Frster Resonance Energy Transfer FRET Marek Scholz Activation




- Slides: 18

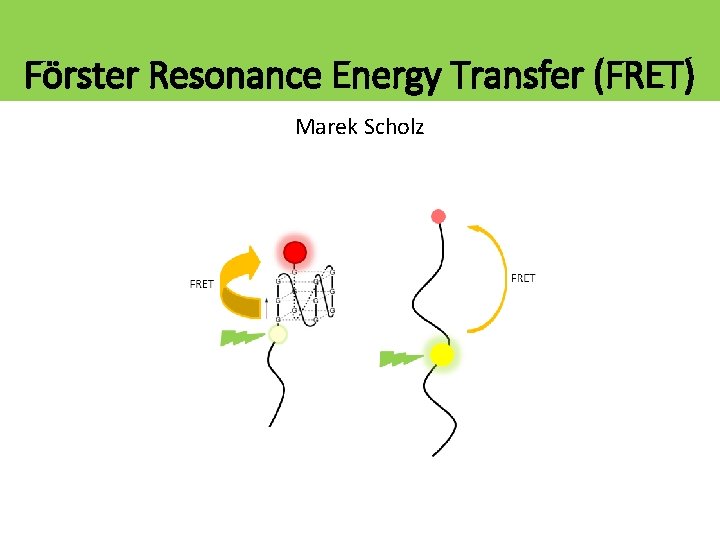
Förster Resonance Energy Transfer (FRET) Marek Scholz

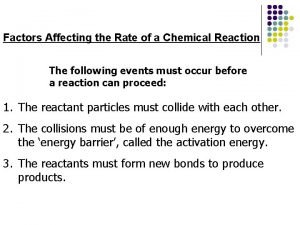
Activation of prior knowledge • What is rate constant, what is its unit and how deactivation rate relates to the excited state lifetime? • What is Stokes shift? • What wavelengths (approximately) have blue, green and red color? • How the quantum yield (QY) of fluorescence is defined? • What is typical for molecular structure of fluorophores? • What is CFP and YFP? • What is usually denoted as S 1 state? Why S? • By which processes the S 1 state can be depleted? • Which processes can cause fluorescence depolarization?

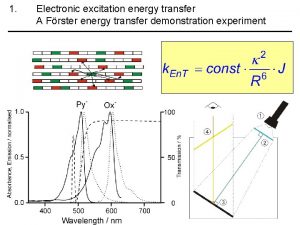
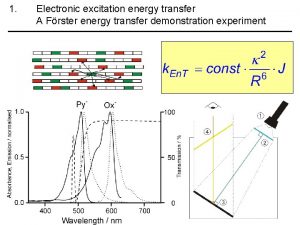
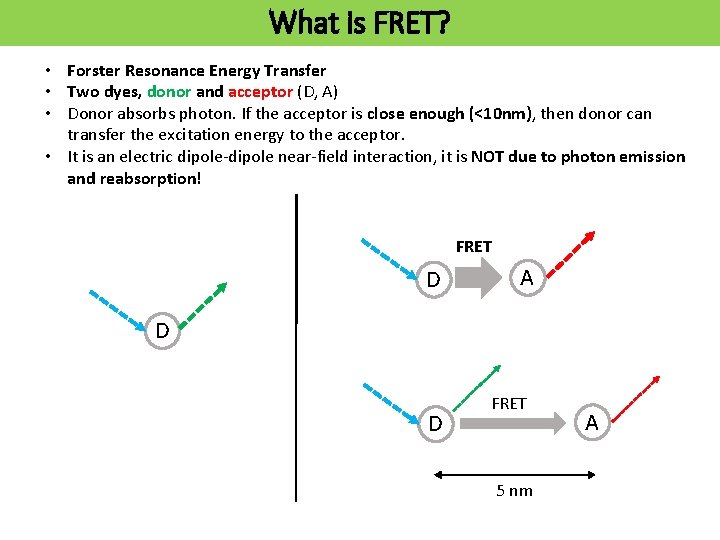
What is FRET? • Forster Resonance Energy Transfer • Two dyes, donor and acceptor (D, A) • Donor absorbs photon. If the acceptor is close enough (<10 nm), then donor can transfer the excitation energy to the acceptor. • It is an electric dipole-dipole near-field interaction, it is NOT due to photon emission and reabsorption! FRET D A D D FRET 5 nm A

