Fuel cell in the energy production Fuel cell











- Slides: 19

Fuel cell in the energy production

Fuel cell • A Fuel is a device that converts directly the chemical energy of reactants (fuel and oxidant) into electricity. • high efficiency (el, el+ heat) • flexibility in the source of fuel (Hx. Cy, biomass, H 2 O) • efficiency independent of plant size • installation of big and small modules • application in stationary, transport and small • electronic devices and systems • non-pollutant and noiseless

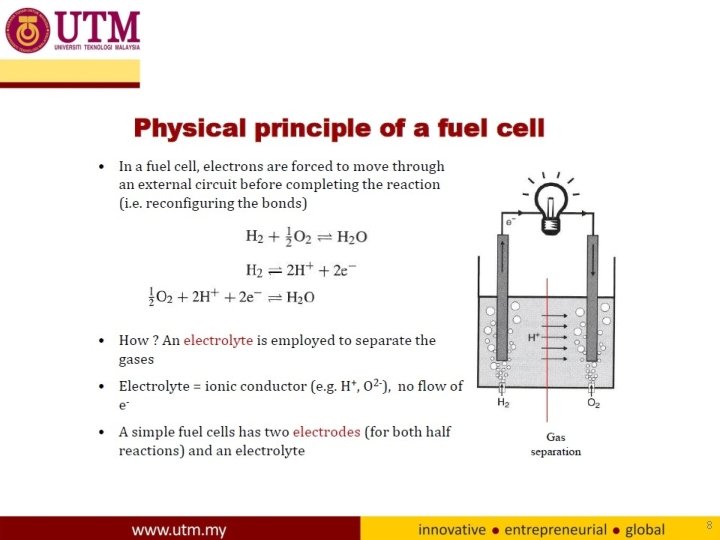
Fuel cell fundamentals Electrochemistry H 2=2 H++2 e-(anode) 1/2 O 2+ 2 H++2 e-=H 2 O(cathode) Overall cell reaction 1/2 O 2+ H 2=H 2 O

Power supply and stacks

5

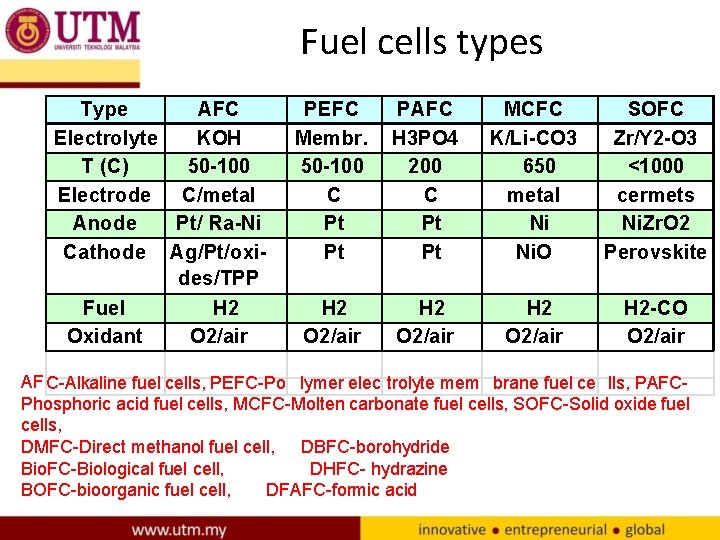
Fuel cells types Type AFC Electrolyte KOH T (C) 50 -100 Electrode C/metal Anode Pt/ Ra-Ni Cathode Ag/Pt/oxides/TPP Fuel H 2 Oxidant O 2/air PEFC Membr. 50 -100 C Pt Pt PAFC H 3 PO 4 200 C Pt Pt MCFC K/Li-CO 3 650 metal Ni Ni. O SOFC Zr/Y 2 -O 3 <1000 cermets Ni. Zr. O 2 Perovskite H 2 O 2/air H 2 -CO O 2/air AF C-Alkaline fuel cells, PEFC-Po lymer elec trolyte mem brane fuel ce lls, PAFCPhosphoric acid fuel cells, MCFC-Molten carbonate fuel cells, SOFC-Solid oxide fuel cells, DMFC-Direct methanol fuel cell, DBFC-borohydride Bio. FC-Biological fuel cell, DHFC- hydrazine BOFC-bioorganic fuel cell, DFAFC-formic acid

