL 2, 4 • Post-menopausal • HR+, HER 2– metastatic breast cancer • Resistant to non-steroidal AIs: – Recurrence on or ≤ 12 months after end of adjuvant treatment with non-steroidal AI – Progression on or ≤ 1 month after end of treatment with non-steroidal AI for advanced disease RANDOMIZATION N=521 • HR+, HER 2– metastatic breast cancer • Premenopausal or post-menopausal • Failure of prior hormonal treatment: – Progressed within 12 months after prior N=348 RANDOMIZATION Phase III Studies of Palbociclib in Endocrine-resistant Metastatic Breast Cancer IM, intramuscularly; q 4 w, every 4 weeks a. In collaboration with GEICAM (Spain) Palbociclib (125 mg QD, 3/1 schedule) + fulvestrant (500 mg IM q 4 w) 2: 1 Placebo (3/1 schedule) + fulvestrant (500 mg IM q 4 w) Palbociclib (125 mg QD, 3/1 schedule) + exemestane (25 mg QD) 1: 1 Capecitabine (1250 mg/m 2 BID, 2/1 schedule) Primary endpoint: PFS Secondary/ exploratory endpoints: response, OS, safety, drug–drug interactions, biomarkers, patientreported outcomes Stratification factors: both studies: visceral metastases, prior sensitivity to hormonal therapy; PALOMA 3 only: menopausal status; PEARL only: prior chemotherapy for metastatic breast cancer, country 1. Turner NC, et al. N Engl J Med. 2015; 373: 209 -219. 2. Cristofanilli M, et al, Lancet Oncology, 2016; Apr; 17(4): 425 -39. 3. Martin M, et al. ASCO 2015 (Abstract TPS 631) 4. clinicaltrials. gov NCT 020285 07
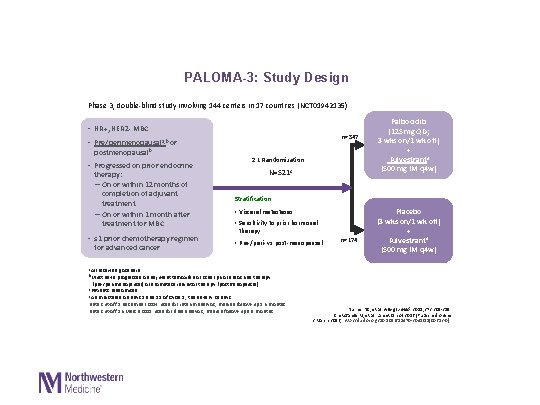
PALOMA-3: Study Design Phase 3, double-blind study involving 144 centers in 17 countries (NCT 01942135) • HR+, HER 2 - MBC n=347 • Pre/perimenopausala, b or postmenopausalb • Progressed on prior endocrine therapy: – On or within 12 months of completion of adjuvant treatment – On or within 1 month after treatment for MBC • ≤ 1 prior chemotherapy regimen for advanced cancer 2: 1 Randomization N=521 c Palbociclib (125 mg QD; 3 wks on/1 wk off) + Fulvestrantd (500 mg IM q 4 w) Stratification: • Visceral metastases • Sensitivity to prior hormonal therapy • Pre-/peri- vs post-menopausal n=174 Placebo (3 wks on/1 wk off) + Fulvestrantd (500 mg IM q 4 w) a. All received goserelin b. Must have progressed on adjuvant tamoxifen or other prior endocrine therapy (pre-/perimenopausal) or aromatase inhibitor therapy (postmenopausal) c. Patients randomised d. Administered on days 1 and 15 of Cycle 1, then every 28 days Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months Data cut-off 16 March 2015 used for final analysis; median follow-up 8. 9 months Turner NC, et al. N Engl J Med. 2015; 373: 209 -219 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]

12/7/2020 8

PALOMA-3: Study Endpoints • Primary endpoint - Progression-free survival (PFS) by investigator assessment • Secondary endpoints - Overall survival - Survival probability at 1, 2, and 3 years - Objective response and duration of response - Rate of clinical benefit (clinical benefit response) - Pharmacokinetics - Safety and tolerability - Tumor tissue biomarkers (i. e. PIK 3 CA mutation status, quantitative expression of estrogen and progesterone receptors) - Patient-reported outcomes Turner NC, et al. N Engl J Med. 2015; 373: 209 -219 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
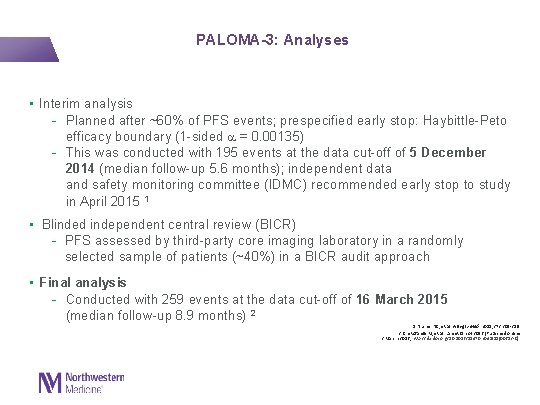
PALOMA-3: Analyses • Interim analysis - Planned after ~60% of PFS events; prespecified early stop: Haybittle-Peto efficacy boundary (1 -sided = 0. 00135) - This was conducted with 195 events at the data cut-off of 5 December 2014 (median follow-up 5. 6 months); independent data and safety monitoring committee (IDMC) recommended early stop to study in April 2015 1 • Blinded independent central review (BICR) - PFS assessed by third-party core imaging laboratory in a randomly selected sample of patients (~40%) in a BICR audit approach • Final analysis - Conducted with 259 events at the data cut-off of 16 March 2015 (median follow-up 8. 9 months) 2 1. Turner NC, et al. N Engl J Med. 2015; 373: 209 -219 2. Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
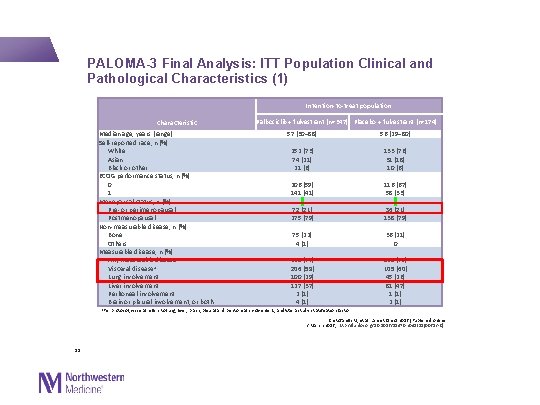
PALOMA-3 Final Analysis: ITT Population Clinical and Pathological Characteristics (1) Intention-to-treat population Characteristic Median age, years (range) Self-reported race, n (%) White Asian Black or other ECOG performance status, n (%) 0 1 Menopausal status, n (%) Pre- or perimenopausal Postmenopausal Non-measurable disease, n (%) Bone Others Measurable disease, n (%) Any measurable disease Visceral diseasea Lung involvement Liver involvement Peritoneal involvement Brain or pleural involvement, or both Palbociclib + fulvestrant (n=347) Placebo + fulvestrant (n=174) 57 (30− 88) 56 (29− 80) 252 (73) 74 (21) 21 (6) 206 (59) 141 (41) 72 (21) 275 (79) 133 (76) 31 (18) 10 (6) 116 (67) 58 (33) 36 (21) 138 (79) 75 (22) 4 (1) 268 (77) 206 (59) 100 (29) 127 (37) 2 (1) 4 (1) 36 (21) 0 138 (79) 105 (60) 45 (26) 81 (47) 1 (1) 2 (1) a. Per protocol, visceral refers to lung, liver, brain, pleural and peritoneal involvement, and was a study stratification factor Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] 11
