FROM EVIDENCE SYNTHESIS TO NATIONAL GUIDELINES POLICY CHIPS













































- Slides: 65

FROM EVIDENCE SYNTHESIS TO NATIONAL GUIDELINES & POLICY CHIPS Seminar April 4, 2019 Beth Devine, Ph. D, Pharm. D, MBA Professor, UW CHOICE Institute Slides courtesy of Marian Mc. Donaugh, Pharm. D, Professor, OHSU

HOW EVIDENCE SYNTHESIS IS USED IN POLICYMAKING > AHRQ Effective Healthcare Program > US Preventive Services Task Force > Drug Effectiveness Review Project > Medicaid Evidence-based Decisions Project > VA Evidence-based Synthesis Program > Professional Society Clinical Guidelines > Other government agencies


AGENCY FOR HEALTHCARE RESEARCH AND QUALITY (AHRQ) Evidence-based Practice Centers (EPCs) > Created in 1997; 5 -year cycles; now EPC VI – EPCs conduct evidence reports on clinical, behavioral, organization, and financing topics – EPCs conduct research on and produce guidance about methodology of systematic reviews and meta-analyses. – Competitive bid process to be an EPC > Recognizes methodological expertise, experience with evidence synthesis, and depth and breadth of research resources > Only these EPCs can compete to conduct evidence synthesis projects for AHRQ https: //www. ahrq. gov/research/findings/evid ence-based-reports/overview/index. html

CURRENT EPCs (EPC V): > > > > Brown University, Center for Evidence-based Medicine, Providence, RI Duke University, Durham, NC ECRI – Penn Medicine Evidence-based Practice Center, PA Johns Hopkins University, Baltimore, MD. Kaiser Permanente Research Affiliates, Portland, OR Mayo Clinic Evidence-based Practice Center, Rochester, MN Pacific Northwest Evidence-based Practice Center - Oregon Health & Science University, Portland, OR RTI International--University of North Carolina, Chapel Hill, NC Southern California Evidence-based Practice Center-RAND, CA University of Alberta, Edmonton, Alberta, Canada. Minnesota Evidence-based Practice Center, Minneapolis, MN. University of Connecticut Evidence-based Practice Center, Storrs, CT Vanderbilt University Medical Center, Nashville, TN

AHRQ EPC REPORTS AND POLICYMAKING > Center for Medicaid and Medicare Services (CMS) – Medicare requests topics typically relevant to a specific policy decision Example: Hyperbaric Oxygen > National Institutes of Health – NIH requests topics, typically for planned Evidence Forums Example: Chronic Fatigue Syndrome > Others: US PSTF, Clinical Guidelines Committees








U. S. PREVENTIVE SERVICES TASK FORCE > An independent, non-governmental panel of experts in primary care and prevention that systematically reviews the evidence of effectiveness and develops recommendations for clinical preventive services. > A and B recommendations are currently mandated for insurance coverage under the Affordable Care Act. https: //www. us preventiveservic estaskforce. org/

U. S. PREVENTIVE SERVICES TASK FORCE > Makes recommendations on clinical preventive services to primary care clinicians. > Recommendations apply to adults and children with no signs or symptoms. > The USPSTF scope for clinical preventive services includes: – Screening tests – Counseling – Preventive medications

USPSTF MEMBERS > 16 volunteer members represent primary care including family medicine, internal medicine, nursing, obstetrics and gynecology, pediatrics, and behavioral medicine. > Serve 4 -year terms. > Appointed by AHRQ Director with guidance from Chair & Vice Chairs. > Current members include deans, medical directors, chief health officers, practicing clinicians, and professors.

EVIDENCE-BASED PRACTICE CENTER (EPC) > EPCs are funded by AHRQ to conduct systematic reviews for the USPSTF. > The Pacific Northwest EPC at OHSU has worked with the USPSTF since 1998; UW more recently. > Current methods of the USPSTF were developed with the EPC during the initial years and are refined periodically.