Electrocatalyst An electrocatalyst is a substance which increases the rate of the reaction by providing an alternative reaction path with a lower activation energy barrier (Appelby, Foulkes) • • Electronic conductivity Stability in the electrolyte Selective for the oxidation/reduction reactions Suitable adsorption characteristics

8

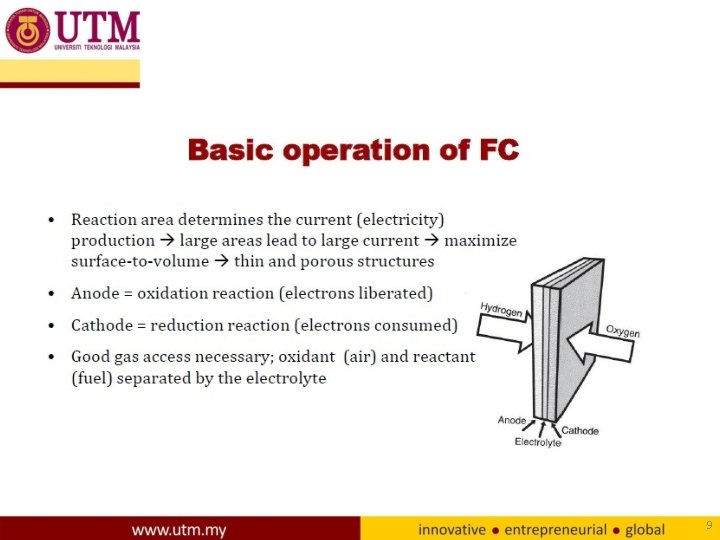
9

10

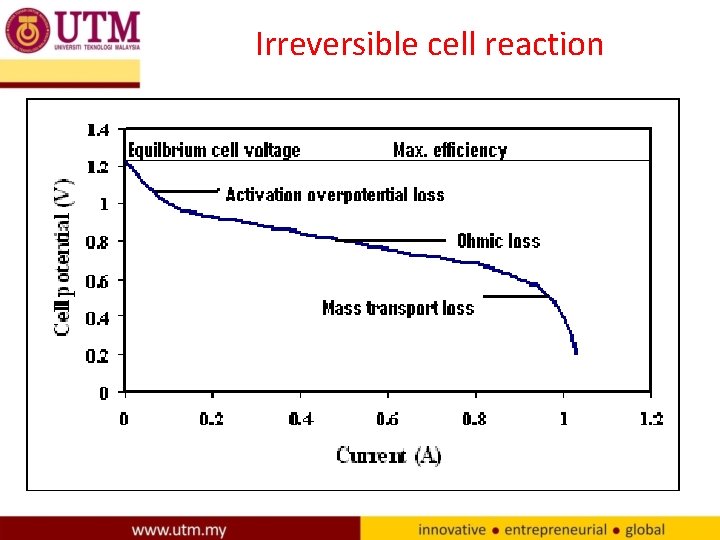
Irreversible cell reaction

12

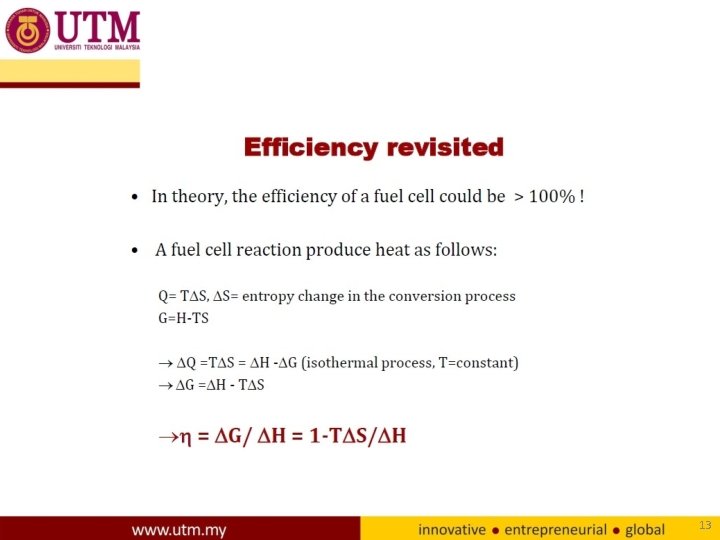
13

14

15

Electrode reactions for selected fuel cells Type Anode Ion Cathode AFC H 2+2 OH-=2 H 2 O+2 e- <-OH- 1/2 O 2+H 2 O+2 e-=2 OH- PEFC H 2=2 H++2 e- H+-> 1/2 O 2+2 H++2 e-=H 2 O DMFC CH 3 OH+H 2 O=CO 2+6 H++6 e- H+-> <-OH- 3/2 O 2+6 H++6 e-=3 H 2 O 3/2 O 2+ 3 H 2 O +6 e-=6 OH- <-OH- 2 O 2+4 H 2 O+8 e-=8 OH- H+-> <-OH- O 2+4 H-+4 e-=2 H 2 O O 2+2 H 2 O+4 e-=4 OH- CH 3 OH+6 OH-=CO 2+5 H 2 O+6 e- - DBFC BH 4 +8 OH =8 BO 2 +H 2 O+8 e DHFC N 2 H 4=N 2+4 H-+4 e. N 2 H 4+4 OH-=N 2+4 H 2 O -

17

18

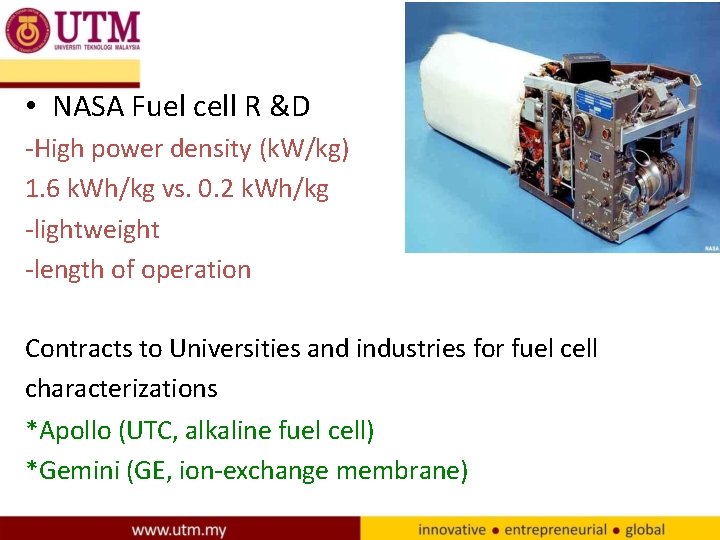
• NASA Fuel cell R &D -High power density (k. W/kg) 1. 6 k. Wh/kg vs. 0. 2 k. Wh/kg -lightweight -length of operation Contracts to Universities and industries for fuel cell characterizations *Apollo (UTC, alkaline fuel cell) *Gemini (GE, ion-exchange membrane)
