FRISC II FRagmin and Fast Revascularization during In






- Slides: 37

FRISC II FRagmin® and Fast Revascularization during In. Stablity in Coronary artery disease

FRISC II Trial Design • Assessment of the efficacy of long-term treatment with Fragmin® vs. placebo in patients managed with a noninvasive treatment strategy – Enrolled 2276 patients with unstable coronary artery disease – Patients recruited from June, 1996 - August 1998 – 58 Scandinavian Centers – Randomized – Double Blind – Placebo Controlled – Intention to Treat Analysis Lancet 1999; 354: 701 -07

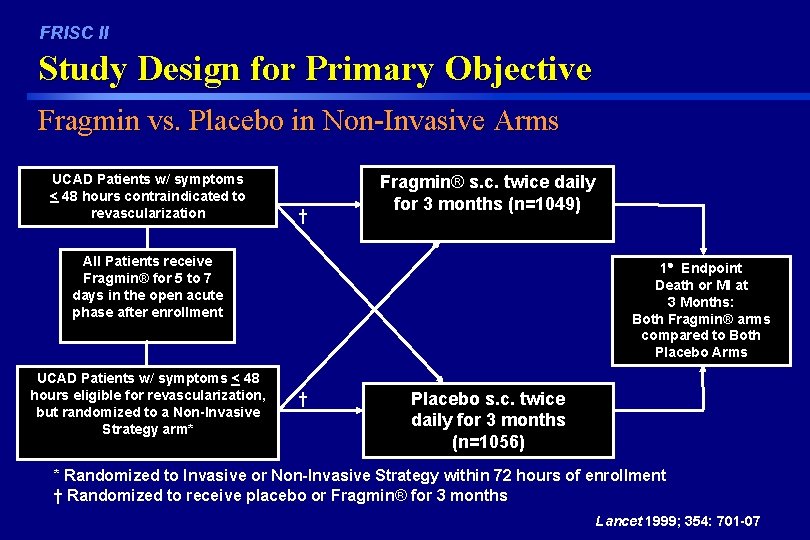
FRISC II Study Design for Primary Objective Fragmin vs. Placebo in Non-Invasive Arms UCAD Patients w/ symptoms < 48 hours contraindicated to revascularization † Fragmin® s. c. twice daily for 3 months (n=1049) All Patients receive Fragmin® for 5 to 7 days in the open acute phase after enrollment UCAD Patients w/ symptoms < 48 hours eligible for revascularization, but randomized to a Non-Invasive Strategy arm* 1 Endpoint Death or MI at 3 Months: Both Fragmin® arms compared to Both Placebo Arms † Placebo s. c. twice daily for 3 months (n=1056) * Randomized to Invasive or Non-Invasive Strategy within 72 hours of enrollment † Randomized to receive placebo or Fragmin® for 3 months Lancet 1999; 354: 701 -07

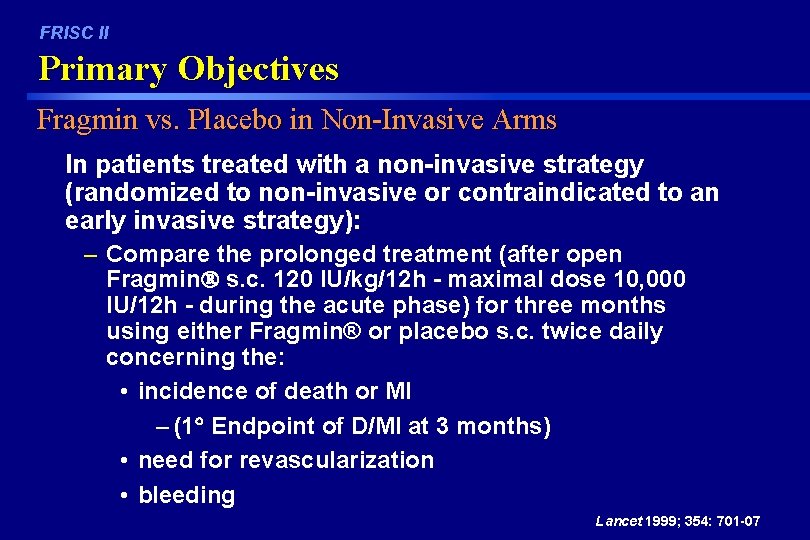
FRISC II Primary Objectives Fragmin vs. Placebo in Non-Invasive Arms In patients treated with a non-invasive strategy (randomized to non-invasive or contraindicated to an early invasive strategy): – Compare the prolonged treatment (after open Fragmin s. c. 120 IU/kg/12 h - maximal dose 10, 000 IU/12 h - during the acute phase) for three months using either Fragmin® or placebo s. c. twice daily concerning the: • incidence of death or MI – (1 Endpoint of D/MI at 3 months) • need for revascularization • bleeding Lancet 1999; 354: 701 -07