TOPIC NOMINATION > Anyone can nominate a topic for the USPSTF to consider via its Web site. > The public may suggest a new preventive service topic or recommend reconsideration of an existing topic: – Availability of new evidence – Changes in the public health burden of the condition – Availability of new screening tests supported by new evidence

RESEARCH PLAN Draft Research Plan > Task Force members work with researchers from an Evidence -based Practice Center (EPC) to create a draft Research Plan that guides the recommendation process. Opportunity for Public Comment > The draft Research Plan is posted on the USPSTF Web site for public comment. Finalize Research Plan > The Task Force and EPC review all comments, address them as appropriate, and create a final Research Plan.

EVIDENCE REVIEW Draft Evidence Review > Using the final Research Plan, the research team independently gathers and reviews the available evidence and creates a draft Evidence Review. Opportunity for Public Comment > The draft Evidence Review and draft Recommendation Statement are posted on the USPSTF Web site for public comment. Finalize Evidence Review > The EPC reviews all comments on the draft Evidence Review, addresses them as appropriate, and creates a final Evidence Review.

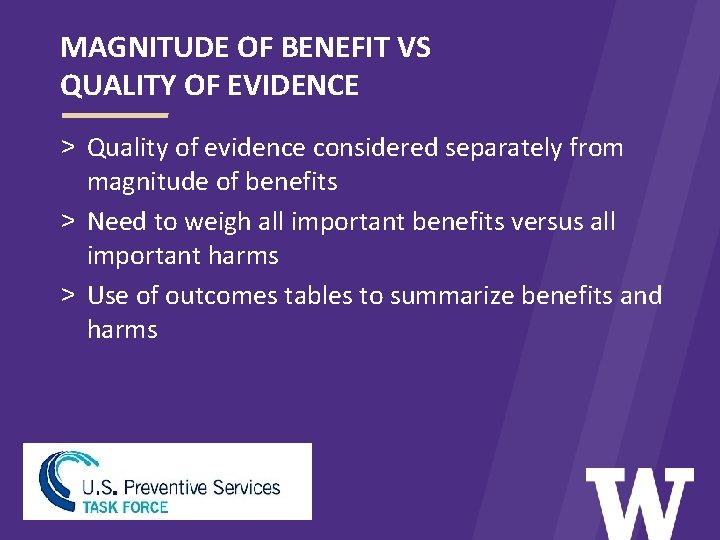
MAGNITUDE OF BENEFIT VS QUALITY OF EVIDENCE > Quality of evidence considered separately from magnitude of benefits > Need to weigh all important benefits versus all important harms > Use of outcomes tables to summarize benefits and harms

QUALITY OF EVIDENCE The USPSTF grades the quality of the overall evidence for a service on a 3 -point scale Good Evidence includes consistent results from well-designed, wellconducted studies in representative populations that directly assess effects on health outcomes. Fair Evidence is sufficient to determine effects on health outcomes, but the strength of the evidence is limited by the number, quality, or consistency of the individual studies, generalizability to routine care. Poor Evidence is insufficient to assess the effects on health outcomes because of limited number or power of studies, important flaws in their design or conduct, gaps in the chain of evidence, or lack of information on important health outcomes.

