FST 151 FOOD FREEZING FOOD SCIENCE AND TECHNOLOGY






















- Slides: 57

FST 151 FOOD FREEZING FOOD SCIENCE AND TECHNOLOGY 151 Food Freezing - Basic concepts (cont’d) Lecture Notes Prof. Vinod K. Jindal (Formerly Professor, Asian Institute of Technology) Visiting Professor Chemical Engineering Department Mahidol University Salaya, Nakornpathom Thailand Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 1

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 2

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 3

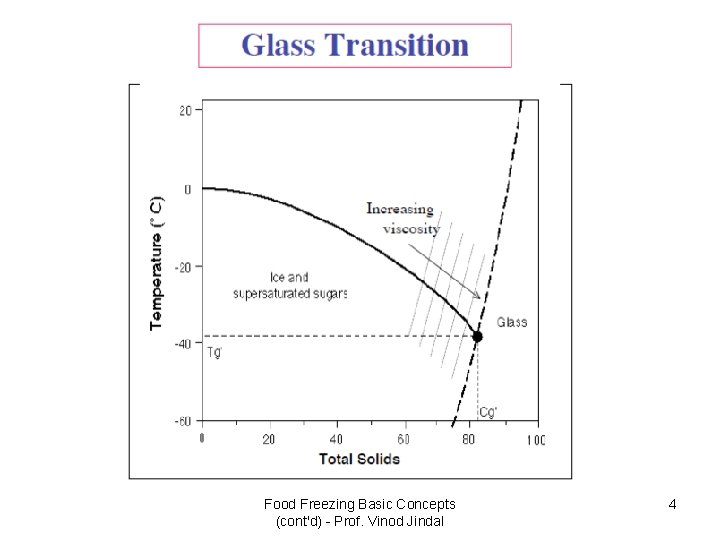
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 4

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 5

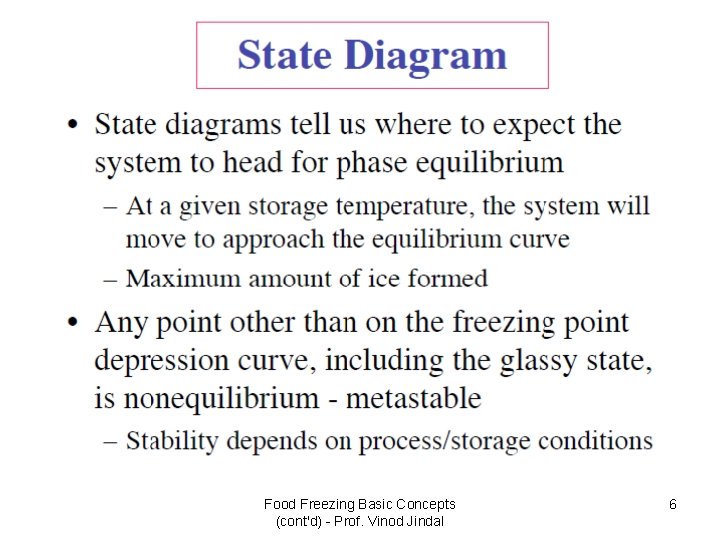
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 6

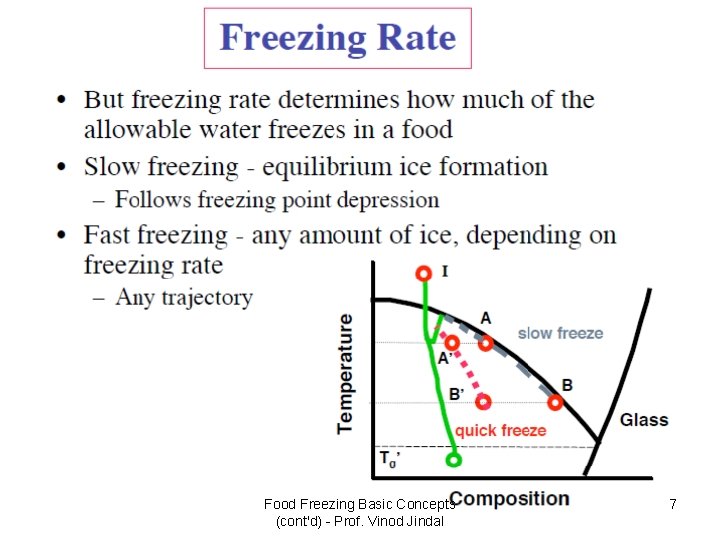
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 7

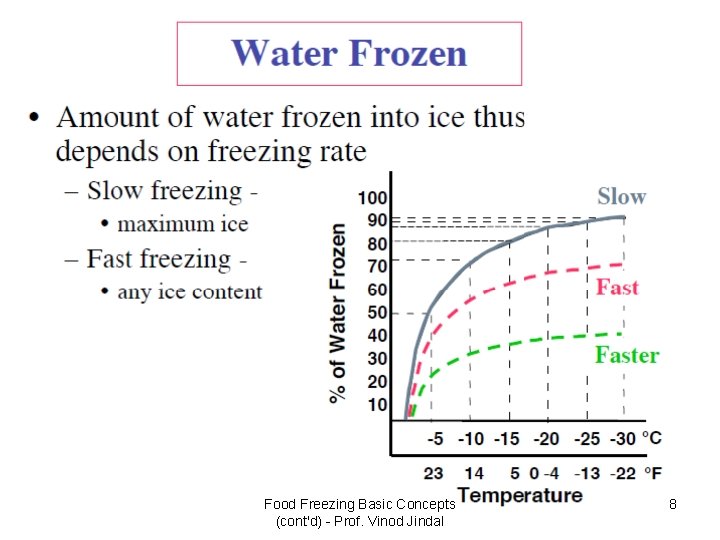
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 8

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 9

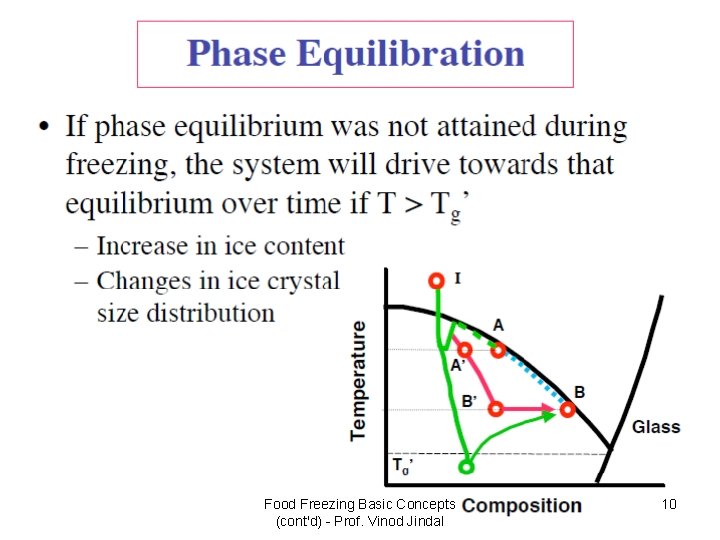
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 10