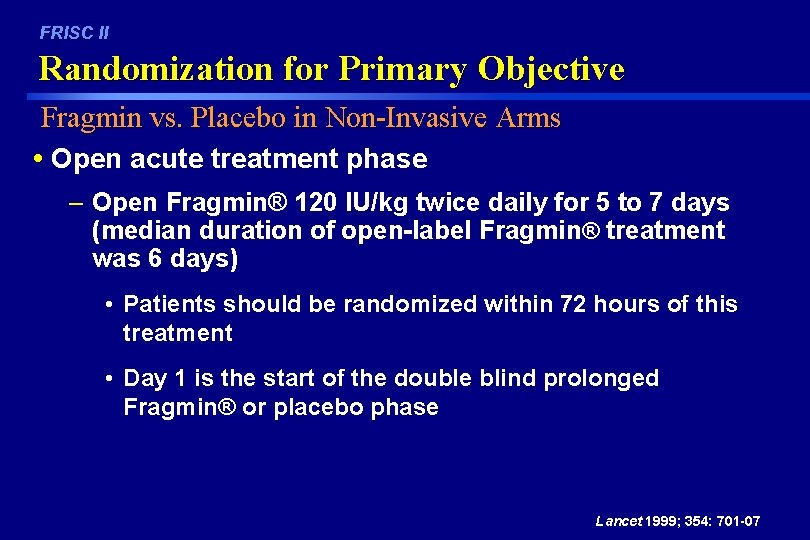
FRISC II Randomization for Primary Objective Fragmin vs. Placebo in Non-Invasive Arms • Open acute treatment phase – Open Fragmin® 120 IU/kg twice daily for 5 to 7 days (median duration of open-label Fragmin® treatment was 6 days) • Patients should be randomized within 72 hours of this treatment • Day 1 is the start of the double blind prolonged Fragmin® or placebo phase Lancet 1999; 354: 701 -07

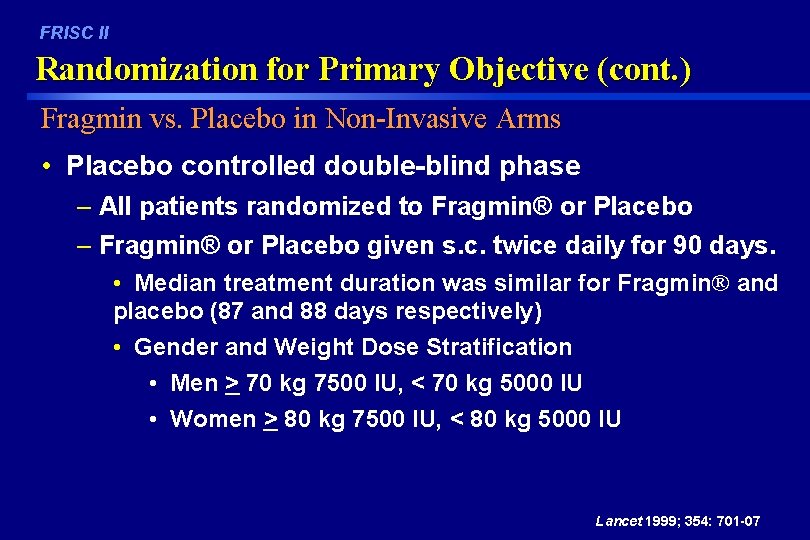
FRISC II Randomization for Primary Objective (cont. ) Fragmin vs. Placebo in Non-Invasive Arms • Placebo controlled double-blind phase – All patients randomized to Fragmin® or Placebo – Fragmin® or Placebo given s. c. twice daily for 90 days. • Median treatment duration was similar for Fragmin® and placebo (87 and 88 days respectively) • Gender and Weight Dose Stratification • Men > 70 kg 7500 IU, < 70 kg 5000 IU • Women > 80 kg 7500 IU, < 80 kg 5000 IU Lancet 1999; 354: 701 -07