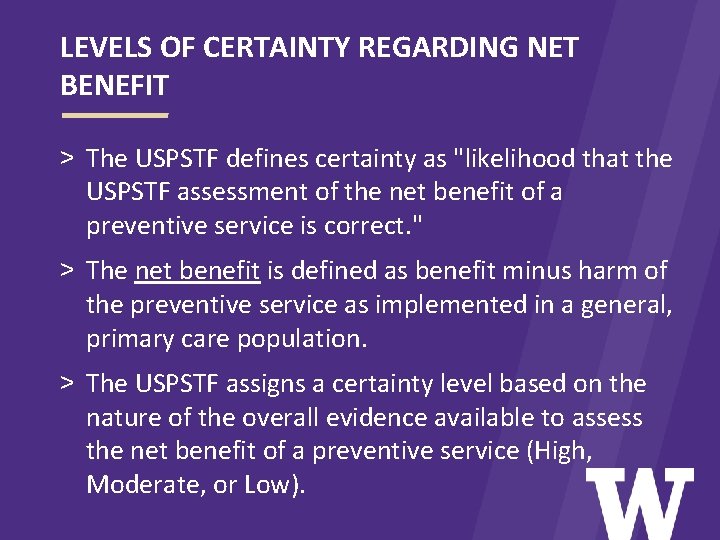
LEVELS OF CERTAINTY REGARDING NET BENEFIT > The USPSTF defines certainty as "likelihood that the USPSTF assessment of the net benefit of a preventive service is correct. " > The net benefit is defined as benefit minus harm of the preventive service as implemented in a general, primary care population. > The USPSTF assigns a certainty level based on the nature of the overall evidence available to assess the net benefit of a preventive service (High, Moderate, or Low).

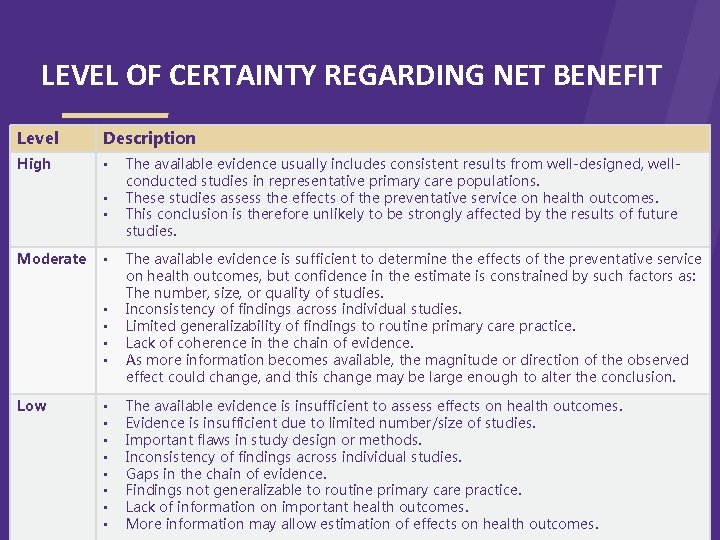
LEVEL OF CERTAINTY REGARDING NET BENEFIT Level High Description > • • • Moderate • • • Low • • The available evidence usually includes consistent results from well-designed, wellconducted studies in representative primary care populations. These studies assess the effects of the preventative service on health outcomes. This conclusion is therefore unlikely to be strongly affected by the results of future studies. The available evidence is sufficient to determine the effects of the preventative service on health outcomes, but confidence in the estimate is constrained by such factors as: The number, size, or quality of studies. Inconsistency of findings across individual studies. Limited generalizability of findings to routine primary care practice. Lack of coherence in the chain of evidence. As more information becomes available, the magnitude or direction of the observed effect could change, and this change may be large enough to alter the conclusion. The available evidence is insufficient to assess effects on health outcomes. Evidence is insufficient due to limited number/size of studies. Important flaws in study design or methods. Inconsistency of findings across individual studies. Gaps in the chain of evidence. Findings not generalizable to routine primary care practice. Lack of information on important health outcomes. More information may allow estimation of effects on health outcomes.