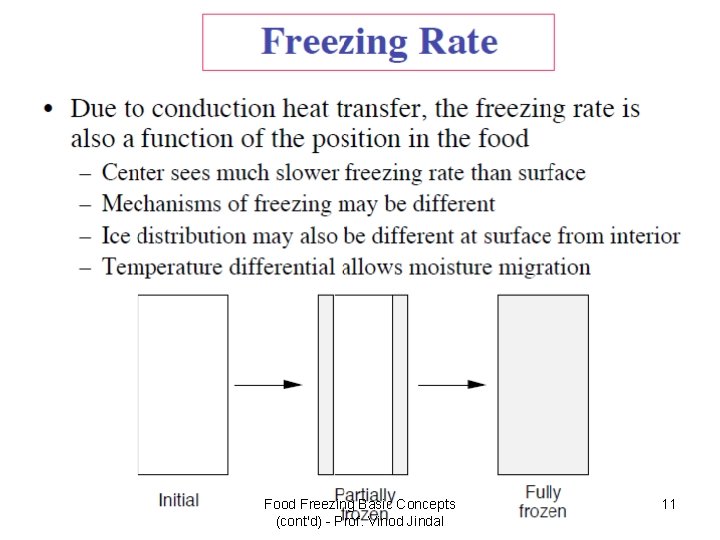
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 11

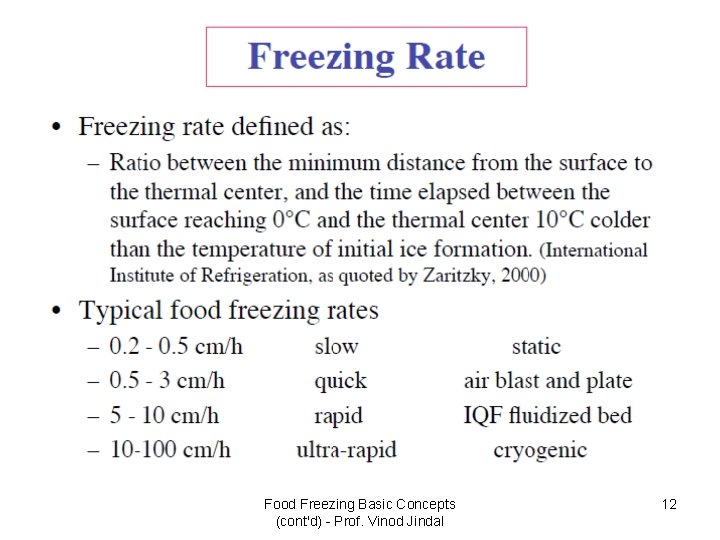
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 12

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 13

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 14

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 15

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 16

Frozen-Food Properties • • Depend on thermal properties of the food product Phase change: Liquid (water) change to solid, the density, thermal conductivity, heat content (enthalpy), specific heat of the product change as temperature decreases below the initial freezing point for water in the food. • 1. Density – The density of solid water is less than that of liquid water – The density of a frozen food is less than the unfrozen product Intensive properties – The magnitude of change in density is proportional to the moisture content of the product • 2. Thermal conductivity – The thermal conductivity of ice is about four times larger than that of liquid water. – Same influence in thermal conductivity of a frozen food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 17

Frozen-Food Properties • • • 3. Enthalpy (heat content) – Important parameter for refrigeration requirement – The heat content normally zero at -40 o. C and increases with increasing temperature – Significant changes in enthalpy occur in 10 o. C below the initial freezing temperature. 4. Apparent specific heat – Depend on function of temperature and phase changes for water in the product – The specific heat of a frozen food at a temperature greater than 20 below the initial point (-2. 61 o. C) 5. Apparent thermal diffusivity – The apparent thermal diffusivity increases as the temperature decreases below the initial freezing point – Frozen product shows larger magnitude than unfrozen product Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 18

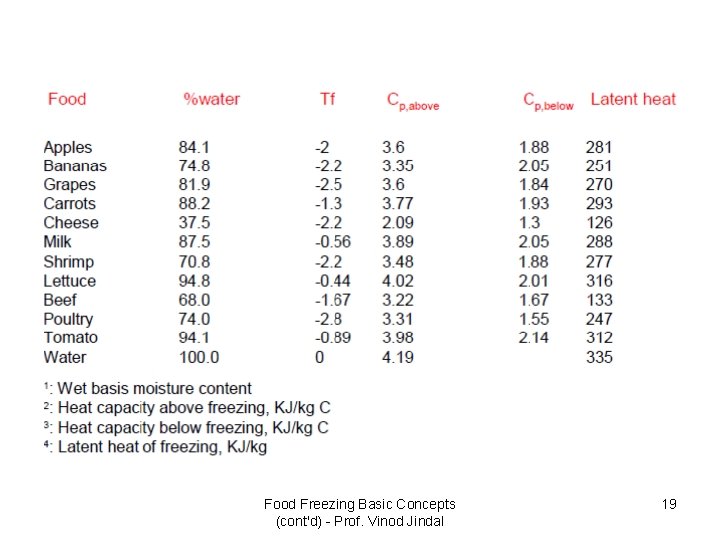
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 19

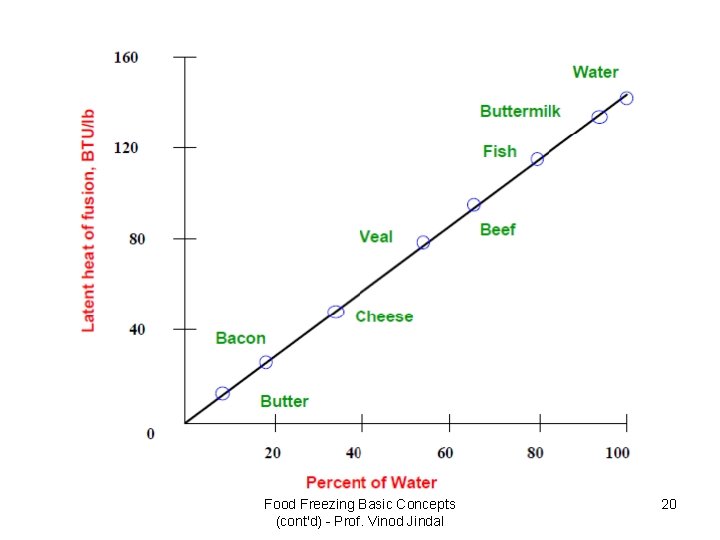
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 20