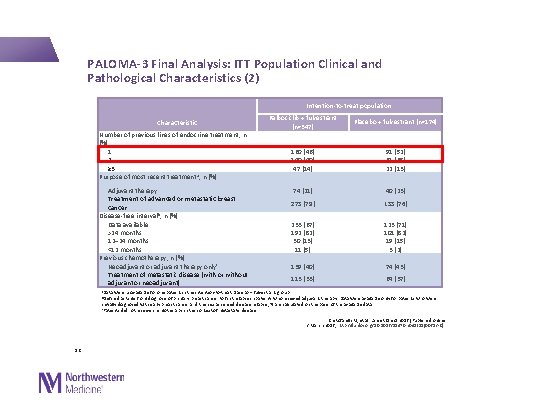
PALOMA-3 Final Analysis: ITT Population Clinical and Pathological Characteristics (2) Intention-to-treat population Characteristic Number of previous lines of endocrine treatment, n (%) 1 2 ≥ 3 Purpose of most recent treatment a, n (%) Palbociclib + fulvestrant (n=347) Placebo + fulvestrant (n=174) 160 (46) 140 (40) 47 (14) 91 (52) 61 (35) 22 (13) Adjuvant therapy Treatment of advanced or metastatic breast cancer Disease-free interval b, n (%) Data available >24 months 12– 24 months <12 months Previous chemotherapy, n (%) Neoadjuvant or adjuvant therapy onlyc Treatment of metastatic disease (with or without adjuvant or neoadjuvant) 74 (21) 40 (23) 273 (79) 133 (76) 233 (67) 192 (82) 30 (13) 11 (5) 123 (71) 101 (82) 19 (15) 3 (2) 139 (40) 74 (43) 113 (33) 64 (37) a. Data were unavailable for one patient in the intention-to-treat placebo + fulvestrant group b. Defined as time from diagnosis of primary breast cancer to first relapse in patients who received adjuvant therapy. Data were available only for patients who were initially diagnosed with early breast cancer and then experienced disease relapse; % are calculated on the basis of the available data c. Patients did not receive chemotherapy in the context of metastatic disease Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] 12
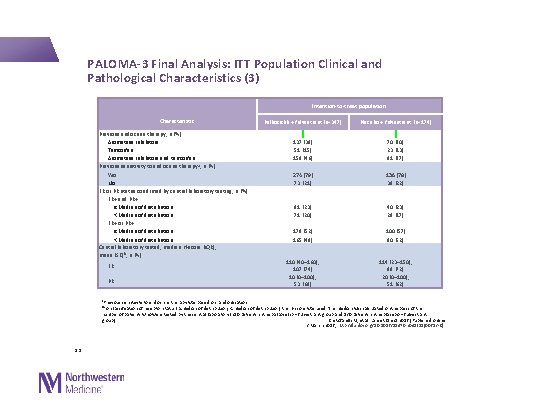
PALOMA-3 Final Analysis: ITT Population Clinical and Pathological Characteristics (3) Intention-to-treat population Characteristic Previous endocrine therapy, n (%) Aromatase inhibitors Tamoxifen Aromatase inhibitors and tamoxifen Previous sensitivity to endocrine therapy a, n (%) Yes No ER or PR status confirmed by central laboratory testing, n (%) ER+ and PR+ ≥Median of distribution <Median of distribution ER+ or PR+ ≥Median of distribution <Median of distribution Central laboratory tested, median H-score (IQR); mean (SD)b, n (%) ER PR Palbociclib + fulvestrant (n=347) Placebo + fulvestrant (n=174) 137 (39) 51 (15) 159 (46) 70 (40) 23 (13) 81 (47) 274 (79) 73 (21) 136 (78) 38 (22) 81 (23) 71 (20) 40 (23) 29 (17) 179 (52) 165 (48) 100 (57) 90 (52) 110 (40– 160); 107 (74) 10 (0– 100); 53 (68) 114 (23– 150); 99 (72) 20 (0– 100); 51 (62) a. Previous sensitivity to endocrine therapy was based on randomisation b. For classification of receptor status (≥median of distribution, <median of distribution) the H-score was used. The median was calculated on the basis of the number of patients who were tested by the central laboratory (250 patients in the palbociclib + fulvestrant group and 130 patients in the placebo + fulvestrant Cristofanilli M, et al. Lancet Oncol 2016 [Published online group) 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] 13
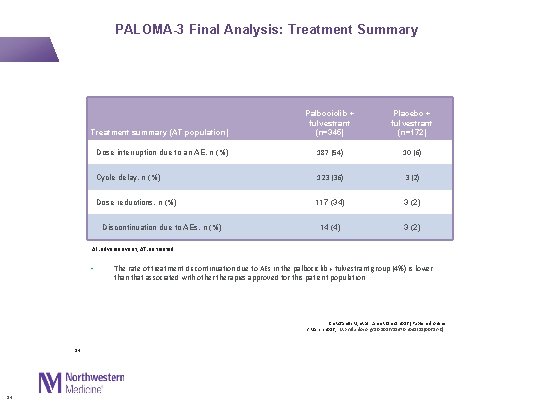
PALOMA-3 Final Analysis: Treatment Summary Treatment summary (AT population) Palbociclib + fulvestrant (n=345) Placebo + fulvestrant (n=172) Dose interruption due to an AE, n (%) 187 (54) 10 (6) Cycle delay, n (%) 123 (36) 3 (2) Dose reductions, n (%) 117 (34) 3 (2) 14 (4) 3 (2) Discontinuation due to AEs, n (%) AE: adverse event; AT: as treated • The rate of treatment discontinuation due to AEs in the palbociclib + fulvestrant group (4%) is lower than that associated with otherapies approved for this patient population Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] 14 14
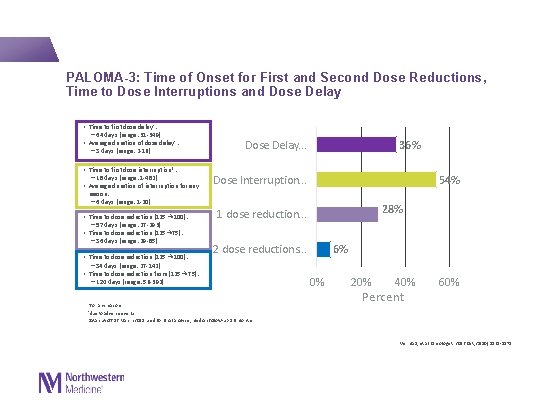
PALOMA-3: Time of Onset for First and Second Dose Reductions, Time to Dose Interruptions and Dose Delay • Time to first dose delay† : − 64 days (range: 31 -349) • Average duration of dose delay† : − 3 days (range: 2 -16) • Time to first dose interruption*: − 18 days (range: 1 -482) • Average duration of interruption for any Dose Delay. . . 36% Dose Interruption. . . 54% reason: − 6 days (range: 1 -20) • Time to dose reduction (125 → 100): − 57 days (range: 27 -293) • Time to dose reduction (125→ 75): − 36 days (range: 29 -85) • Time to dose reduction (125 → 100): − 34 days (range: 27 -142) • Time to dose reduction from (125→ 75): − 120 days (range: 56 -392) 28% 1 dose reduction. . . 2 dose reductions. . . *for any reasons. †due to adverse events. Data cut-off 16 March 2015 used for final analysis; median follow-up 8. 9 months 6% 0% 20% 40% Percent 60% Verma S, et al. Oncologist. 2016 Oct; 21(10): 1165 -1175.