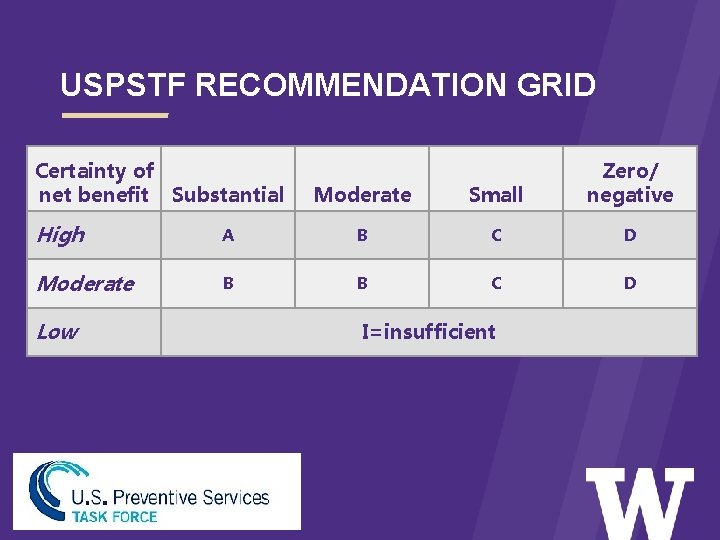
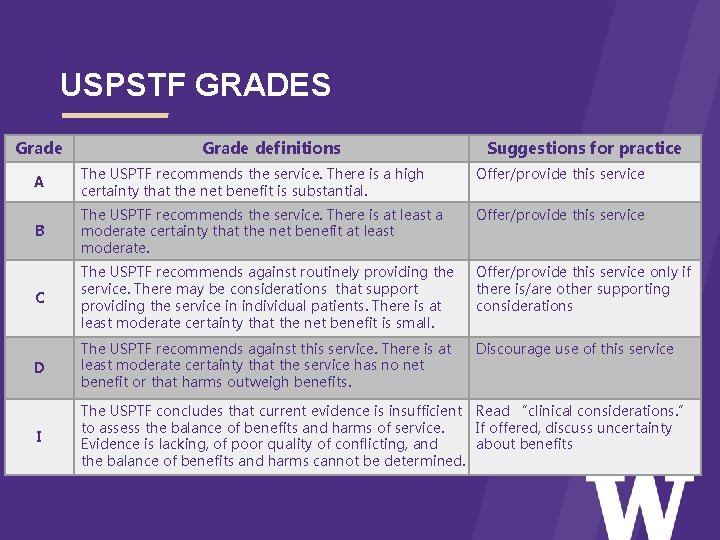
USPSTF RECOMMENDATION GRID > Certainty of net benefit Substantial Moderate Small Zero/ negative High A B C D Moderate B B C D Low I=insufficient

USPSTF GRADES Grade > Grade definitions Suggestions for practice A The USPTF recommends the service. There is a high certainty that the net benefit is substantial. Offer/provide this service B The USPTF recommends the service. There is at least a moderate certainty that the net benefit at least moderate. Offer/provide this service C The USPTF recommends against routinely providing the service. There may be considerations that support providing the service in individual patients. There is at least moderate certainty that the net benefit is small. Offer/provide this service only if there is/are other supporting considerations Discourage use of this service D The USPTF recommends against this service. There is at least moderate certainty that the service has no net benefit or that harms outweigh benefits. I The USPTF concludes that current evidence is insufficient Read “clinical considerations. ” to assess the balance of benefits and harms of service. If offered, discuss uncertainty Evidence is lacking, of poor quality of conflicting, and about benefits the balance of benefits and harms cannot be determined.

RECOMMENDATION STATEMENT Draft Recommendation Statement > The Task Force discusses the draft Evidence Review and the effectiveness of the service. Based on the discussion, the Task Force creates a draft Recommendation Statement. Opportunity for Public Comment > The draft Evidence Review and draft Recommendation Statement are posted simultaneously on the USPSTF Web site for public comment. Finalize Recommendation Statement > The Task Force discusses the final Evidence Review and any new evidence. The Task Force then reviews all comments on the draft Recommendation Statement, addresses them as appropriate, and creates a final Recommendation Statement.