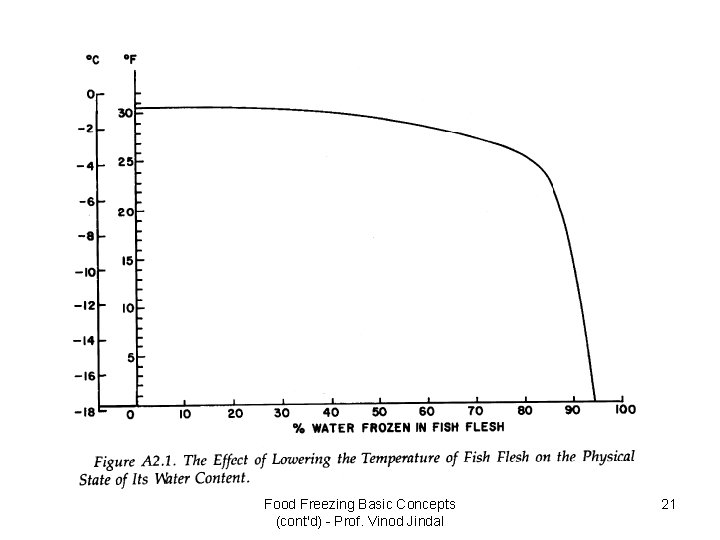
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 21

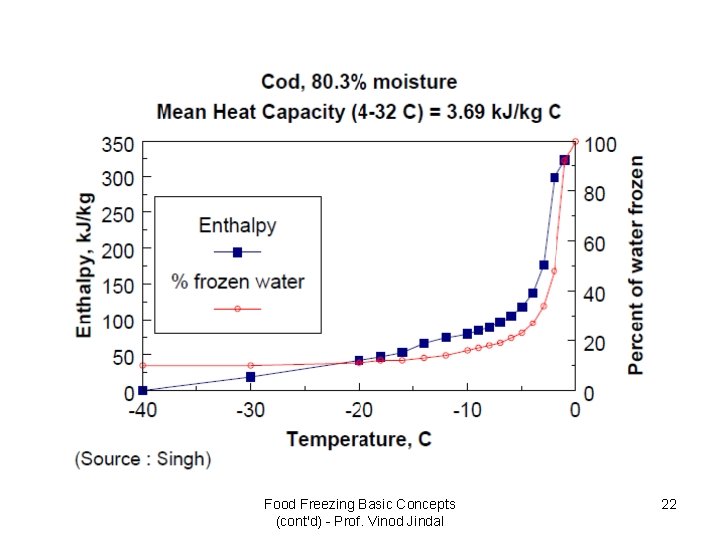
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 22

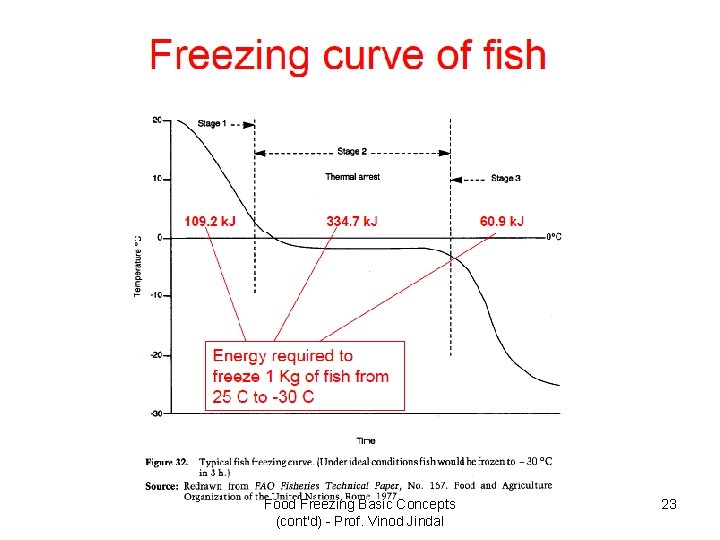
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 23

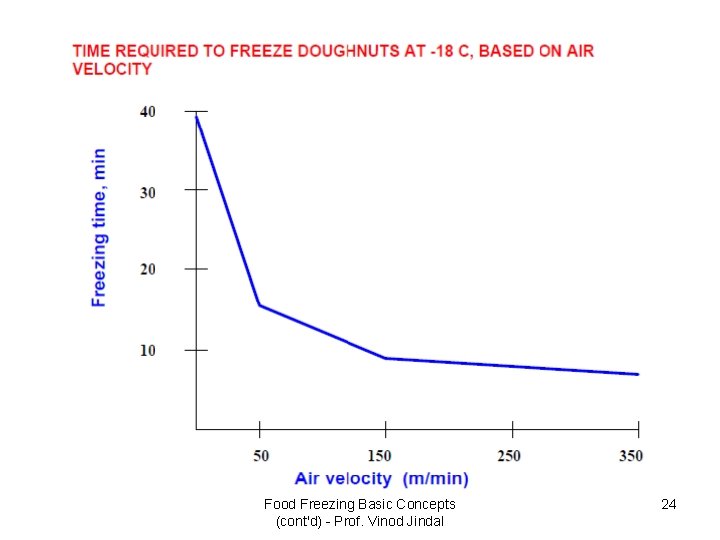
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 24

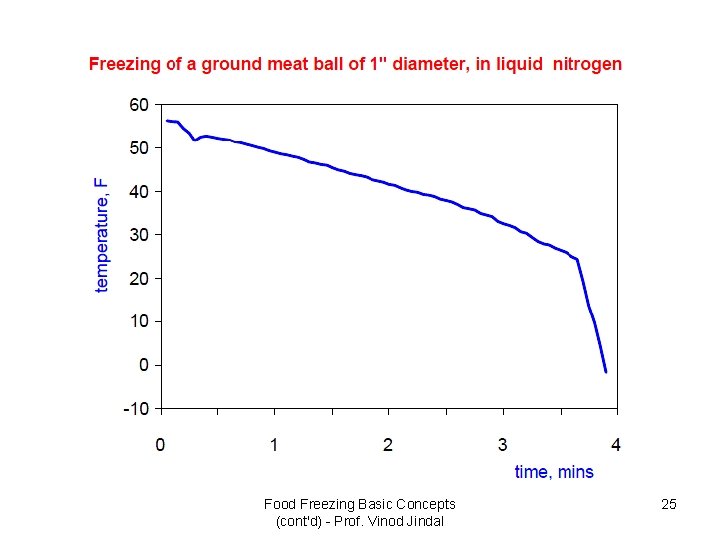
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 25