Efficacy Data 12/7/2020
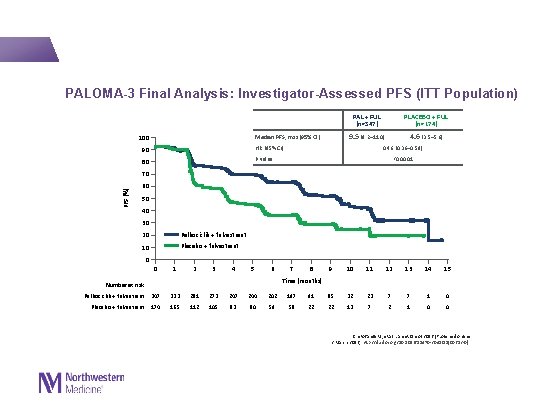
PALOMA-3 Final Analysis: Investigator-Assessed PFS (ITT Population) PAL + FUL (n=347) 9. 5 (9. 2─11. 0) Median PFS, mos (95% CI) 100 90 HR (95% CI) 80 P value PLACEBO + FUL (n=174) 4. 6 (3. 5─5. 6) 0. 46 (0. 36─0. 59) <0. 0001 PFS (%) 70 60 50 40 30 20 Palbociclib + fulvestrant 10 Placebo + fulvestrant 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time (months) Number at risk Palbociclib + fulvestrant 347 333 281 273 247 244 202 197 91 85 32 23 7 7 1 0 Placebo + fulvestrant 174 165 112 105 83 80 59 58 22 22 13 7 2 1 0 0 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
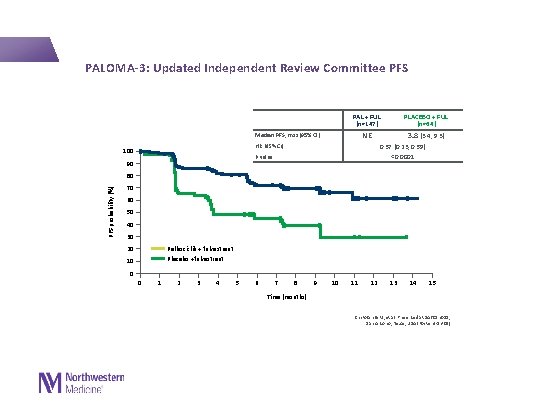
PALOMA-3: Updated Independent Review Committee PFS PAL + FUL (n=147) NE Median PFS, mos (95% CI) HR (95% CI) 100 3. 8 (3. 4, 9. 3) 0. 37 (0. 23, 0. 59) <0. 0001 P value 90 PLACEBO + FUL (n=64) PFS probability (%) 80 70 60 50 40 30 Palbociclib + fulvestrant Placebo +fulvestrant 20 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time (months) Cristofanilli M, et al. Presented at SABCS 2015; San Antonio, Texas, USA (Poster 4 -13 -01)
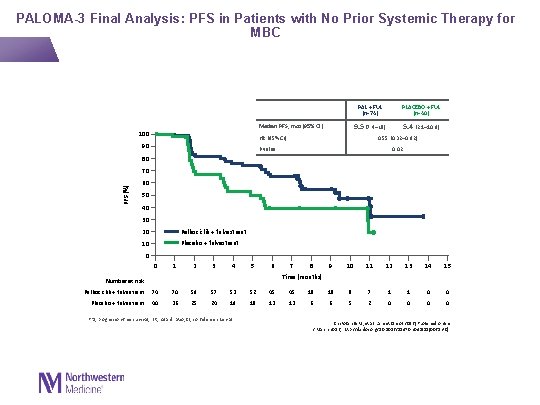
PALOMA-3 Final Analysis: PFS in Patients with No Prior Systemic Therapy for MBC Median PFS, mos (95% CI) 100 PAL + FUL (n=74) PLACEBO + FUL (n=40) 9. 5 (7. 4–NE) 5. 4 (2. 1– 10. 9) HR (95% CI) 90 0. 55 (0. 32– 0. 92) P value 0. 02 80 PFS (%) 70 60 50 40 30 20 Palbociclib + fulvestrant 10 Placebo + fulvestrant 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time (months) Number at risk Palbociclib + fulvestrant 74 70 59 57 53 52 45 45 18 18 8 7 1 1 0 0 Placebo + fulvestrant 40 36 25 24 19 18 13 13 6 6 5 2 0 0 PFS, progression-free survival; HR, hazard ratio; CI, confidence interval Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
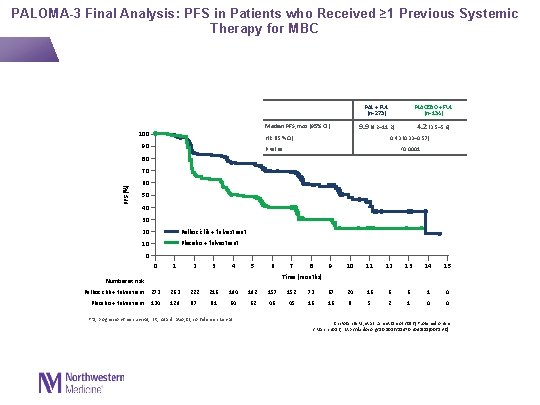
PALOMA-3 Final Analysis: PFS in Patients who Received ≥ 1 Previous Systemic Therapy for MBC PAL + FUL (n=273) 9. 9 (9. 2– 11. 2) Median PFS, mos (95% CI) 100 HR (95% CI) 90 PLACEBO + FUL (n=134) 4. 2 (3. 5– 5. 6) 0. 43 (0. 33– 0. 57) P value <0. 0001 80 PFS (%) 70 60 50 40 30 20 Palbociclib + fulvestrant 10 Placebo + fulvestrant 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time (months) Number at risk Palbociclib + fulvestrant 273 263 222 216 194 192 157 152 73 67 24 16 6 6 1 0 Placebo + fulvestrant 134 129 87 81 64 62 46 45 16 16 8 5 2 1 0 0 PFS, progression-free survival; HR, hazard ratio; CI, confidence interval Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
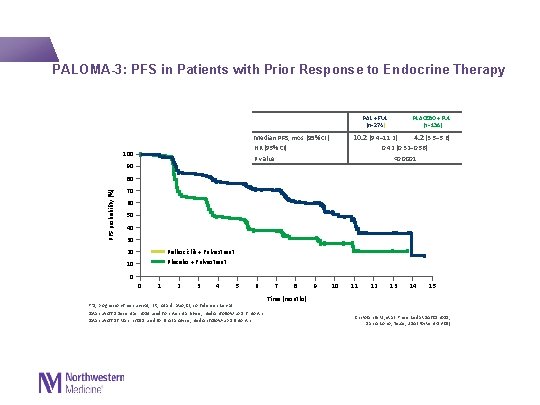
PALOMA-3: PFS in Patients with Prior Response to Endocrine Therapy PLACEBO + FUL (n=136) PAL + FUL (n=274) 10. 2 (9. 4– 11. 2) 4. 2 (3. 5– 5. 6) 0. 42 (0. 32– 0. 56) <0. 0001 Median PFS, mos (95% CI) HR (95% CI) P value 100 90 PFS probability (%) 80 70 60 50 40 30 Palbociclib + Fulvestrant Placebo + Fulvestrant 20 10 0 0 1 2 3 4 5 6 PFS, progression-free survival; HR, hazard ratio; CI, confidence interval Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months Data cut-off 16 March 2015 used for final analysis; median follow-up 8. 9 months 7 8 9 10 11 12 13 14 15 Time (months) Cristofanilli M, et al. Presented at SABCS 2015; San Antonio, Texas, USA (Poster 4 -13 -01)
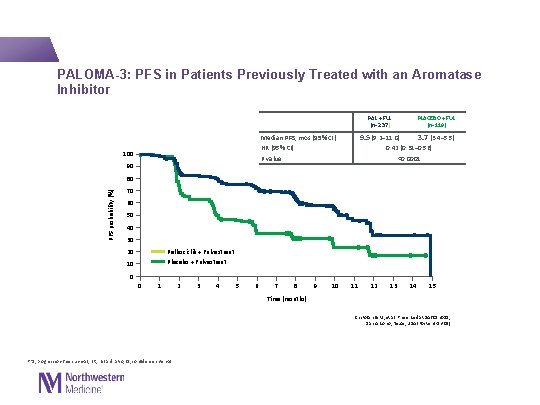
PALOMA-3: PFS in Patients Previously Treated with an Aromatase Inhibitor PLACEBO + FUL (n=119) PAL + FUL (n=237) 9. 5 (9. 2– 11. 0) 3. 7 (3. 4– 5. 5) 0. 42 (0. 31– 0. 56) <0. 0001 Median PFS, mos (95% CI) HR (95% CI) P value 100 90 PFS probability (%) 80 70 60 50 40 30 Palbociclib + Fulvestrant Placebo + Fulvestrant 20 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Time (months) Cristofanilli M, et al. Presented at SABCS 2015; San Antonio, Texas, USA (Poster 4 -13 -01) PFS, progression-free survival; HR, hazard ratio; CI, confidence interval
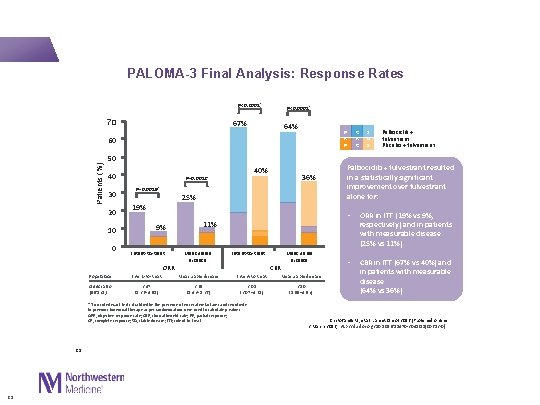