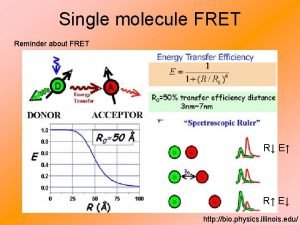
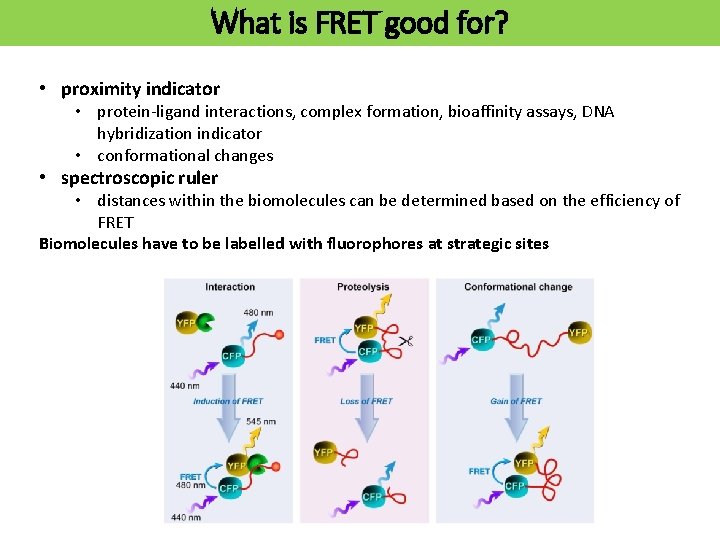
What is FRET good for? • proximity indicator • protein-ligand interactions, complex formation, bioaffinity assays, DNA hybridization indicator • conformational changes • spectroscopic ruler • distances within the biomolecules can be determined based on the efficiency of FRET Biomolecules have to be labelled with fluorophores at strategic sites

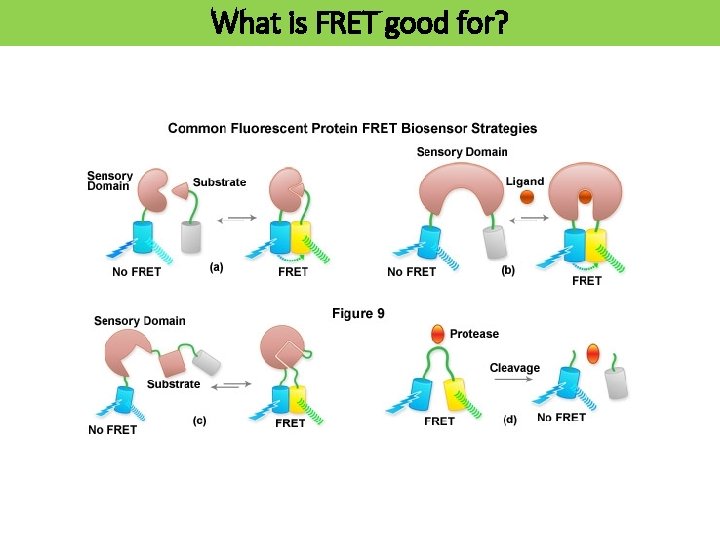
What is FRET good for?

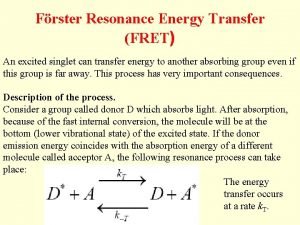
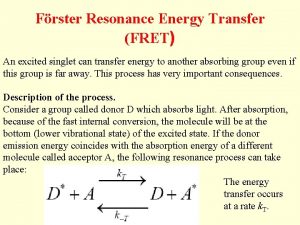
Under what conditions FRET occurs? • The interaction is of dipole nature (coupled oscillators in resonance) • It depends on the distance R of the molecules and the orientation of their transition dipoles. There also has to be spectral overlap of donor emission and acceptor absorption.