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 26

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 27

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 28

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 29

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 30

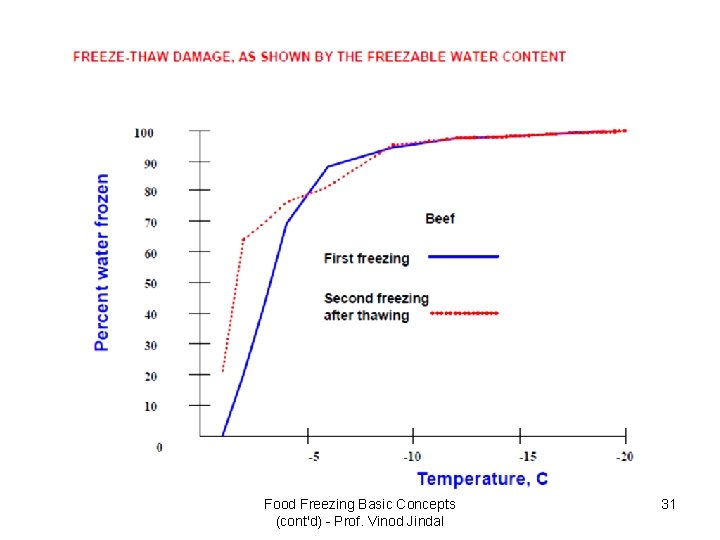
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 31

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 32

Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 33

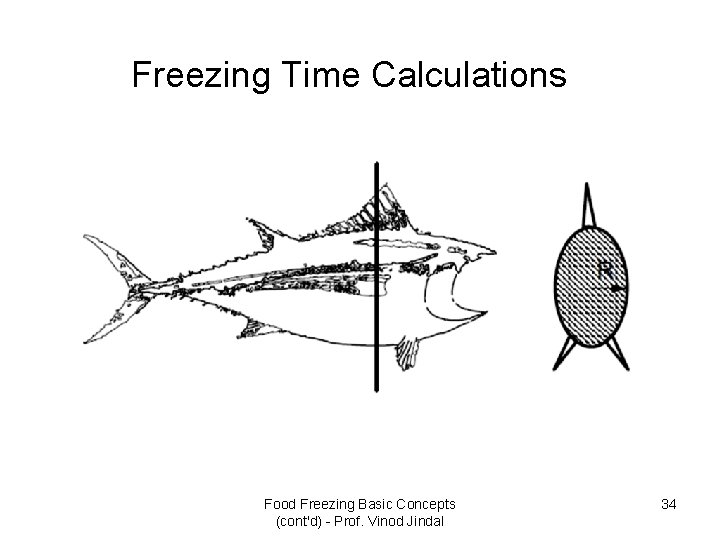
Freezing Time Calculations Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 34

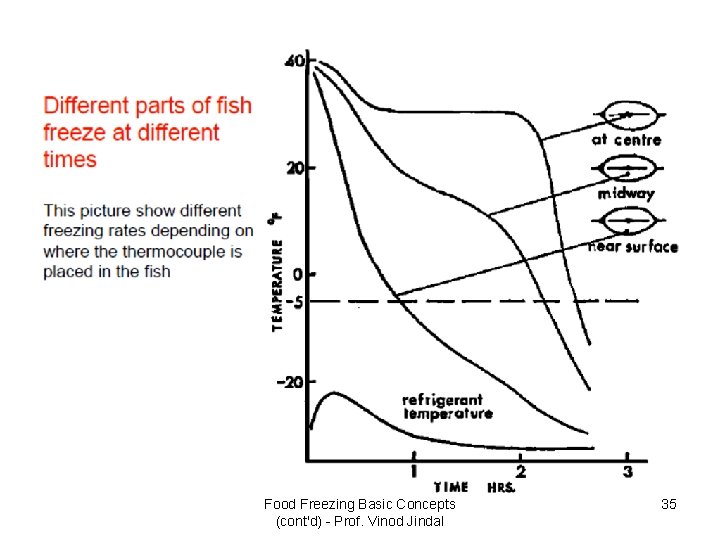
Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 35

Freezing Time Calculation • In freezing time calculations, the imprecise control of freezing conditions and uncertainty in thermal properties data of foods are mainly responsible for not so accurate predictions. • The overall accuracy of prediction is governed more by the uncertainty in thermal properties data rather than the calculation procedure. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 36

• There are three alternatives for obtaining thermal properties data of foods: 1) Use data from literature 2) Direct measurement 3) Using prediction equations based on the composition information Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 37

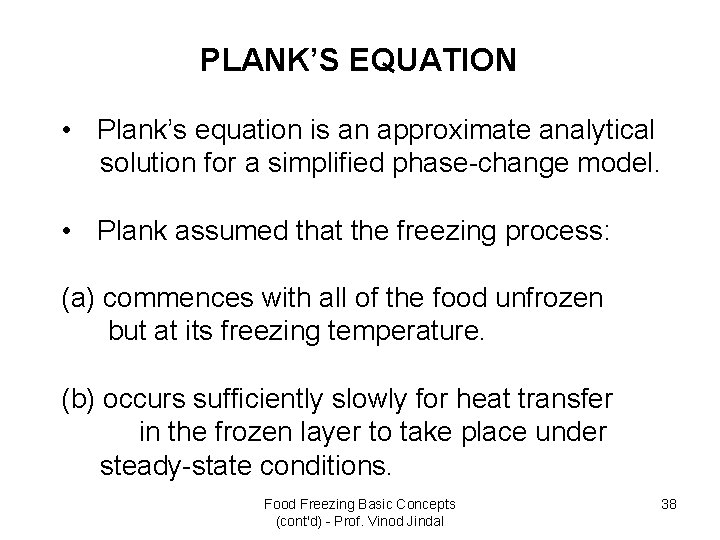
PLANK’S EQUATION • Plank’s equation is an approximate analytical solution for a simplified phase-change model. • Plank assumed that the freezing process: (a) commences with all of the food unfrozen but at its freezing temperature. (b) occurs sufficiently slowly for heat transfer in the frozen layer to take place under steady-state conditions. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 38