PALOMA-3 Final Analysis: Response Rates P<0. 0001* 70 P<0. 0001* 67% 64% P R Patients (%) 60 50 40 30 20 10 0 40% P=0. 0012* P=0. 0019* 25% 19% 9% Intent-to-treat ORR Measurable disease Intent-to-treat CBR 23 Measurable disease Population: Intent-to-treat Measurable disease Odds ratio (95% CI) 2. 47 (1. 36– 4. 91) 2. 69 (1. 43– 5. 26) 3. 05 (2. 07– 4. 61) 3. 10 (1. 99– 4. 92) S D Palbociclib + fulvestrant Placebo + fulvestrant Palbociclib + fulvestrant resulted in a statistically significant improvement over fulvestrant alone for: • ORR in ITT (19% vs 9%, respectively) and in patients with measurable disease (25% vs 11%) • CBR in ITT (67% vs 40%) and in patients with measurable disease (64% vs 36%) 11% * Two-sided exact tests stratified by the presence of visceral metastases and sensitivity to previous hormonal therapy as per randomisation were used to calculate p values. ORR, objective response rate; CBR, clinical benefit rate; PR, partial response; CR, complete response; SD, stable disease; ITT; intent-to-treat 23 36% C R Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
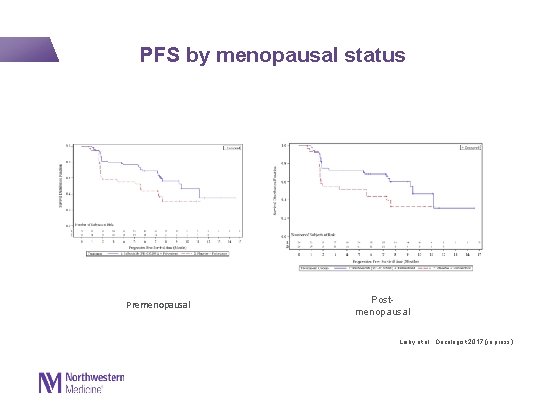
PFS by menopausal status Premenopausal Postmenopausal Loiby et al. , Oncologist 2017 (in press)
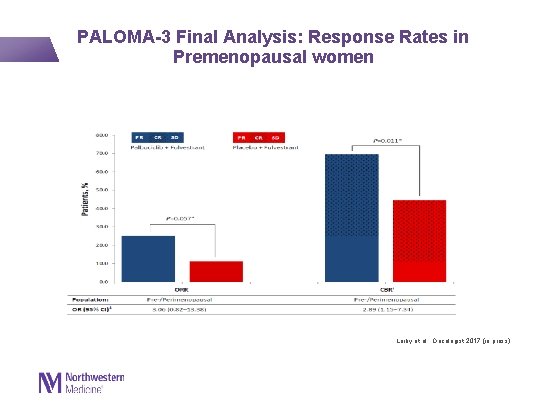
PALOMA-3 Final Analysis: Response Rates in Premenopausal women Loiby et al. , Oncologist 2017 (in press)
Biomarkers analysis in PALOMA-3 12/7/2020
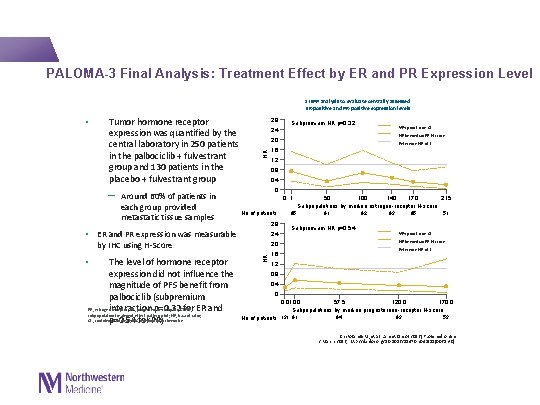
PALOMA-3 Final Analysis: Treatment Effect by ER and PR Expression Level STEPP analysis to evaluate centrally assessed ER-positive and PR-positive expression levels Tumor hormone receptor expression was quantified by the central laboratory in 250 patients in the palbociclib + fulvestrant group and 130 patients in the placebo + fulvestrant group 2. 8 2. 4 Reference HR of 1 1. 2 0 0 1 No of patients 2. 8 2. 4 by IHC using H-Score 2. 0 HR The level of hormone receptor expression did not influence the magnitude of PFS benefit from palbociclib (subpremium ER, estrogen receptor; PR, progesterone receptor; STEPP, interaction p=0. 32 for ER and subpopulation treatment effect pattern plot; HR, hazard ratio; CI , confidence interval; IHC, immunohistochemistry p=0. 54 for PR) HR by median ER H-score 1. 6 0. 4 • ER and PR expression was measurable • 95% point-wise CI 0. 8 ─ Around 60% of patients in each group provided metastatic tissue samples Subpremium HR p=0. 32 2. 0 HR • 1. 6 95 50 100 140 170 215 Subpopulations by median estrogen-receptor H-score 91 92 93 95 51 Subpremium HR p=0. 54 95% point-wise CI HR by median PR H-score Reference HR of 1 1. 2 0. 8 0. 4 0 0. 010. 0 57. 5 120. 0 170. 0 Subpopulations by median progesterone-receptor H-score 94 93 53 No of patients 131 91 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
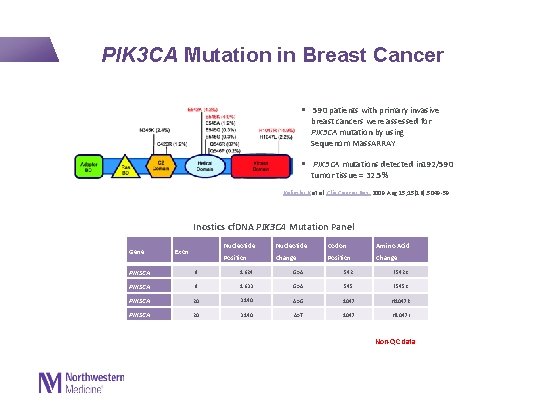
PIK 3 CA Mutation in Breast Cancer § 590 patients with primary invasive breast cancers were assessed for PIK 3 CA mutation by using Sequenom Mass. ARRAY § PIK 3 CA mutations detected in 192/590 tumor tissue = 32. 5% Kalinsky K et al. Clin Cancer Res. 2009 Aug 15; 15(16): 5049 -59. Inostics cf. DNA PIK 3 CA Mutation Panel Gene Exon Nucleotide Codon Amino Acid Position Change 9 1624 G>A PIK 3 CA 9 1633 G>A 545 E 545 K PIK 3 CA 20 3140 A>G 1047 H 1047 R PIK 3 CA 20 3140 A>T 1047 H 1047 L PIK 3 CA 542 E 542 K Non-QC data
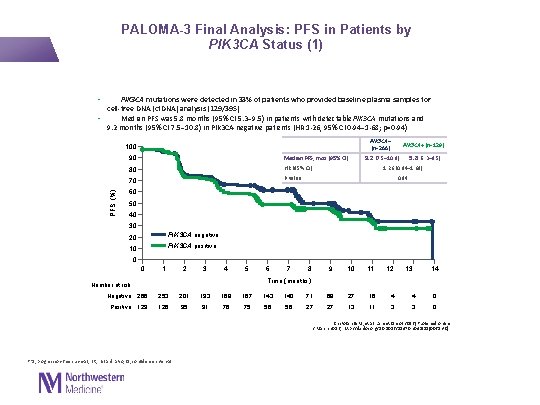
PALOMA-3 Final Analysis: PFS in Patients by PIK 3 CA Status (1) • PIK 3 CA mutations were detected in 33% of patients who provided baseline plasma samples for cell-free DNA (cf. DNA) analysis (129/395) • Median PFS was 5. 8 months (95% CI 5. 3– 9. 5) in patients with detectable PIK 3 CA mutations and 9. 2 months (95% CI 7. 5– 10. 8) in PIK 3 CA-negative patients (HR 1· 26, 95% CI 0· 94– 1· 68; p=0· 94) PIK 3 CA– (n=266) PFS (%) 100 90 Median PFS, mos (95% CI) 80 HR (95% CI) 70 P value PIK 3 CA+ (n=129) 9. 2 (7. 5– 10. 8) 5. 8 (5. 3– 9. 5) 1. 26 (0. 94─1. 68) 0. 94 60 50 40 30 20 PIK 3 CA negative 10 PIK 3 CA positive 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Time (months) Number at risk Negative 266 253 201 193 168 167 143 140 71 68 27 16 4 4 0 Positive 129 126 95 91 76 75 56 56 27 27 13 11 3 3 0 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] PFS, progression-free survival; HR, hazard ratio; CI, confidence interval