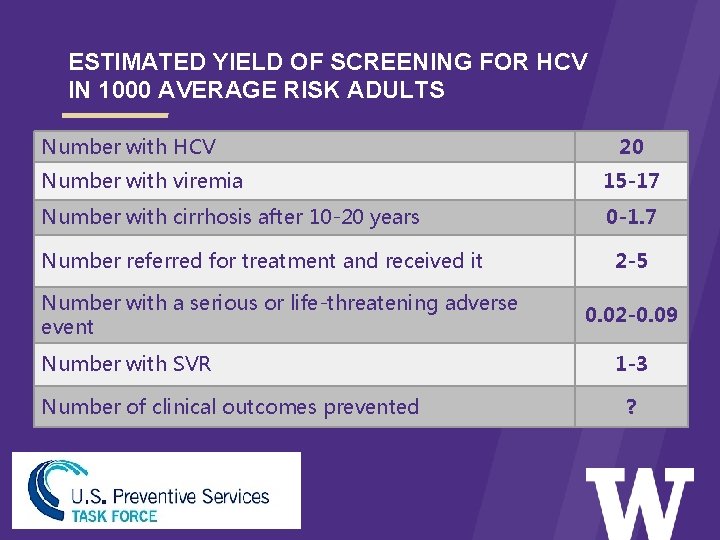
ESTIMATED YIELD OF SCREENING FOR HCV IN 1000 AVERAGE RISK ADULTS Number with HCV > 20 Number with viremia 15 -17 Number with cirrhosis after 10 -20 years 0 -1. 7 Number referred for treatment and received it Number with a serious or life-threatening adverse event Number with SVR Number of clinical outcomes prevented 2 -5 0. 02 -0. 09 1 -3 ?

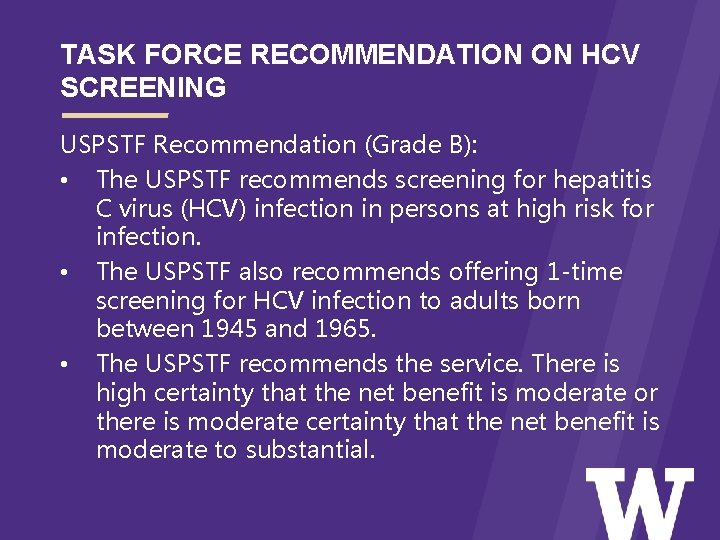
TASK FORCE RECOMMENDATION ON HCV SCREENING USPSTF Recommendation (Grade B): • The USPSTF recommends screening for hepatitis C virus (HCV) infection in persons at high risk for infection. • The USPSTF also recommends offering 1 -time screening for HCV infection to adults born between 1945 and 1965. • The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.