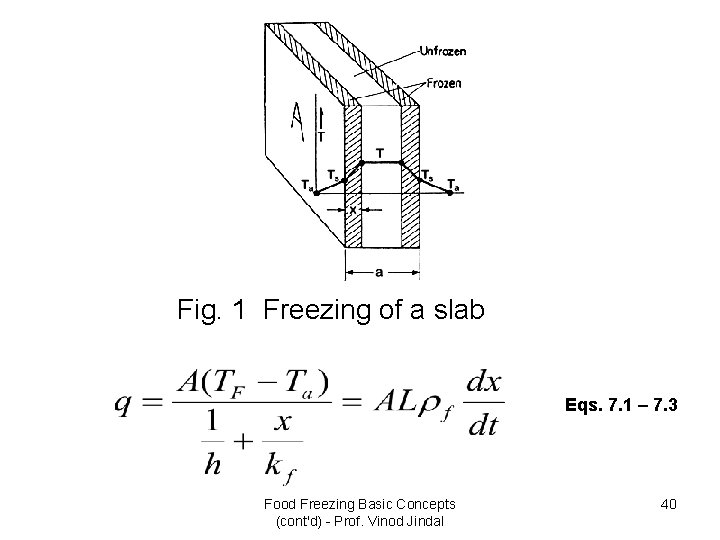
• Plank’s equation considers only phase change period during freezing process. However, Plank’s approximate solution is sufficient for many practical purposes. • This method when applied to calculate the time taken to freeze to the centre of a slab (Fig. 1) whose length and breadth are large compared with the thickness, results in the following equation: Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 39

Fig. 1 Freezing of a slab Eqs. 7. 1 – 7. 3 Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 40

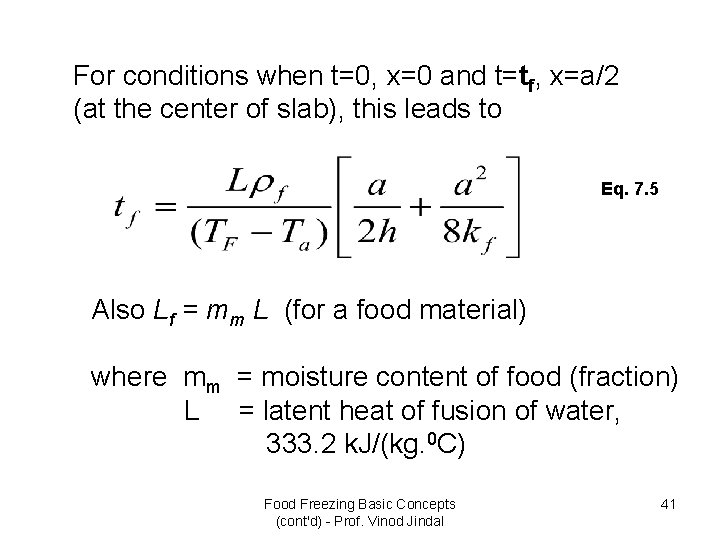
For conditions when t=0, x=0 and t=tf, x=a/2 (at the center of slab), this leads to Eq. 7. 5 Also Lf = mm L (for a food material) where mm = moisture content of food (fraction) L = latent heat of fusion of water, 333. 2 k. J/(kg. 0 C) Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 41

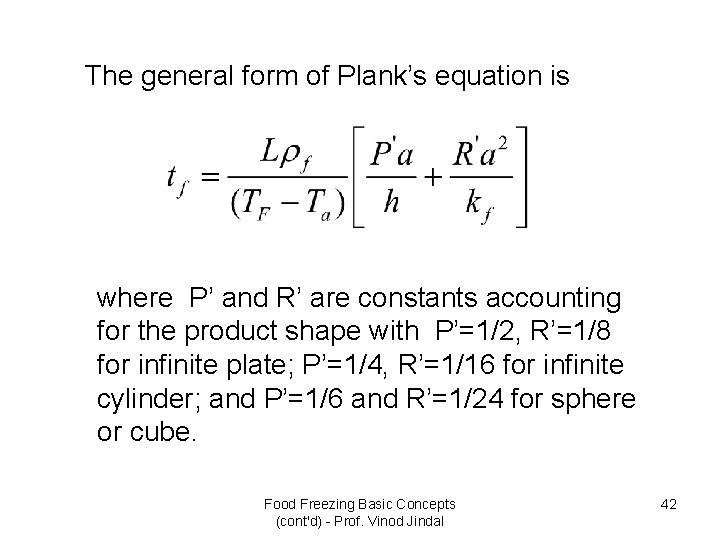
The general form of Plank’s equation is where P’ and R’ are constants accounting for the product shape with P’=1/2, R’=1/8 for infinite plate; P’=1/4, R’=1/16 for infinite cylinder; and P’=1/6 and R’=1/24 for sphere or cube. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 42

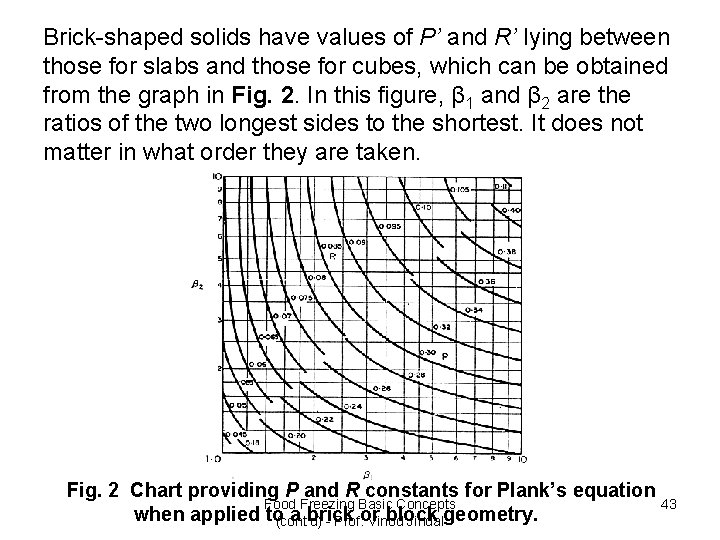
Brick-shaped solids have values of P’ and R’ lying between those for slabs and those for cubes, which can be obtained from the graph in Fig. 2. In this figure, β 1 and β 2 are the ratios of the two longest sides to the shortest. It does not matter in what order they are taken. Fig. 2 Chart providing P and R constants for Plank’s equation Food Freezing Basic Concepts 43 when applied to(cont'd) a brick or block geometry. - Prof. Vinod Jindal