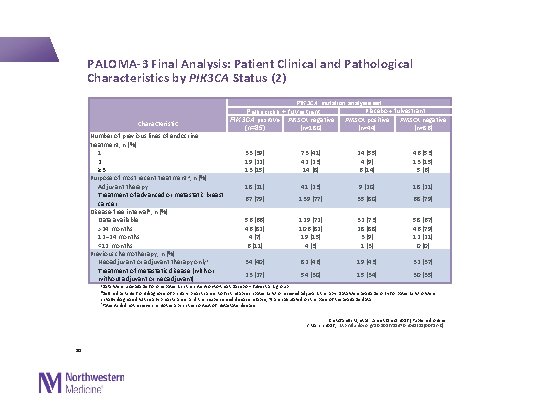
PALOMA-3 Final Analysis: Patient Clinical and Pathological Characteristics by PIK 3 CA Status (2) PIK 3 CA mutation analysis set Characteristic Number of previous lines of endocrine treatment, n (%) 1 2 ≥ 3 Purpose of most recent treatment a, n (%) Adjuvant therapy Treatment of advanced or metastatic breast cancer Disease-free interval b, n (%) Data available >24 months 12– 24 months <12 months Previous chemotherapy, n (%) Neoadjuvant or adjuvant therapy onlyc Treatment of metastatic disease (with or without adjuvant or neoadjuvant) Palbociclib + fulvestrant PIK 3 CA positive PIK 3 CA negative (n=180) (n=85) 33 (39) 19 (22) 13 (15) 73 (41) 42 (23) 14 (8) Placebo + fulvestrant PIK 3 CA positive PIK 3 CA negative (n=44) (n=86) 24 (55) 4 (9) 6 (14) 46 (53) 13 (15) 5 (6) 18 (21) 41 (23) 9 (20) 18 (21) 67 (79) 139 (77) 35 (80) 68 (79) 56 (66) 46 (82) 4 (7) 6 (11) 129 (72) 106 (82) 19 (15) 4 (3) 32 (73) 28 (88) 3 (9) 1 (3) 58 (67) 46 (79) 12 (21) 0 (0) 34 (40) 82 (46) 19 (43) 32 (37) 23 (27) 54 (30) 15 (34) 30 (35) a. Data were unavailable for one patient in the intention-to-treat placebo + fulvestrant group b. Defined as time from diagnosis of primary breast cancer to first relapse in patients who received adjuvant therapy. Data were available only for patients who were initially diagnosed with early breast cancer and then experienced disease relapse; % are calculated on the basis of the available data c. Patients did not receive chemotherapy in the context of metastatic disease Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] 30
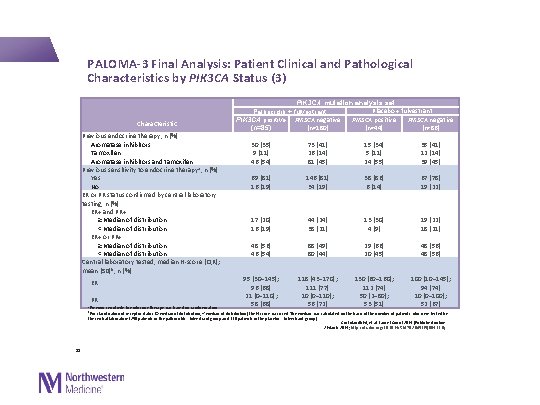
PALOMA-3 Final Analysis: Patient Clinical and Pathological Characteristics by PIK 3 CA Status (3) Characteristic Previous endocrine therapy, n (%) Aromatase inhibitors Tamoxifen Aromatase inhibitors and tamoxifen Previous sensitivity to endocrine therapy a, n (%) Yes No ER or PR status confirmed by central laboratory testing, n (%) ER+ and PR+ ≥Median of distribution <Median of distribution ER+ or PR+ ≥Median of distribution <Median of distribution Central laboratory tested, median H-score (IQR); mean (SD)b, n (%) ER PR a. Previous sensitivity to endocrine therapy was based on randomisation PIK 3 CA mutation analysis set Placebo + fulvestrant Palbociclib + fulvestrant PIK 3 CA positive PIK 3 CA negative (n=180) (n=44) (n=86) (n=85) 30 (35) 9 (11) 46 (54) 73 (41) 26 (14) 81 (45) 15 (34) 5 (11) 24 (55) 35 (41) 12 (14) 39 (45) 69 (81) 16 (19) 146 (81) 34 (19) 38 (86) 6 (14) 67 (78) 19 (22) 17 (20) 16 (19) 44 (24) 38 (21) 13 (30) 4 (9) 19 (22) 18 (21) 48 (56) 46 (54) 88 (49) 80 (44) 29 (66) 20 (45) 48 (56) 95 (30– 145); 96 (68) 21 (0– 110); 58 (68) 118 (43– 170); 111 (77) 10 (0– 110); 56 (72) 130 (60– 160); 112 (74) 50 (2– 80); 53 (51) 100 (10– 145); 94 (74) 10 (0– 100); 52 (67) b. For classification of receptor status (≥median of distribution, <median of distribution) the H-score was used. The median was calculated on the basis of the number of patients who were tested by the central laboratory (250 patients in the palbociclib + fulvestrant group and 130 patients in the placebo + fulvestrant group) 31 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
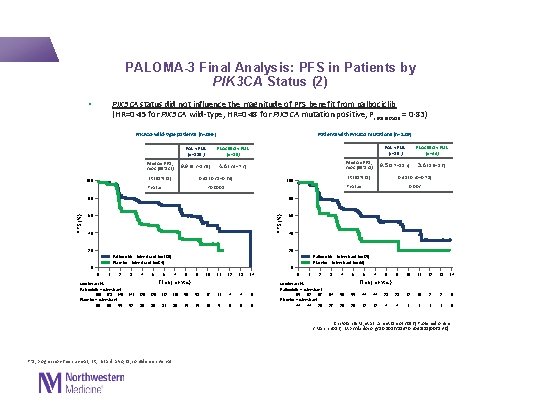
PALOMA-3 Final Analysis: PFS in Patients by PIK 3 CA Status (2) • PIK 3 CA status did not influence the magnitude of PFS benefit from palbociclib (HR=0· 45 for PIK 3 CA wild-type, HR=0· 48 for PIK 3 CA mutation positive, Pinteraction = 0· 83) PIK 3 CA-wild-type patients (n=266) Median PFS, mos (95% CI) PLACEBO + FUL (n=86) 9. 9 (9. 2– 13. 9) 4. 6 (3. 4– 7. 3) HR (95% CI) 100 Median PFS, mos (95% CI) 0. 45 (0. 31– 0. 64) P value PAL + FUL (n=85 ) PLACEBO + FUL (n=44) 9. 5 (5. 7– 11. 2) 3. 6 (1. 9– 5. 6) 0. 48 (0. 30– 0. 78) HR (95% CI) 100 0. 002 P value <0. 0001 80 80 60 60 PFS (%) Patients with PIK 3 CA mutations (n=129) PAL + FUL (n=180 ) 40 40 20 20 Palbociclib + fulvestrant (n=180) Placebo + fulvestrant (n=86) Palbociclib + fulvestrant (n=85) Placebo + fulvestrant (n=44) 0 0 0 1 2 Number at risk Palbociclib + fulvestrant 180 173 146 Placebo + fulvestrant 86 80 55 3 4 5 6 7 8 9 10 11 12 13 14 Time (months) 141 129 112 110 56 53 17 11 4 4 0 52 39 38 31 30 15 15 10 5 0 0 1 2 Number at risk Palbociclib + fulvestrant 85 82 67 Placebo + fulvestrant 44 44 28 3 4 5 6 7 8 9 10 11 12 13 14 Time (months) 64 56 55 44 44 23 23 12 10 2 2 0 27 20 20 12 12 4 4 1 1 0 Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0] PFS, progression-free survival; HR, hazard ratio; CI, confidence interval