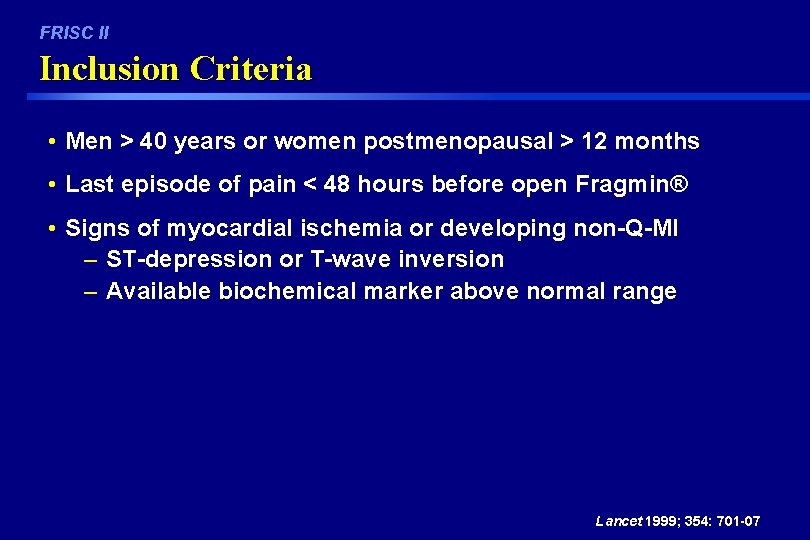
FRISC II Inclusion Criteria • Men > 40 years or women postmenopausal > 12 months • Last episode of pain < 48 hours before open Fragmin® • Signs of myocardial ischemia or developing non-Q-MI – ST-depression or T-wave inversion – Available biochemical marker above normal range Lancet 1999; 354: 701 -07

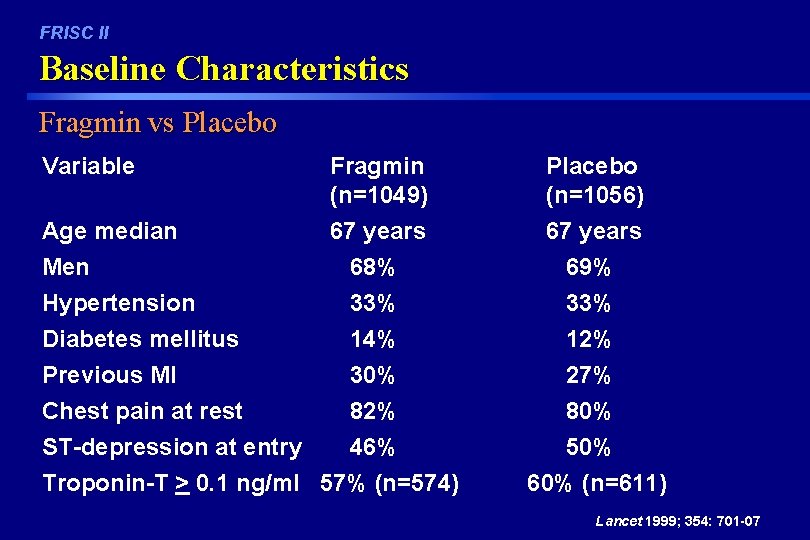
FRISC II Baseline Characteristics Fragmin vs Placebo Variable Age median Men Hypertension Diabetes mellitus Previous MI Chest pain at rest ST-depression at entry Fragmin (n=1049) 67 years 68% 33% 14% 30% 82% Placebo (n=1056) 67 years 69% 33% 12% 27% 80% 46% 50% Troponin-T > 0. 1 ng/ml 57% (n=574) 60% (n=611) Lancet 1999; 354: 701 -07