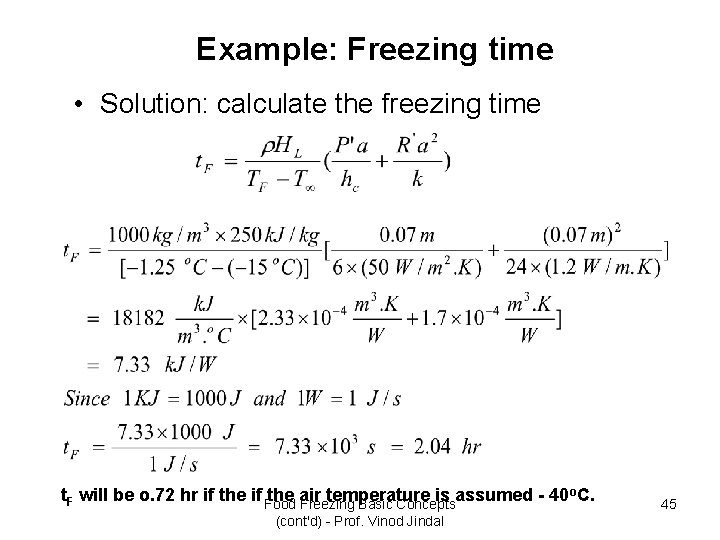
Example: Freezing time) Example 7. 1( • A spherical food product is being frozen in an air-blast wind tunnel. The initial product temperature is 10 o. C and the cold air -15 o. C. The product has a 7 -cm diameter with density of 1, 000 kg/m 3. The initial freezing temperature is -1. 25 o. C, and the latent heat of fusion is 250 k. J/kg. Compute the freezing time. Given: Initial product temperature Ti = 10 o. C Air temperature T = -15 o. C (Not – 40 o. C) Initial freezing temperature TF = -1. 25 o. C Product diameter a = 7 cm (0. 07 m) Product density = 1000 kg/m 3 Thermal conductivity of frozen product k = 1. 2 W/m. k Latent heat HL = 250 k. J/kg Shape constants for spheres: P’ = 1/6, R’ = 1/24 Convective heat-transfer coefficient hc = 50 W/m 2. k Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 44

Example: Freezing time • Solution: calculate the freezing time o. C. t. F will be o. 72 hr if the if Food the air temperature is assumed 40 Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 45

• Plank's equation results in the underestimation of freezing times because of the assumptions made in its derivation. • The initial freezing temperature (TF) for most foods is not reliably known. Although the initial freezing temperature is tabulated for many foods, the initial and final product temperatures are not accounted for in the computation of freezing times. • Also we often do not know for sure what values of ρf and kf to select. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 46

• Despite the limitations, Plank’s equation is the most popular method for predicting freezing time. • Most other available methods are based on the modification of Plank’s equation. • Because of data uncertainty alone, freezing time estimates should be treated as being accurate to within ± 20% at best. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 47

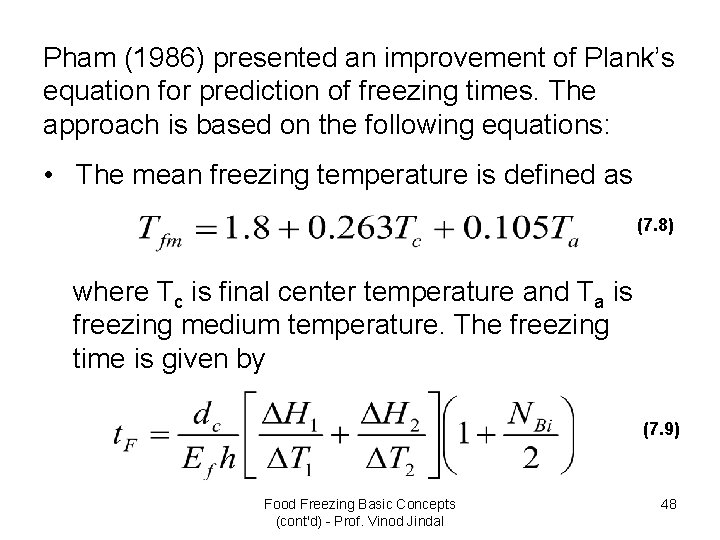
Pham (1986) presented an improvement of Plank’s equation for prediction of freezing times. The approach is based on the following equations: • The mean freezing temperature is defined as (7. 8) where Tc is final center temperature and Ta is freezing medium temperature. The freezing time is given by (7. 9) Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 48

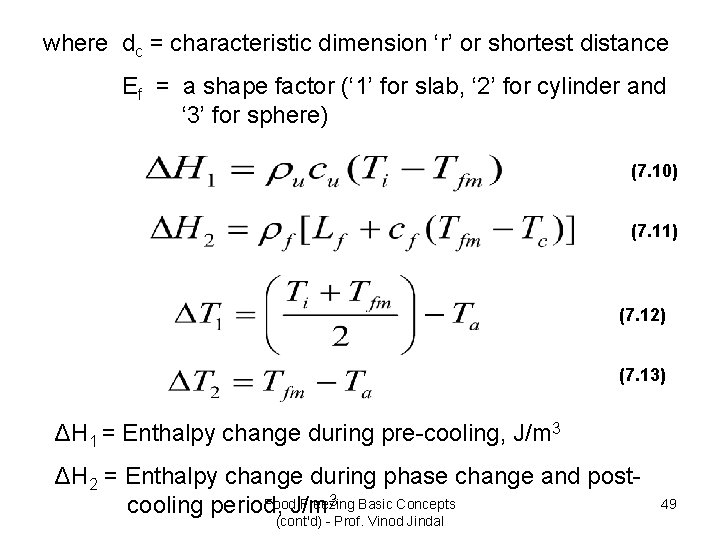
where dc = characteristic dimension ‘r’ or shortest distance Ef = a shape factor (‘ 1’ for slab, ‘ 2’ for cylinder and ‘ 3’ for sphere) (7. 10) (7. 11) (7. 12) (7. 13) ΔH 1 = Enthalpy change during pre-cooling, J/m 3 ΔH 2 = Enthalpy change during phase change and post 3 Basic Concepts Food. J/m Freezing cooling period, (cont'd) - Prof. Vinod Jindal 49