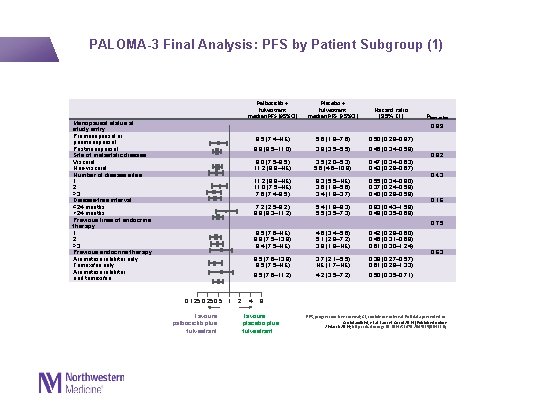
PALOMA-3 Final Analysis: PFS by Patient Subgroup (1) Palbociclib + fulvestrant median PFS (95%CI) Menopausal status at study entry Premenopausal or perimenopausal Postmenopausal Site of metastatic disease Visceral Non-visceral Number of disease sites 1 2 ≥ 3 Disease-free interval ≤ 24 months >24 months Previous lines of endocrine therapy 1 2 ≥ 3 Previous endocrine therapy Aromatase inhibitor only Tamoxifen only Aromatase inhibitor and tamoxifen Placebo + fulvestrant median PFS (95%CI) Hazard ratio (95% CI) Pinteraction 0. 89 9. 5 (7. 4–NE) 5. 6 (1. 8– 7. 6) 0. 50 (0. 29– 0. 87) 9. 9 (8. 5– 11. 0) 3. 9 (3. 5– 5. 5) 0. 45 (0. 34– 0. 59) 8. 0 (7. 5– 9. 5) 11. 2 (9. 9–NE) 3. 5 (2. 0– 5. 3) 5. 6 (4. 6– 10. 9) 0. 47 (0. 34– 0. 63) 0. 43 (0. 28– 0. 67) 11. 2 (9. 9–NE) 11. 0 (7. 5–NE) 7. 6 (7. 4– 9. 5) 9. 3 (5. 5–NE) 3. 6 (1. 9– 5. 6) 3. 4 (1. 9– 3. 7) 0. 55 (0. 34– 0. 90) 0. 37 (0. 24– 0. 59) 0. 40 (0. 28– 0. 59) 7. 2 (2. 5– 9. 2) 9. 9 (9. 3– 11. 2) 5. 4 (1. 8– 9. 3) 5. 5 (3. 5– 7. 3) 0. 83 (0. 43– 1. 59) 0. 48 (0. 35– 0. 68) 9. 5 (7. 6–NE) 9. 9 (7. 5– 13. 9) 9. 4 (7. 5–NE) 4. 6 (3. 4– 5. 6) 5. 1 (2. 8– 7. 2) 3. 9 (1. 8–NE) 0. 42 (0. 29– 0. 60) 0. 46 (0. 31– 0. 69) 0. 61 (0. 30– 1. 24) 9. 5 (7. 6– 13. 9) 9. 5 (7. 5–NE) 3. 7 (2. 1– 5. 5) NE (1. 7–NE) 0. 39 (0. 27– 0. 57) 0. 61 (0. 28– 1. 33) 9. 5 (7. 6– 11. 2) 4. 2 (3. 5– 7. 2) 0. 50 (0. 35– 0. 71) 0. 82 0. 43 0. 16 0. 75 0. 125 0. 5 1 Favours palbociclib plus fulvestrant 2 4 0. 63 8 Favours placebo plus fulvestrant PFS, progression-free survival; CI, confidence interval. Full data presented in: Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
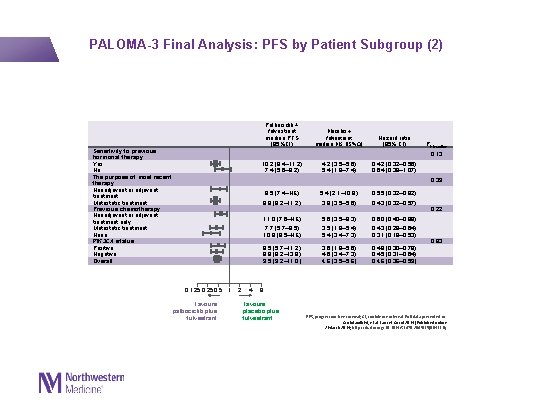
PALOMA-3 Final Analysis: PFS by Patient Subgroup (2) Sensitivity to previous hormonal therapy Yes No The purpose of most recent therapy Neoadjuvant or adjuvant treatment Metastatic treatment Previous chemotherapy Neoadjuvant or adjuvant treatment only Metastatic treatment None PIK 3 CA status Positive Negative Overall Palbociclib + fulvestrant median PFS (95%CI) Placebo + fulvestrant median PFS (95%CI) Hazard ratio (95% CI) 10. 2 (9. 4– 11. 2) 7. 4 (5. 6– 9. 2) 4. 2 (3. 5– 5. 6) 5. 4 (1. 9– 7. 4) 0. 42 (0. 32– 0. 56) 0. 64 (0. 39– 1. 07) 9. 5 (7. 4–NE) 5. 4 (2. 1– 10. 9) 0. 55 (0. 32– 0. 92) 9. 9 (9. 2– 11. 2) 3. 9 (3. 5– 5. 6) 0. 43 (0. 32– 0. 57) 11. 0 (7. 6–NE) 5. 6 (3. 5– 9. 3) 0. 60 (0. 40– 0. 88) 7. 7 (5. 7– 9. 5) 10. 8 (9. 5–NE) 3. 5 (1. 9– 5. 4) 5. 4 (3. 4– 7. 3) 0. 43 (0. 29– 0. 64) 0. 31 (0. 18– 0. 53) 9. 5 (5. 7– 11. 2) 9. 9 (9. 2– 13. 9) 9. 5 (9. 2– 11. 0) 3. 6 (1. 9– 5. 6) 4. 6 (3. 4– 7. 3) 4. 6 (3. 5– 5. 6) 0. 48 (0. 30– 0. 78) 0. 45 (0. 31– 0. 64) 0. 46 (0. 36– 0. 59) Pinteraction 0. 13 0. 39 0. 125 0. 5 1 Favours palbociclib plus fulvestrant 2 4 0. 22 0. 83 8 Favours placebo plus fulvestrant PFS, progression-free survival; CI, confidence interval. Full data presented in: Cristofanilli M, et al. Lancet Oncol 2016 [Published online 2 March 2016; http: //dx. doi. org/10. 1016/S 1470 -2045(15)00613 -0]
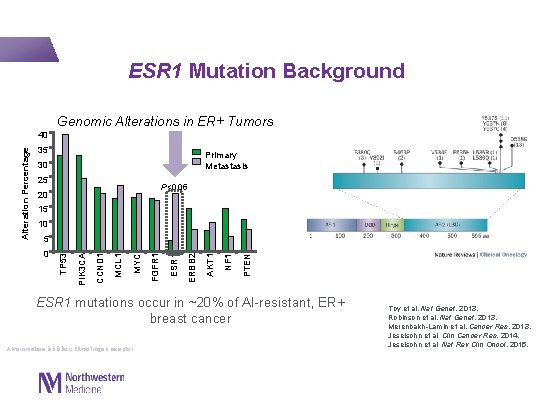
ESR 1 Mutation Background Genomic Alterations in ER+ Tumors 35 Primary Metastasis 30 25 P<0. 05 20 15 10 PTEN NF 1 AKT 1 ERBB 2 ESR 1 FGFR 1 MYC MCL 1 CCND 1 0 PIK 3 CA 5 TP 53 Alteration Percentage 40 ESR 1 mutations occur in ~20% of AI-resistant, ER+ breast cancer AI=aromatase inhibitor; ER=estrogen receptor. Toy et al. Nat Genet, 2013. Robinson et al. Nat Genet, 2013. Merenbakh-Lamin et al. Cancer Res, 2013. Jeselsohn et al. Clin Cancer Res, 2014. Jeselsohn et al. Nat Rev Clin Oncol, 2015.