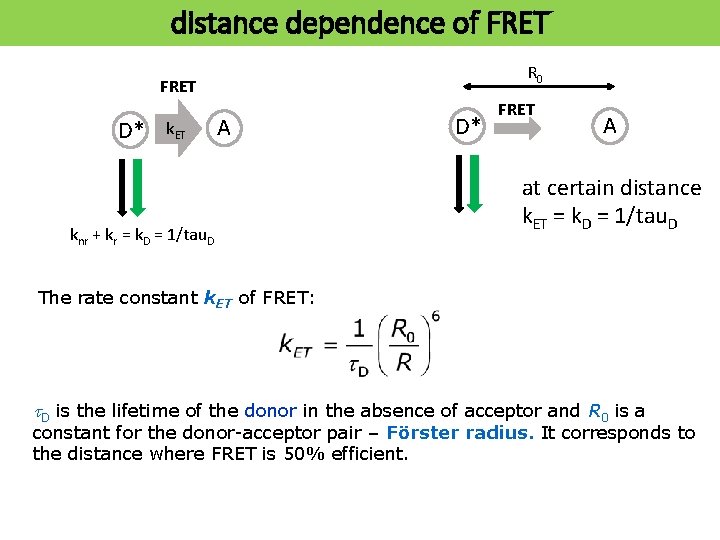
distance dependence of FRET R 0 FRET D* k. ET A knr + kr = k. D = 1/tau. D D* FRET A at certain distance k. ET = k. D = 1/tau. D The rate constant k. ET of FRET: t. D is the lifetime of the donor in the absence of acceptor and R 0 is a constant for the donor-acceptor pair – Förster radius. It corresponds to the distance where FRET is 50% efficient.

FRET efficiency Where k. ET is the rate of energy transfer and ki of all other deactivation processes. Distances can generally be measured between ~0. 5 R 0 and ~1. 5 R 0

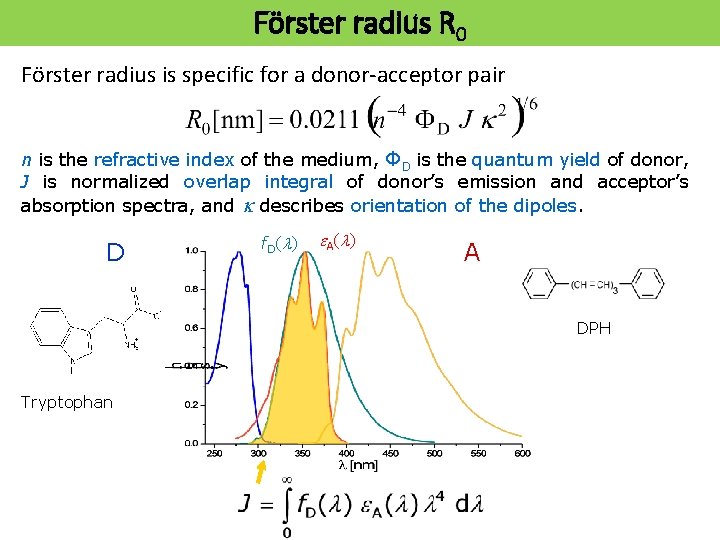
Förster radius R 0 Förster radius is specific for a donor-acceptor pair n is the refractive index of the medium, ΦD is the quantum yield of donor, J is normalized overlap integral of donor’s emission and acceptor’s absorption spectra, and k describes orientation of the dipoles. D f. D(l) e A (l ) A DPH Tryptophan

orientation factor If the molecules undergo fast isotropic movement (dynamic averaging) k 2 = 2/3