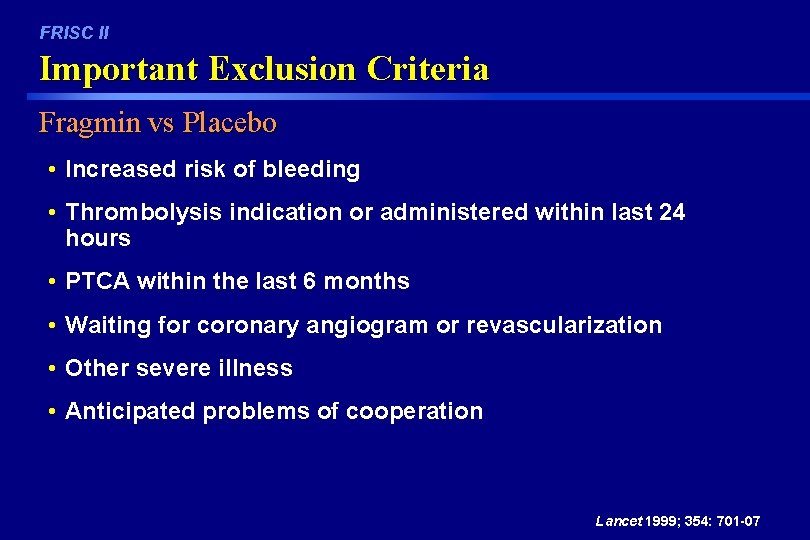
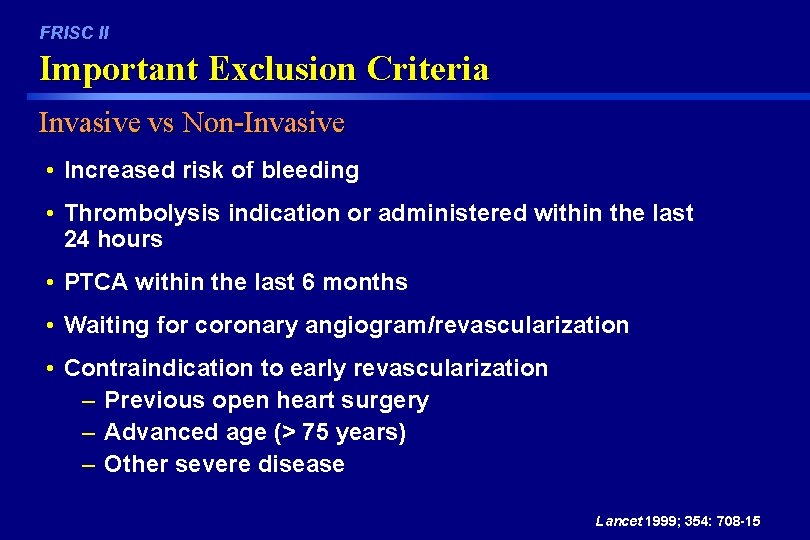
FRISC II Important Exclusion Criteria Fragmin vs Placebo • Increased risk of bleeding • Thrombolysis indication or administered within last 24 hours • PTCA within the last 6 months • Waiting for coronary angiogram or revascularization • Other severe illness • Anticipated problems of cooperation Lancet 1999; 354: 701 -07