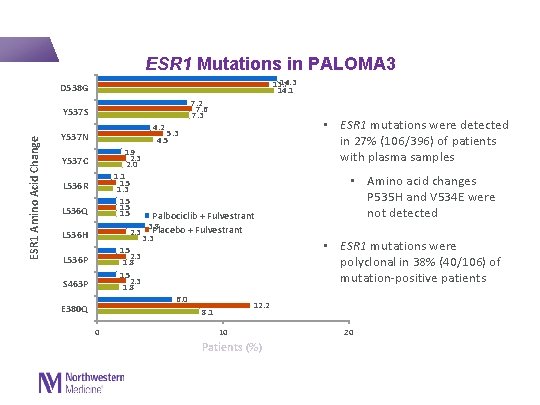
ESR 1 Mutations in PALOMA 3 14. 3 13. 7 14. 1 D 538 G 7. 2 7. 6 7. 3 ESR 1 Amino Acid Change Y 537 S • ESR 1 mutations were detected in 27% (106/396) of patients with plasma samples 4. 2 5. 3 4. 5 Y 537 N 1. 9 2. 3 2. 0 1. 1 1. 5 1. 3 1. 5 Y 537 C L 536 R L 536 Q Palbociclib + Fulvestrant 3. 8 2. 3 Placebo + Fulvestrant 3. 3 1. 5 2. 3 1. 8 6. 0 12. 2 8. 1 L 536 H L 536 P S 463 P E 380 Q 0 10 Patients (%) • Amino acid changes P 535 H and V 534 E were not detected • ESR 1 mutations were polyclonal in 38% (40/106) of mutation-positive patients 20
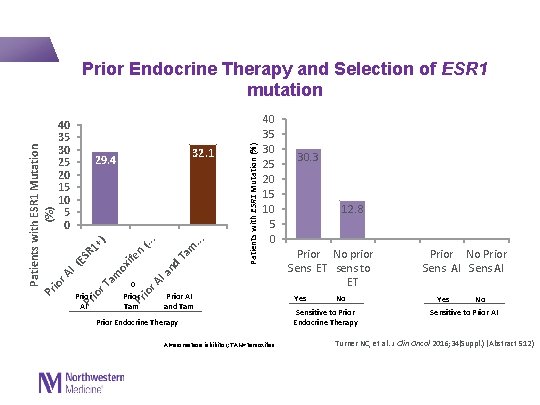
Patients with ESR 1 Mutation (%) io r A I (E SR Pr 1+ io r T ) am ox ife Pr n (. . io r A. I a nd T am. . . 40 35 30 25 20 15 10 5 0 32. 1 29. 4 0 Patients with ESR 1 Mutation (%) Prior Endocrine Therapy and Selection of ESR 1 mutation 40 35 30 25 20 15 10 5 0 Prior AI Prior Tam Prior AI and Tam Prior Endocrine Therapy AI=aromatase inhibitor; TAM=tamoxifen. 30. 3 12. 8 Prior No prior Sens ET sens to ET Prior No Prior Sens AI Yes No Yes No Sensitive to Prior Endocrine Therapy Sensitive to Prior AI Turner NC, et al. J Clin Oncol 2016; 34(Suppl. ) (Abstract 512)
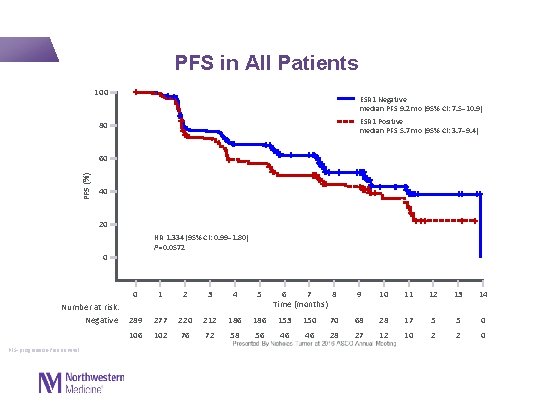
PFS in All Patients 100 ESR 1 Negative median PFS 9. 2 mo (95% CI: 7. 5– 10. 9) ESR 1 Positive median PFS 5. 7 mo (95% CI: 3. 7– 9. 4) 80 PFS (%) 60 40 20 HR 1. 334 (95% CI: 0. 99– 1. 80) P=0. 0572 0 Number at risk: Negative PFS=progression-free survival. 0 1 2 3 4 5 6 7 8 Time (months) 289 277 220 212 186 153 150 106 102 76 72 58 56 46 46 9 10 11 12 13 14 70 68 28 17 5 5 0 28 27 12 10 2 2 0
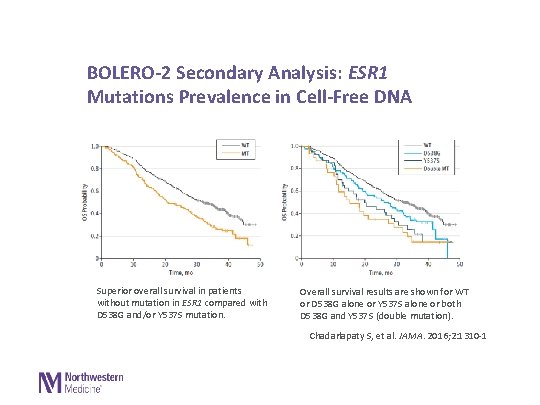
BOLERO-2 Secondary Analysis: ESR 1 Mutations Prevalence in Cell-Free DNA Superior overall survival in patients without mutation in ESR 1 compared with D 538 G and/or Y 537 S mutation. Overall survival results are shown for WT or D 538 G alone or Y 537 S alone or both D 538 G and Y 537 S (double mutation). Chadarlapaty S, et al. JAMA. 2016; 2: 1310 -1 Copyright © 2016 American Medical Association. All righ

PFS by ESR 1 Mutation Status ESR 1 positive ESR 1 negative Interaction P-values P=0. 1772 P=0. 4877 Palbociclib + Fulvestrant median PFS 9. 4 mo (95% CI: 4. 1– 11. 2) Placebo + Fulvestrant median PFS 4. 1 mo (95% CI: 2. 8– 5. 6) 100 80 60 PFS (%) 60 40 20 0 Palbociclib + Fulvestrant median PFS 9. 5 mo (95% CI: 9. 2– 13. 9) Placebo + Fulvestrant median PFS 3. 8 mo (95% CI: 3. 4– 7. 4) 40 20 HR 0. 524 (95% CI: 0. 32– 0. 87) P=0. 0052 0 1 Number at risk: 2 3 4 5 6 7 8 9 10 11 12 13 14 Time (months) Palbociclib + 67 65 49 47 39 38 35 35 23 22 10 Fulvestrant Placebo + 39 37 27 25 19 18 11 11 5 5 2 Fulvestrant PFS=progression-free survival. 0 9 1 1 0 1 1 1 0 HR 0. 438 (95% CI: 0. 31– 0. 62) P<0. 0001 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Time (months) Number at risk: Palbociclib + 198 190 164 158 146 121 119 56 54 19 12 Fulvestrant Placebo + 91 87 56 54 40 40 32 31 14 14 9 5 Fulvestrant Presented By Nicholas Turner at 2016 ASCO Annual Meeting 5 5 0 0 March 2015 final PFS data cut, Cristofanilli et al. Lancet Oncol, 2015.

Updated analysis 12/7/2020
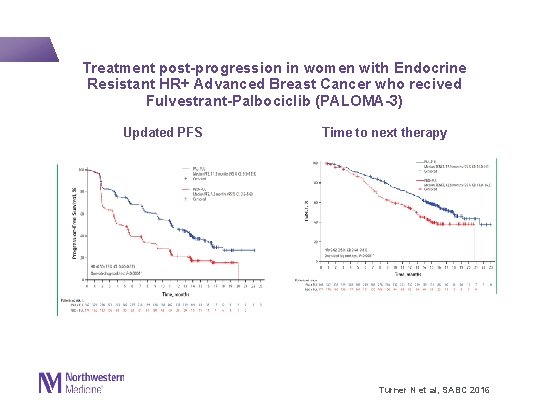
Treatment post-progression in women with Endocrine Resistant HR+ Advanced Breast Cancer who recived Fulvestrant-Palbociclib (PALOMA-3) Updated PFS Time to next therapy Turner N et al, SABC 2016
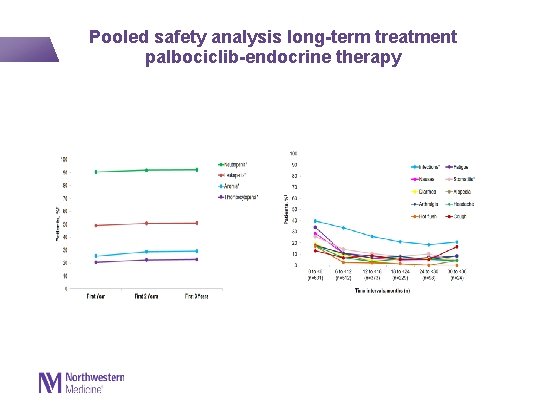
Pooled safety analysis long-term treatment palbociclib-endocrine therapy
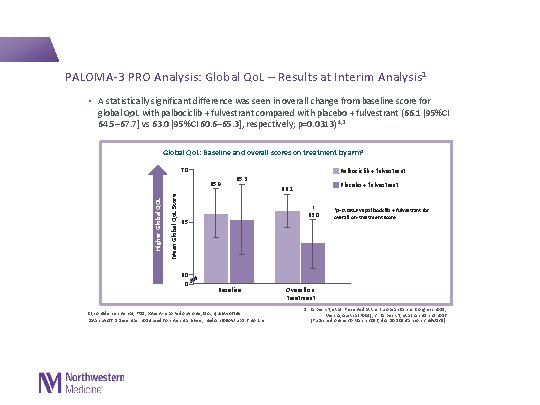
PALOMA-3 PRO Analysis: Global Qo. L – Results at Interim Analysis 1 • A statistically significant difference was seen in overall change from baseline score for global Qo. L with palbociclib + fulvestrant compared with placebo + fulvestrant (66. 1 [95%CI 64. 5– 67. 7] vs 63. 0 [95%CI 60. 6– 65. 3], respectively; p=0. 0313) 1, 2 Global Qo. L: Baseline and overall scores on treatment by arm 1 70 Palbociclib + fulvestrant Mean Global Qo. L Score Higher Global QOL 65. 9 65. 3 * 63. 0 65 60 0 Placebo + fulvestrant 66. 1 Baseline CI, confidence interval; PRO, patient-reported outcome; Qo. L, quality of life Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months *p=0. 0313 vs palbociclib + fulvestrant for overall on-treatment score Overall on treatment 1. Harbeck N, et al. Presented at the European Cancer Congress 2015; Vienna, Austria (P 004); 2. Harbeck N, et al. Ann Oncol 2016 [Published online 30 March 2016; doi: 10. 1093/annonc/mdw 139].