Forster radius examples

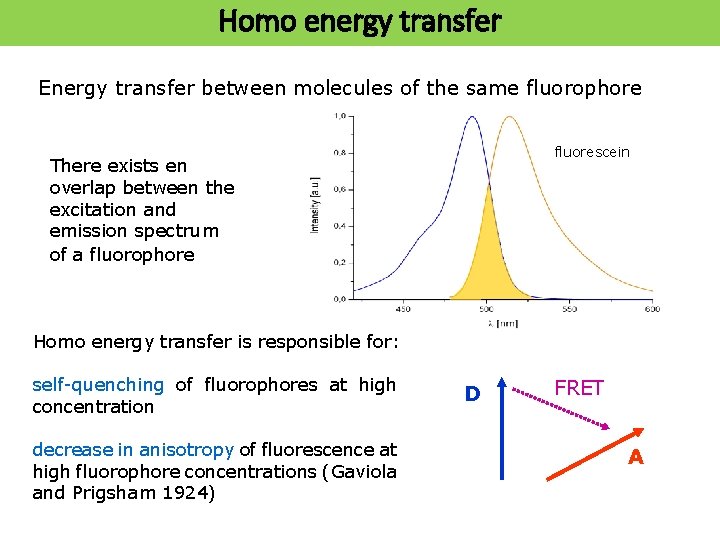
Homo energy transfer Energy transfer between molecules of the same fluorophore fluorescein There exists en overlap between the excitation and emission spectrum of a fluorophore Homo energy transfer is responsible for: self-quenching of fluorophores at high concentration decrease in anisotropy of fluorescence at high fluorophore concentrations (Gaviola and Prigsham 1924) D FRET A

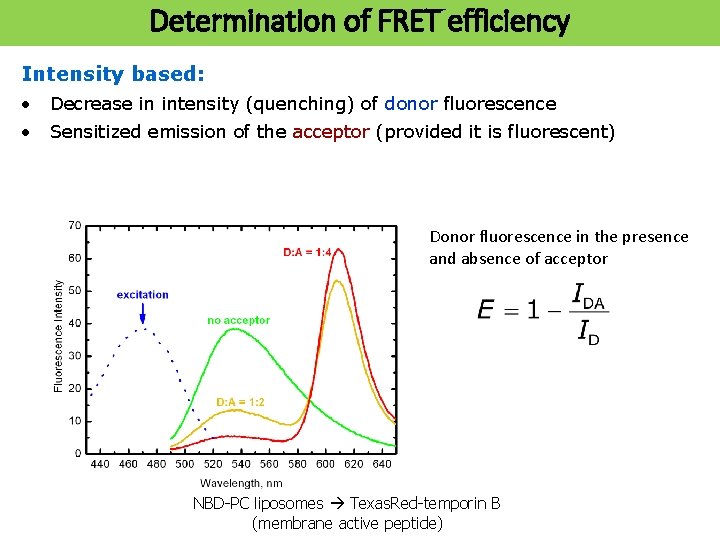
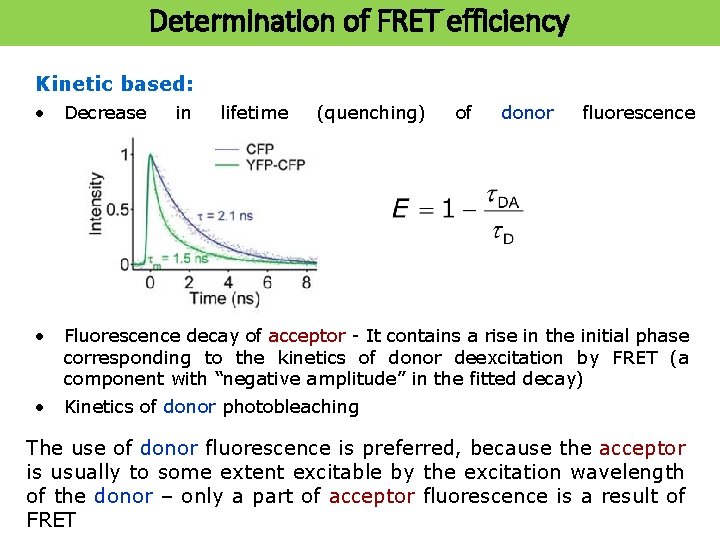
Determination of of FRET efficiency Determination efficiency Intensity based: • Decrease in intensity (quenching) of donor fluorescence • Sensitized emission of the acceptor (provided it is fluorescent) Donor fluorescence in the presence and absence of acceptor NBD-PC liposomes Texas. Red-temporin B (membrane active peptide)