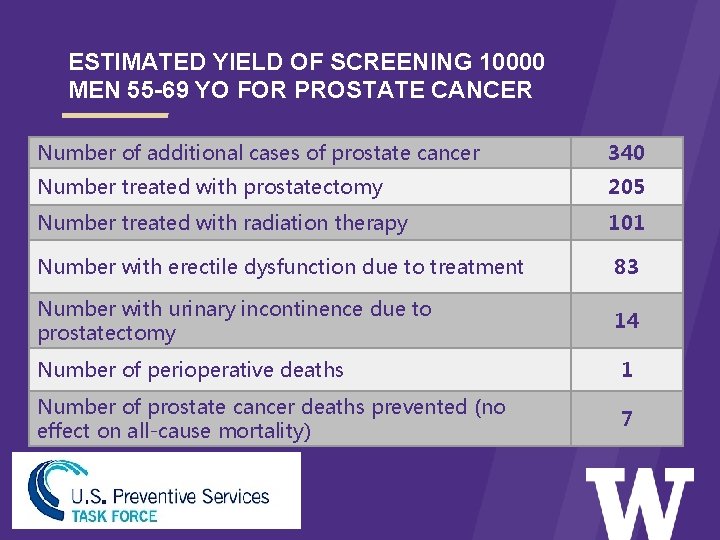
ESTIMATED YIELD OF SCREENING 10000 MEN 55 -69 YO FOR PROSTATE CANCER Number of additional cases of prostate cancer > 340 Number treated with prostatectomy 205 Number treated with radiation therapy 101 Number with erectile dysfunction due to treatment 83 Number with urinary incontinence due to prostatectomy 14 Number of perioperative deaths 1 Number of prostate cancer deaths prevented (no effect on all-cause mortality) 7

TASK FORCE RECOMMENDATION ON SCREENING FOR PROSTATE CANCER USPSTF Recommendation (Grade D): • The U. S. Preventive Services Task Force (USPSTF) recommends against prostatespecific antigen (PSA)-based screening for prostate cancer. • The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits





DRUG EFFECTIVENESS REVIEW PROJECT (DERP) Self-governing collaboration of state Medicaid agencies that: > Pool funds to obtain and synthesize global evidence on the comparative effectiveness, safety, and effects of drugs. > Support policy makers in using the evidence to inform policy in local decision making. – Preferred Drug lists – Prior authorization criteria https: //www. ohsu. edu/evidencebased-practice-center/derp-reports

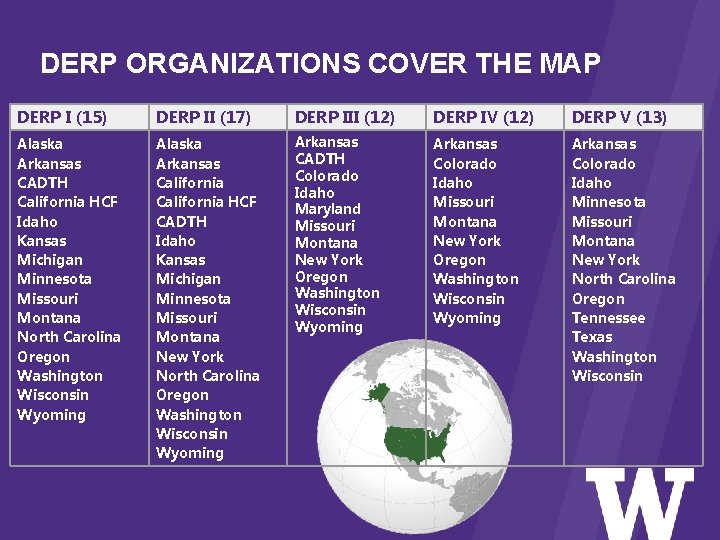
DERP ORGANIZATIONS COVER THE MAP DERP I (15) DERP II (17) DERP III (12) DERP IV (12) DERP V (13) Alaska Arkansas CADTH California HCF Idaho Kansas Michigan Minnesota Missouri Montana North Carolina Oregon Washington Wisconsin Wyoming Alaska Arkansas California HCF CADTH Idaho Kansas Michigan Minnesota Missouri Montana New York North Carolina Oregon Washington Wisconsin Wyoming Arkansas CADTH Colorado Idaho Maryland Missouri Montana New York Oregon Washington Wisconsin Wyoming Arkansas Colorado Idaho Minnesota Missouri Montana New York North Carolina Oregon Tennessee Texas Washington Wisconsin

DRUG EFFECTIVENESS REVIEW PROJECT (DERP) DERP GOVERNANCE > Monthly governance meeting by conference call > Weekly written communication by e-mail (Friday Updates) > Biannual face-to-face conferences > Participant website for posting key questions, drafts, governance materials