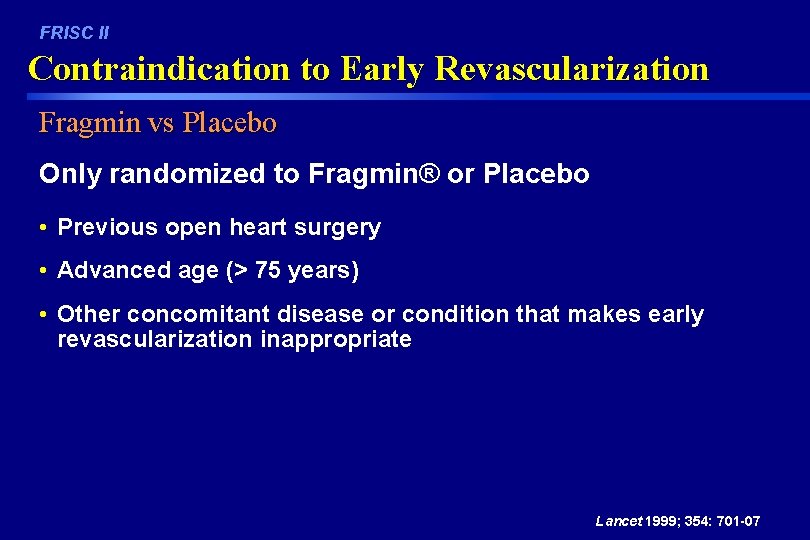
FRISC II Contraindication to Early Revascularization Fragmin vs Placebo Only randomized to Fragmin® or Placebo • Previous open heart surgery • Advanced age (> 75 years) • Other concomitant disease or condition that makes early revascularization inappropriate Lancet 1999; 354: 701 -07

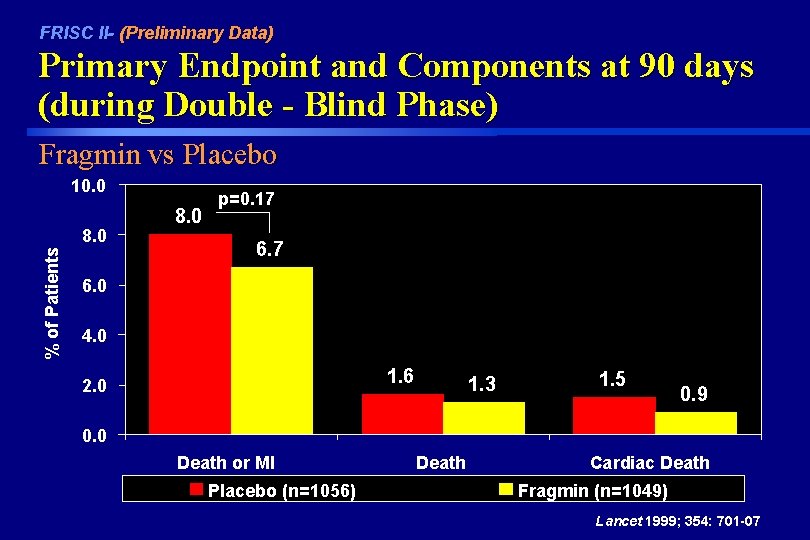
FRISC II- (Preliminary Data) Primary Endpoint and Components at 90 days (during Double - Blind Phase) Fragmin vs Placebo 10. 0 % of Patients 8. 0 p=0. 17 6. 0 4. 0 1. 6 2. 0 1. 3 1. 5 0. 9 0. 0 Death or MI Placebo (n=1056) Death Cardiac Death Fragmin (n=1049) Lancet 1999; 354: 701 -07

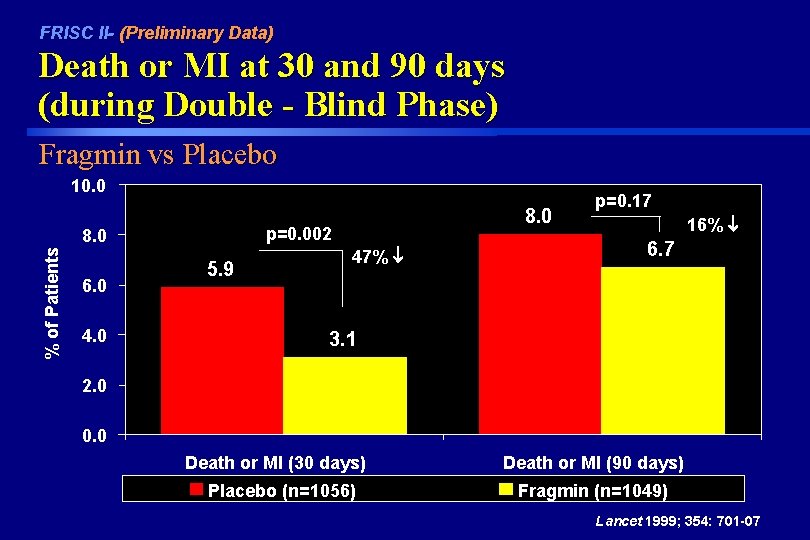
FRISC II- (Preliminary Data) Death or MI at 30 and 90 days (during Double - Blind Phase) Fragmin vs Placebo 10. 0 p=0. 002 % of Patients 8. 0 6. 0 4. 0 8. 0 5. 9 47% p=0. 17 16% 6. 7 3. 1 2. 0 0. 0 Death or MI (30 days) Placebo (n=1056) Death or MI (90 days) Fragmin (n=1049) Lancet 1999; 354: 701 -07