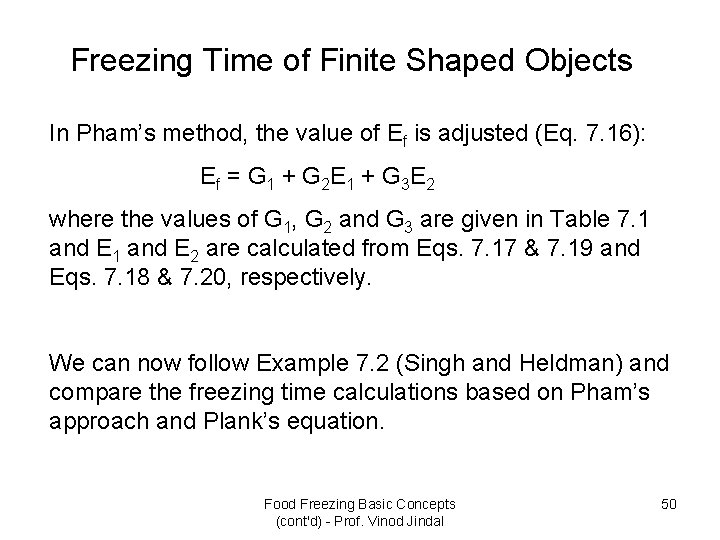
Freezing Time of Finite Shaped Objects In Pham’s method, the value of Ef is adjusted (Eq. 7. 16): E f = G 1 + G 2 E 1 + G 3 E 2 where the values of G 1, G 2 and G 3 are given in Table 7. 1 and E 2 are calculated from Eqs. 7. 17 & 7. 19 and Eqs. 7. 18 & 7. 20, respectively. We can now follow Example 7. 2 (Singh and Heldman) and compare the freezing time calculations based on Pham’s approach and Plank’s equation. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 50

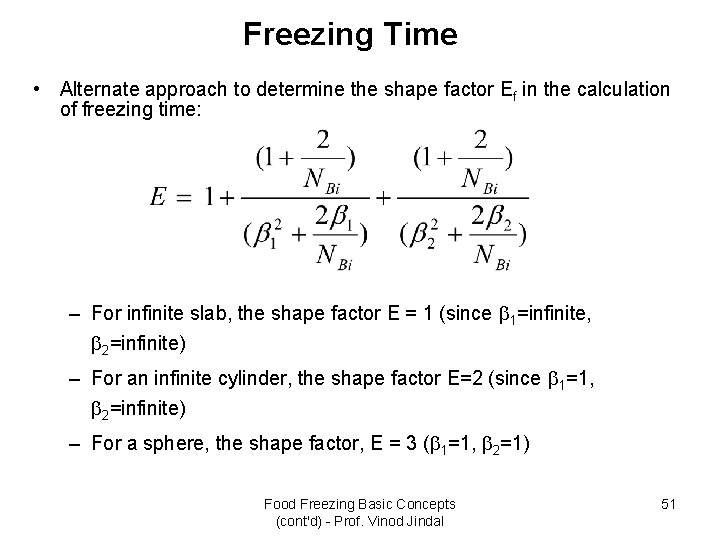
Freezing Time • Alternate approach to determine the shape factor Ef in the calculation of freezing time: – For infinite slab, the shape factor E = 1 (since 1=infinite, 2=infinite) – For an infinite cylinder, the shape factor E=2 (since 1=1, 2=infinite) – For a sphere, the shape factor, E = 3 ( 1=1, 2=1) Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 51

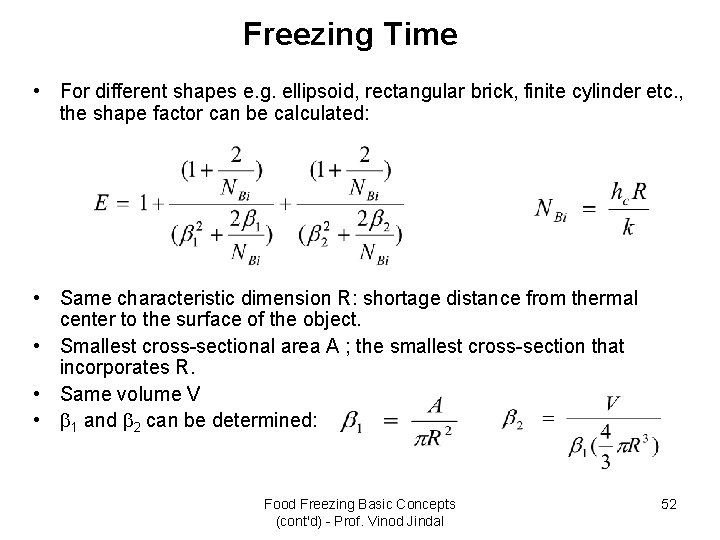
Freezing Time • For different shapes e. g. ellipsoid, rectangular brick, finite cylinder etc. , the shape factor can be calculated: • Same characteristic dimension R: shortage distance from thermal center to the surface of the object. • Smallest cross-sectional area A ; the smallest cross-section that incorporates R. • Same volume V • 1 and 2 can be determined: Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 52