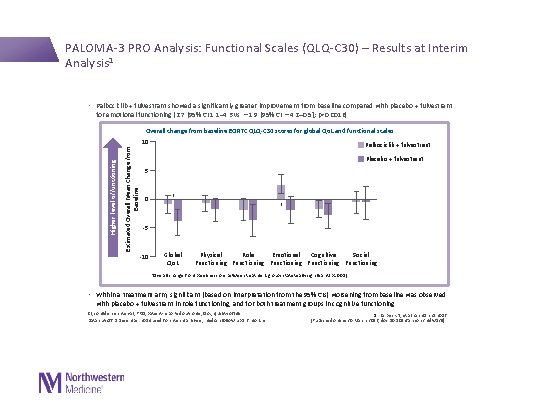
PALOMA-3 PRO Analysis: Functional Scales (QLQ-C 30) – Results at Interim Analysis 1 • Palbociclib + fulvestrant showed a significantly greater improvement from baseline compared with placebo + fulvestrant for emotional functioning (2. 7 [95% CI 1. 1– 4. 3 vs − 1. 9 [95% CI − 4. 2– 0. 5]; p=0. 0016) Estimated Overall Mean Change from Baseline Overall change from baseline EORTC QLQ-C 30 scores for global Qo. L and functional scales Higher level of functioning 10 Palbociclib + fulvestrant Placebo + fulvestrant 5 0 * * -5 -10 Global Qo. L Physical Role Emotional Cognitive Social Functioning Functioning *Overall change from baseline score between treatment groups statistically significant (p<0. 05) • Within a treatment arm, significant (based on interpretation from the 95% CIs) worsening from baseline was observed with placebo + fulvestrant in role functioning, and for both treatment groups in cognitive functioning CI, confidence interval; PRO, patient-reported outcome; Qo. L, quality of life Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months 1. Harbeck N, et al. Ann Oncol 2016 [Published online 30 March 2016; doi: 10. 1093/annonc/mdw 139].

PALOMA-3 PRO Analysis: QLQ-C 30 Symptoms – Results at Interim Analysis 1 • Palbociclib + fulvestrant demonstrated a significant improvement from baseline in pain compared with placebo + fulvestrant (− 3. 3 [95% CI − 5. 1−− 1. 5] vs 2. 0 [95% CI − 0. 6– 4. 6]; p=0. 0011) • Significantly less deterioration from baseline in nausea and vomiting was seen with palbociclib + fulvestrant vs placebo + fulvestrant (1. 7 [95% CI 0. 4– 3. 0] vs 4. 2 [95% CI 2. 3– 6. 1]; p=0. 0369) Between-treatment comparison of changes in score from baseline (PRO analysis set) C 30 Symptom Scales Estimate (95% CI) Fatigue – 1. 5 (– 4. 5– 1. 5) Nausea & vomiting (p=0. 0369) – 2. 5 (– 4. 8–– 0. 2) Pain (p=0. 0011) – 5. 3 (– 8. 5–– 2. 1) Dyspnea – 0. 5 (– 3. 7– 2. 8) Insomnia – 2. 0 (– 5. 5– 1. 6) Appetite loss – 0. 6 (– 4. 1– 2. 9) Constipation 0. 7 (– 2. 5– 3. 9) Diarrhea – 0. 6 (– 2. 8– 1. 7) Financial difficulties 0. 3 (– 3. 1– 3. 6) – 10 Favors palbociclib + fulvestrant – 5 0 Estimate CI, confidence interval; PRO, patient-reported outcome Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months 5 10 15 20 Favors placebo + fulvestrant 1. Harbeck N, et al. Ann Oncol 2016 [Published online 30 March 2016; doi: 10. 1093/annonc/mdw 139].
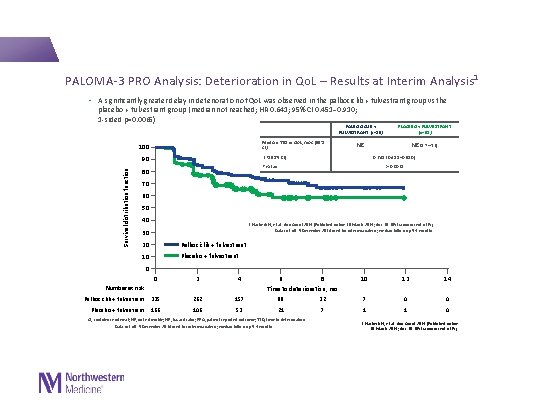
PALOMA-3 PRO Analysis: Deterioration in Qo. L – Results at Interim Analysis 1 • A significantly greater delay in deterioration of Qo. L was observed in the palbociclib + fulvestrant group vs the placebo + fulvestrant group (median not reached; HR 0. 641; 95% CI 0. 451– 0. 910; 1 -sided p=0. 0065) Median TTD in Qo. L, mos (95% CI) 100 PLACEBO + FULVESTRANT (n=52) NE NE (5. 7─NE) HR (95% CI) 90 Survival distribution function PALBOCICLIB + FULVESTRANT (n=80) 0. 641 (0. 451─0. 910) P value 80 p=0. 0065 70 60 50 40 1. Harbeck N, et al. Ann Oncol 2016 [Published online 30 March 2016; doi: 10. 1093/annonc/mdw 139]. Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months 30 20 Palbociclib + fulvestrant 10 Placebo + fulvestrant 0 0 2 4 Palbociclib + fulvestrant 335 262 157 88 Placebo + fulvestrant 166 106 53 21 Number at risk 6 8 Time to deterioration, mo CI, confidence interval; NE, not estimable; HR, hazard ratio; PRO, patient-reported outcome; TTD, time to deterioration Data cut-off 5 December 2014 used for interim analysis; median follow-up 5. 6 months 10 12 14 32 7 0 0 7 1 1 0 1. Harbeck N, et al. Ann Oncol 2016 [Published online 30 March 2016; doi: 10. 1093/annonc/mdw 139].
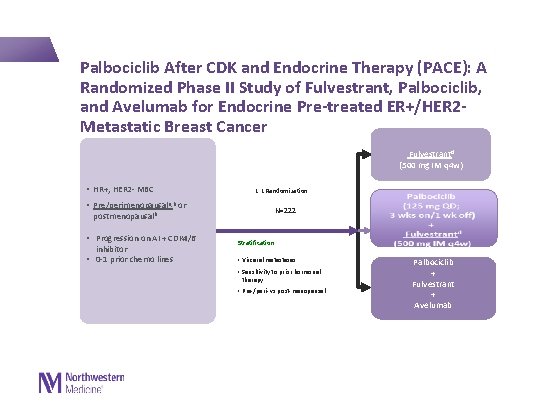
Palbociclib After CDK and Endocrine Therapy (PACE): A Randomized Phase II Study of Fulvestrant, Palbociclib, and Avelumab for Endocrine Pre-treated ER+/HER 2 - Metastatic Breast Cancer Fulvestrant d (500 mg IM q 4 w) • HR+, HER 2 - MBC • 1: 1 Randomization Pre/perimenopausala, b or N=222 postmenopausalb • Progression on AI + CDK 4/6 inhibitor • 0 -1 prior chemo lines Stratification: • Visceral metastases • Sensitivity to prior hormonal therapy • Pre-/peri- vs post-menopausal Palbociclib + Fulvestrant + Avelumab

Conclusions • Palbociclib combined with fulvestrant improved PFS compared to placebo and fulvestrant in women with HR+/HER 2– advanced breast cancer whose disease had progressed on prior endocrine therapy and benefit was demonstrated across pre-specified subgroups, including premenopausal women. • Palbociclib-Fulvestrant demonstrated activity in patients with PI 3 KCA or ESR 1 mutations. • Palbociclib demonstrated well tolerated, improved QOL and did not compromise furtherapeutic interventions. • Treatment with the combination palbociclib significantly improved QOL in HR+ MBC • Palbociclib in combination with fulvestrant represents a new standard of care for women whose cancer progressed on prior endocrine therapy.

Global warming
- Slides: 50