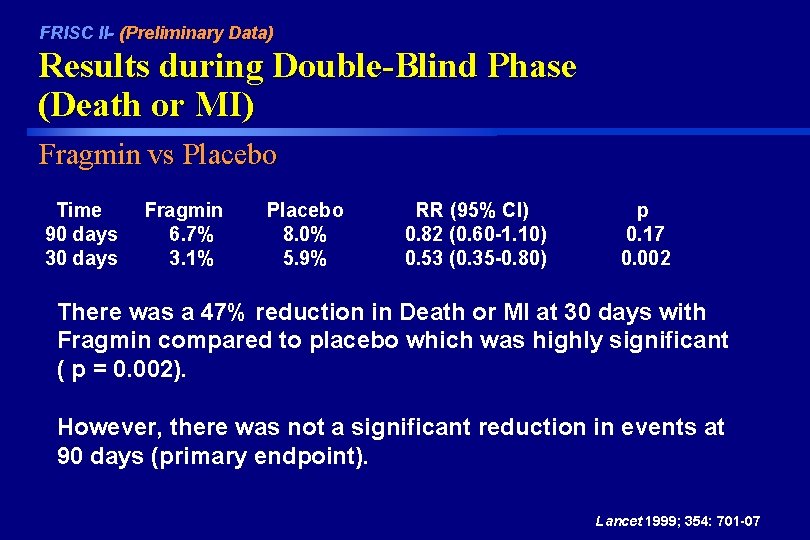
FRISC II- (Preliminary Data) Results during Double-Blind Phase (Death or MI) Fragmin vs Placebo Time 90 days 30 days Fragmin 6. 7% 3. 1% Placebo 8. 0% 5. 9% RR (95% CI) 0. 82 (0. 60 -1. 10) 0. 53 (0. 35 -0. 80) p 0. 17 0. 002 There was a 47% reduction in Death or MI at 30 days with Fragmin compared to placebo which was highly significant ( p = 0. 002). However, there was not a significant reduction in events at 90 days (primary endpoint). Lancet 1999; 354: 701 -07

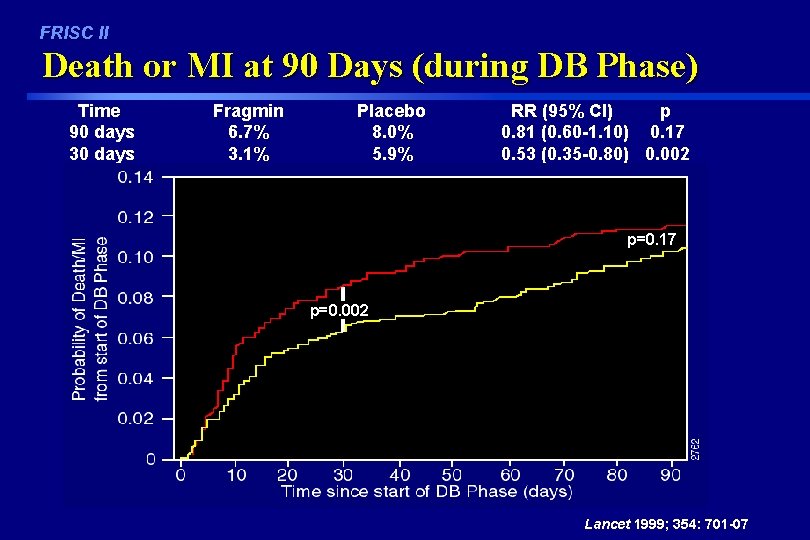
FRISC II Death or MI at 90 Days (during DB Phase) Time 90 days 30 days Fragmin 6. 7% 3. 1% Placebo 8. 0% 5. 9% RR (95% CI) p 0. 81 (0. 60 -1. 10) 0. 17 0. 53 (0. 35 -0. 80) 0. 002 p=0. 17 p=0. 002 Lancet 1999; 354: 701 -07