Determination of FRET efficiency Kinetic based: • Decrease in lifetime (quenching) of donor fluorescence • Fluorescence decay of acceptor - It contains a rise in the initial phase corresponding to the kinetics of donor deexcitation by FRET (a component with “negative amplitude” in the fitted decay) • Kinetics of donor photobleaching The use of donor fluorescence is preferred, because the acceptor is usually to some extent excitable by the excitation wavelength of the donor – only a part of acceptor fluorescence is a result of FRET

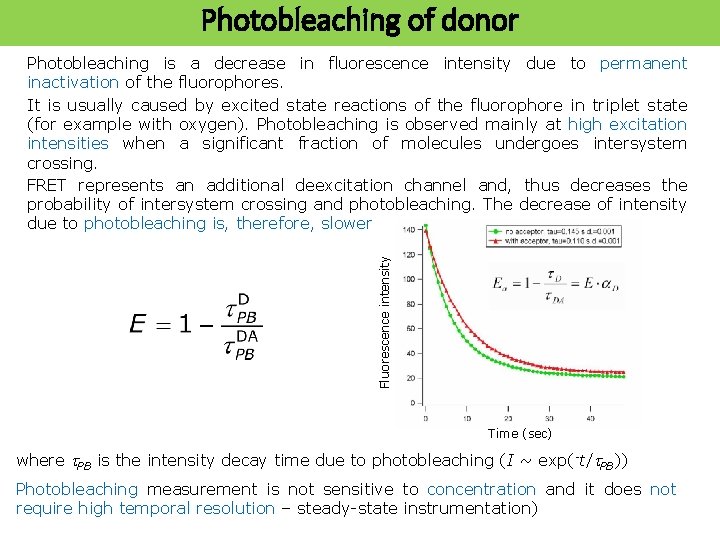
Photobleaching of donor Fluorescence intensity Photobleaching is a decrease in fluorescence intensity due to permanent inactivation of the fluorophores. It is usually caused by excited state reactions of the fluorophore in triplet state (for example with oxygen). Photobleaching is observed mainly at high excitation intensities when a significant fraction of molecules undergoes intersystem crossing. FRET represents an additional deexcitation channel and, thus decreases the probability of intersystem crossing and photobleaching. The decrease of intensity due to photobleaching is, therefore, slower Time (sec) where t. PB is the intensity decay time due to photobleaching (I ~ exp(-t/t. PB)) Photobleaching measurement is not sensitive to concentration and it does not require high temporal resolution – steady-state instrumentation)

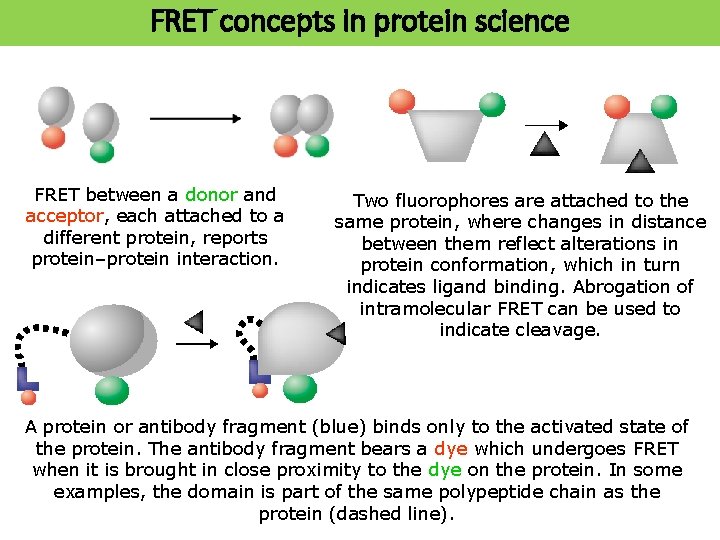
FRET concepts in protein science FRET between a donor and acceptor, each attached to a different protein, reports protein–protein interaction. Two fluorophores are attached to the same protein, where changes in distance between them reflect alterations in protein conformation, which in turn indicates ligand binding. Abrogation of intramolecular FRET can be used to indicate cleavage. A protein or antibody fragment (blue) binds only to the activated state of the protein. The antibody fragment bears a dye which undergoes FRET when it is brought in close proximity to the dye on the protein. In some examples, the domain is part of the same polypeptide chain as the protein (dashed line).