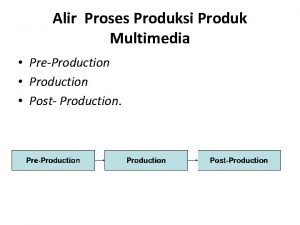
 Production process flow chart
Production process flow chart Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis What is fuel cell in chemistry
What is fuel cell in chemistry Fuel cell apollo
Fuel cell apollo Fuel cell apollo
Fuel cell apollo Roller cell fuel pump
Roller cell fuel pump Hydrogen cycle
Hydrogen cycle Fuel cell uses
Fuel cell uses Fuel cell system
Fuel cell system Molten carbonate fuel cell
Molten carbonate fuel cell Solid oxide fuel cell
Solid oxide fuel cell Fuel value of food
Fuel value of food Parallel flow heat exchanger
Parallel flow heat exchanger Hydrogen energy advantages and disadvantages
Hydrogen energy advantages and disadvantages Fuel cell cartridge
Fuel cell cartridge Fossil fuel energy advantages and disadvantages
Fossil fuel energy advantages and disadvantages How does fossil fuel produce energy
How does fossil fuel produce energy A fuel’s net energy yield is correctly defined as
A fuel’s net energy yield is correctly defined as Electricity pros and cons
Electricity pros and cons