DRUG EFFECTIVENESS REVIEW PROJECT (DERP) MEDICAID PARTICIPANTS INVOLVEMENT IN REPORT DEVELOPMENT > Topic and update selection > Determination of key questions: – Indications, Interventions & Outcomes > Recommendation of clinical experts > Review of draft report > Review and approval of final report

DERP TOPIC AREAS (> 130 REPORTS) 2 nd Generation Antipsychotics Estrogens 2 nd Generation Antidepressants Fibromyalgia Drugs 2 nd Generation Antihistamines Hepatitis C Drugs ADHD Drugs Long-acting Insulins Alzheimer's, Drugs to treat Long-acting Opioids Antiepileptic Drugs(off-label) Macrolide antibiotics Antiplatelet Drugs Newer Antiemetics Beta Blockers Calcium Channel Blockers Newer Drugs for Insomnia NSAIDs Diabetes Drugs (oral) Oral Anticoagulants Direct Renin Inhibitors/ACEIs/ AIIRAs PCSK 9 Inhibitors Drugs for Asthma/COPD Proton Pump Inhibitors Drugs for Constipation Skeletal Muscle Relaxants Drugs for Hepatitis C Statins Drugs for Multiple Sclerosis Targeted Immune Modulators Drugs for Neuropathic Pain Topical Calcineurin Inhibitors Drugs for Overactive Bladder Triptans

USING EVIDENCE: STATE MEDICAID AGENCIES PDLS > Preferred Drug List (PDL) determinations > Independent Pharmacy & Therapeutics Committees review evidence in making recommendations > No drug is excluded, but some are preferred > Cost is considered separately from clinical evidence > Public testimony allowed during committee meetings

USE OF EVIDENCE USE BY MEDICAID PARTICIPANTS > States Differ in how they use DERP reports in their PDL process > Most states use DERP reports in addition to other sources – E. g. Pharmacy Benefit Managers (PBM) services > Others use DERP reports almost exclusively – E. g. Washington legislatively mandated to use only DERP reports

CHALLENGES IN USING EVIDENCE: STATE MEDICAID AGENCIES PDLS > States differ in what drugs can be considered based on local legislation – E. g. Mental health drugs are exempt from PDL process in many states > States differ in how they use evidence in these situations – Developing criteria for prior authorization – Clinician education initiatives

CHALLENGES IN DEVELOPING REVIEW SCOPE: SELECTION OF INCLUSION CRITERIA > Drugs to include in disease-state reviews – States differ on view of products with off-label use > E. g. modafinil for ADHD – States differ in interest in evidence on drugs with limited prescribing > E. g. growth hormone for fibromyalgia > Outcome measures to review – Some variation in view of intermediate or surrogate outcomes > E. g. LDL for statins, radiographic evidence for targeted immune modulators

CHALLENGES IN USING EVIDENCE: P&T COMMITTEES AND DERP > Challenges related directly to Comparative Evidence Reviews – Evidence is often complicated, nuanced > Users request high level summaries, easy to read, shorter reports > Rigorous review takes time and $ – Users request faster delivery – Value for money is an issue

DISSEMINATING DERP EVIDENCE > Several states directly interact with DERP report authors > Presentation of findings by author to Pharmacy & Therapeutics Committees > Q&A with committee members, respond to public testimony when requested. > Arkansas, Idaho, Washington, New York, Oregon

RECENT DEVELOPMENTS IN DERP: WHAT THE STATES NEED NOW > Struggling with high cost drugs, rapid development of new drugs, new evidence – Example: Hepatitis C drugs > Need evaluation of evidence prior to drug approval in some cases – Unpublished evidence – Examples: Hepatitis C and PCSK 9 Inhibitor drugs