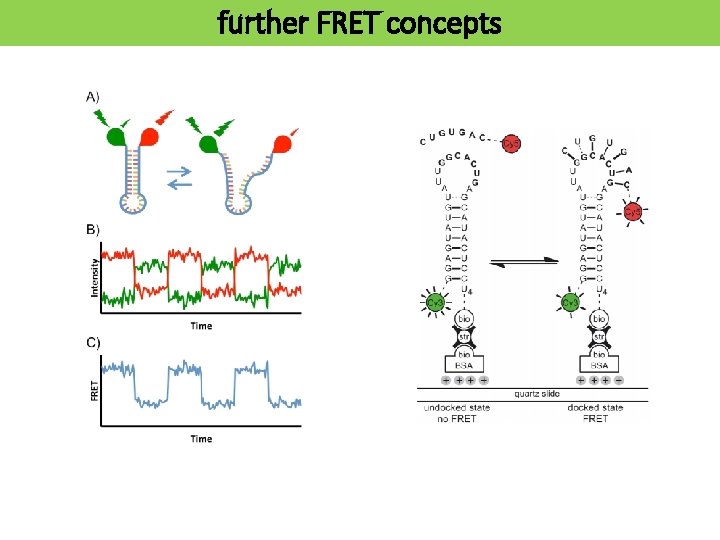
further FRET concepts

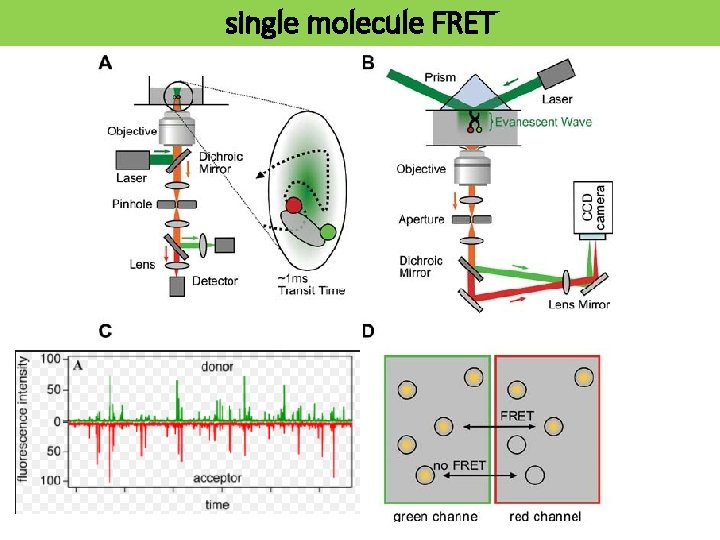
single molecule FRET
 Marek scholz
Marek scholz Energy transfer theory
Energy transfer theory Activation tree and activation record
Activation tree and activation record Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Elfriede scholz
Elfriede scholz Fritz scholz
Fritz scholz Scott scholz
Scott scholz Miklas scholz
Miklas scholz Fret formula
Fret formula Saggio fret
Saggio fret Restools
Restools Konijn zachte keutels
Konijn zachte keutels Activation energy unit
Activation energy unit The lower the activation energy the faster the reaction
The lower the activation energy the faster the reaction Activation energy for reverse reaction
Activation energy for reverse reaction How does temperature affect rate of reaction
How does temperature affect rate of reaction Activation energy definition
Activation energy definition A disturbance that transfers energy
A disturbance that transfers energy