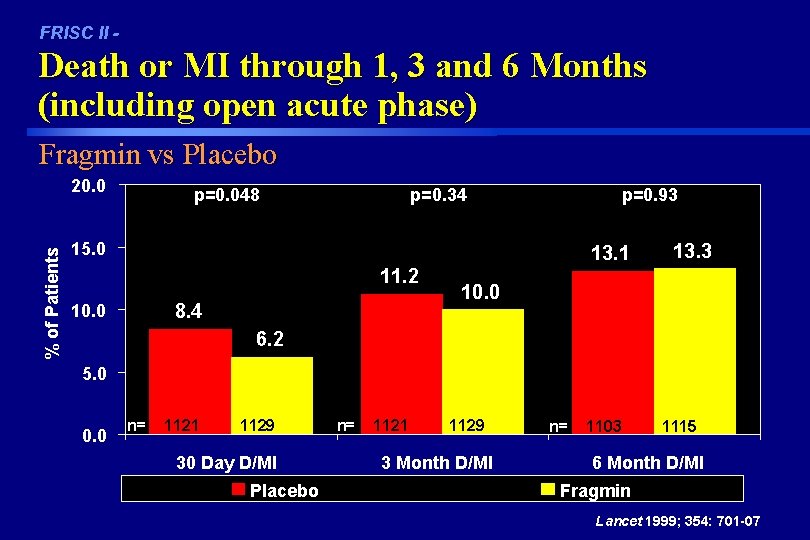
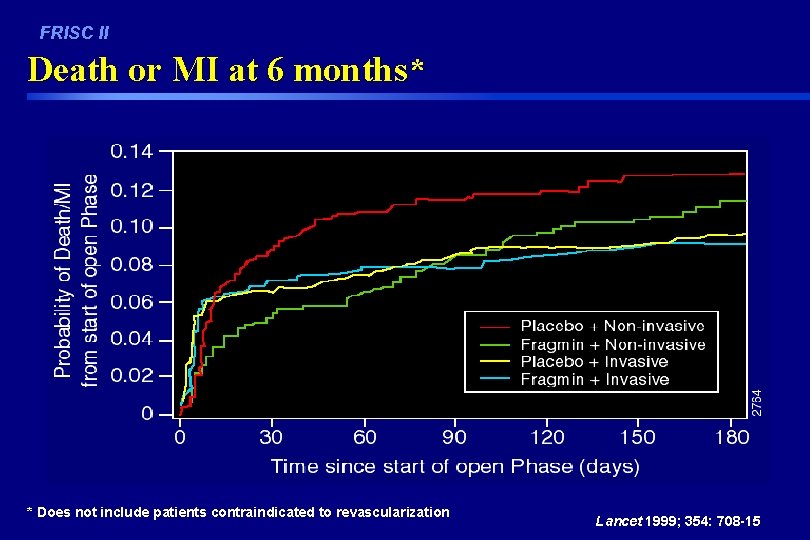
FRISC II - Death or MI through 1, 3 and 6 Months (including open acute phase) Fragmin vs Placebo % of Patients 20. 0 p=0. 048 p=0. 34 p=0. 93 15. 0 13. 1 11. 2 8. 4 10. 0 13. 3 10. 0 6. 2 5. 0 0. 0 n= 1121 1129 30 Day D/MI Placebo n= 1121 1129 3 Month D/MI n= 1103 1115 6 Month D/MI Fragmin Lancet 1999; 354: 701 -07