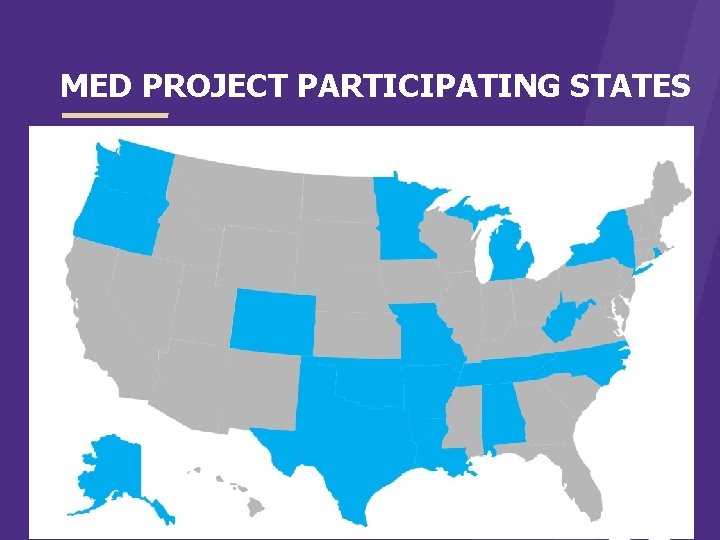
MEDICAID EVIDENCE-BASED DECISIONS PROJECT (MED PROJECT) > Currently eleven state Medicaid collaborative > Started in 2006 > Use evidence to inform benefit and coverage decisions > Over 100 reports produced

MED PROJECT PARTICIPATING STATES

MEDICAID EVIDENCE-BASED DECISIONS PROJECT MED Reports > Systematic Reviews/ Technology Assessments > Rapid Reviews (best evidence syntheses) > Participant Inquiries > Policy Companion Papers/ Policy Briefs > Vendor/ Guideline Reviews

MEDICAID EVIDENCE-BASED DECISIONS PROJECT Policy Companion Papers > Document that may accompany evidence reports or stand alone > Existing policies and guidelines > External and environmental factors > Financial and economic considerations > Policy decision making guide > Examples: ACOs, Coverage of Clinical Trials, Dental Hygiene Scope of Practice

MEDICAID EVIDENCE-BASED DECISIONS PROJECT Example Clinical Topics > Role of percutaneous coronary intervention (PCI) in patients with stable angina > Relationship between adherence to treatment and treatment outcome for chronic hepatitis C > Relationship between extended office hours and emergency department utilization, quality of care, health outcomes > Elective induction of Labor


VA EVIDENCE SYNTHESIS PROGRAM (ESP) Mission: Provide timely and accurate syntheses of targeted healthcare topics of particular importance to VA managers and policymakers, as they work to improve the health and healthcare of Veterans. https: //www. hsrd. research. va. gov/publications/esp/

USE OF REPORTS BY VA > Develop clinical policies informed by evidence, > The implementation of effective services to improve patient outcomes and to support clinical practice guidelines and performance measures, and > Set the direction for future research to address gaps in clinical knowledge.

REVIEWS AND VA POLICY MAKERS > “lay of the land” > Usually, the option to do nothing and wait for more evidence is not an option > Want some evidence to help steer the initial decision

VA ESP REPORTS TO DATE > 84 reports thus far > Broad ranging topics – – Risk prediction models for hospital readmission Suicide prevention interventions Screening pelvic exams Group visits for management of chronic conditions

DISSEMINATION > Reports: > https: //www. hsrd. research. va. gov/publications/esp/ > > > Cyberseminars Management e-briefs Journal manuscripts Conference presentations Guidelines

PROFESSIONAL SOCIETY CLINICAL GUIDELINES > Medical Society Guideline and Consensus Conference Support – American Pain Society – American Psychiatric Association/ American Psychological Association – American Urological Association – American College of Obstetricians and Gynecologists – American College of Chest Physicians – Antithrombotic Guideline – Brain Trauma Foundation

OTHER GOVERNMENT USES > Political influences when funding is an issue > Examples: – British parliament: water fluoridation – US Senate: telehealth, hyperbaric oxygen



THANK YOU!