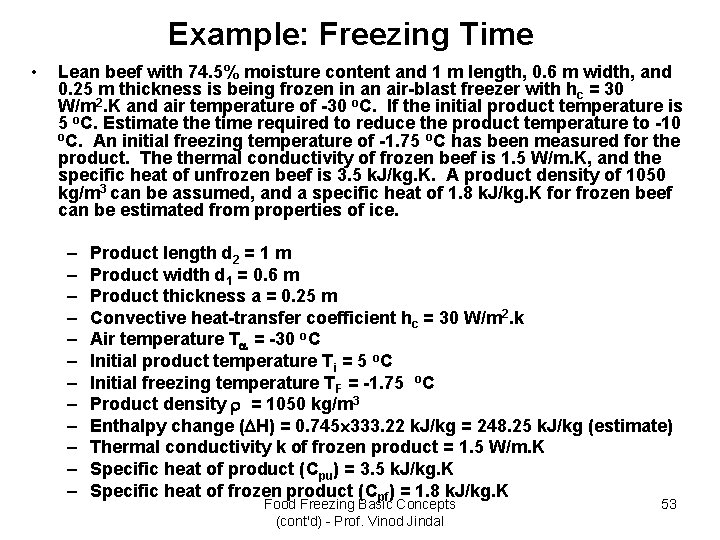
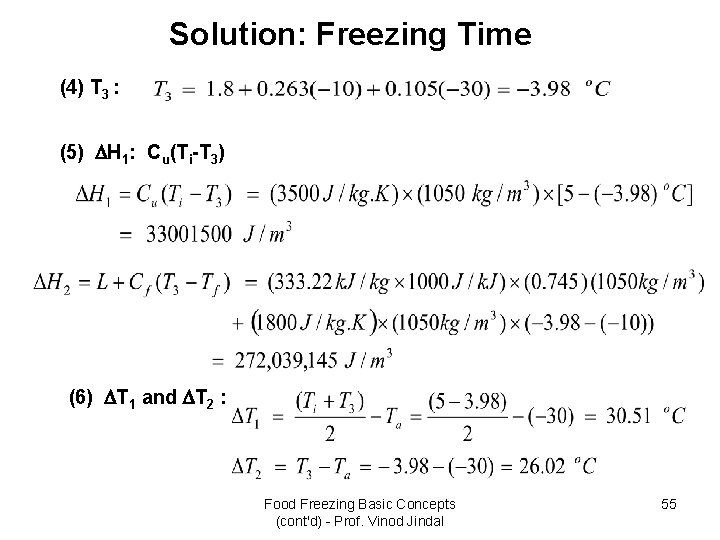
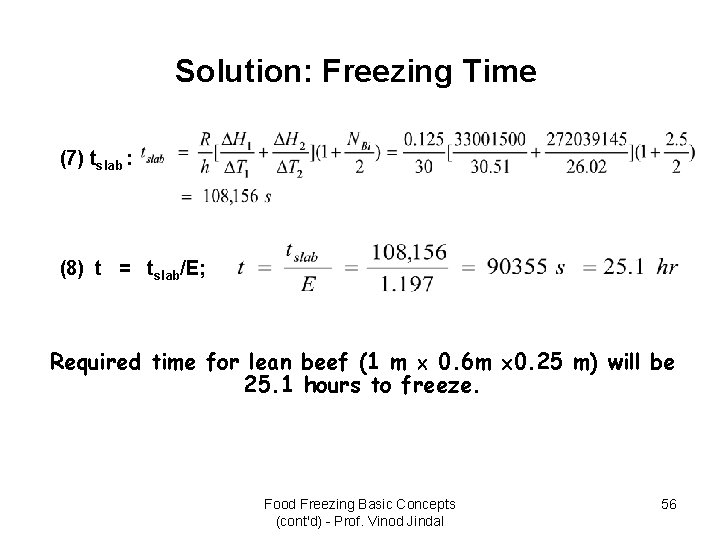
Example: Freezing Time • Lean beef with 74. 5% moisture content and 1 m length, 0. 6 m width, and 0. 25 m thickness is being frozen in an air-blast freezer with hc = 30 W/m 2. K and air temperature of -30 o. C. If the initial product temperature is 5 o. C. Estimate the time required to reduce the product temperature to -10 o. C. An initial freezing temperature of -1. 75 o. C has been measured for the product. The thermal conductivity of frozen beef is 1. 5 W/m. K, and the specific heat of unfrozen beef is 3. 5 k. J/kg. K. A product density of 1050 kg/m 3 can be assumed, and a specific heat of 1. 8 k. J/kg. K for frozen beef can be estimated from properties of ice. – – – Product length d 2 = 1 m Product width d 1 = 0. 6 m Product thickness a = 0. 25 m Convective heat-transfer coefficient hc = 30 W/m 2. k Air temperature T = -30 o. C Initial product temperature Ti = 5 o. C Initial freezing temperature TF = -1. 75 o. C Product density = 1050 kg/m 3 Enthalpy change ( H) = 0. 745 333. 22 k. J/kg = 248. 25 k. J/kg (estimate) Thermal conductivity k of frozen product = 1. 5 W/m. K Specific heat of product (Cpu) = 3. 5 k. J/kg. K Specific heat of frozen product (Cpf) = 1. 8 k. J/kg. K Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 53

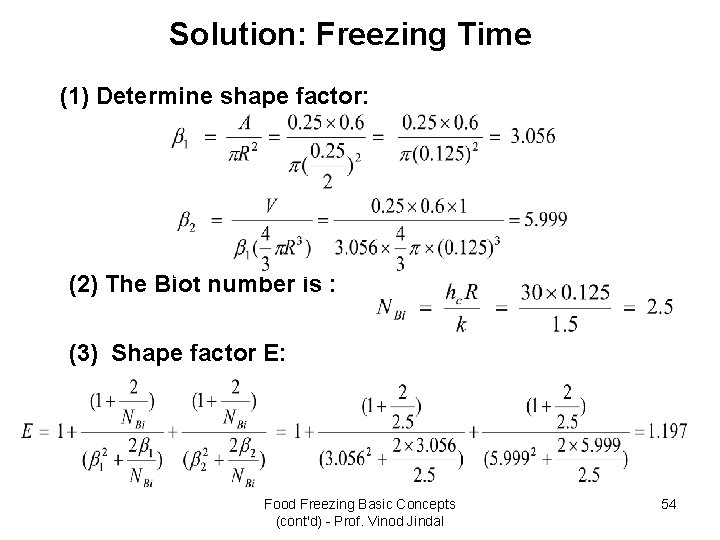
Solution: Freezing Time (1) Determine shape factor: (2) The Biot number is : (3) Shape factor E: Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 54

Solution: Freezing Time (4) T 3 : (5) H 1: Cu(Ti-T 3) (6) T 1 and T 2 : Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 55

Solution: Freezing Time (7) tslab : (8) t = tslab/E; Required time for lean beef (1 m 0. 6 m 0. 25 m) will be 25. 1 hours to freeze. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 56

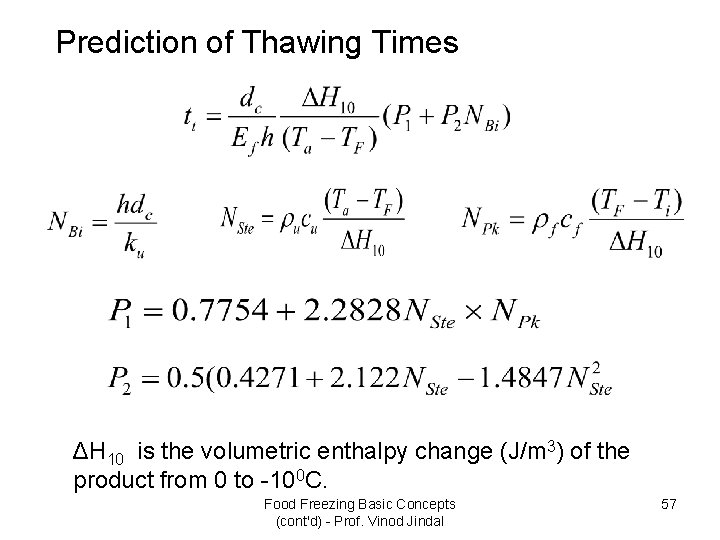
Prediction of Thawing Times ΔH 10 is the volumetric enthalpy change (J/m 3) of the product from 0 to -100 C. Food Freezing Basic Concepts (cont'd) - Prof. Vinod Jindal 57