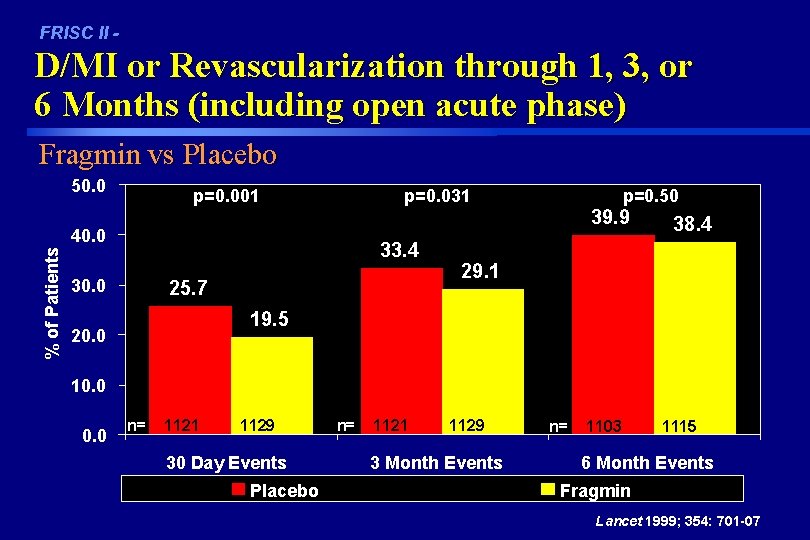
FRISC II - D/MI or Revascularization through 1, 3, or 6 Months (including open acute phase) Fragmin vs Placebo 50. 0 p=0. 001 p=0. 031 % of Patients 40. 0 33. 4 30. 0 25. 7 p=0. 50 39. 9 38. 4 29. 1 19. 5 20. 0 10. 0 n= 1121 1129 30 Day Events Placebo n= 1121 1129 3 Month Events n= 1103 1115 6 Month Events Fragmin Lancet 1999; 354: 701 -07

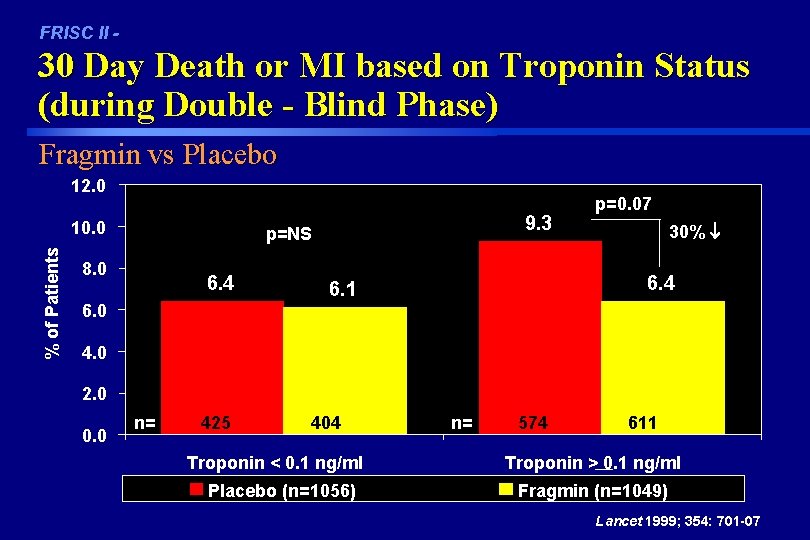
FRISC II - 30 Day Death or MI based on Troponin Status (during Double - Blind Phase) Fragmin vs Placebo 12. 0 % of Patients 10. 0 9. 3 p=NS 8. 0 6. 4 p=0. 07 30% 6. 4 6. 1 6. 0 4. 0 2. 0 0. 0 n= 425 404 Troponin < 0. 1 ng/ml Placebo (n=1056) n= 574 611 Troponin > 0. 1 ng/ml Fragmin (n=1049) Lancet 1999; 354: 701 -07

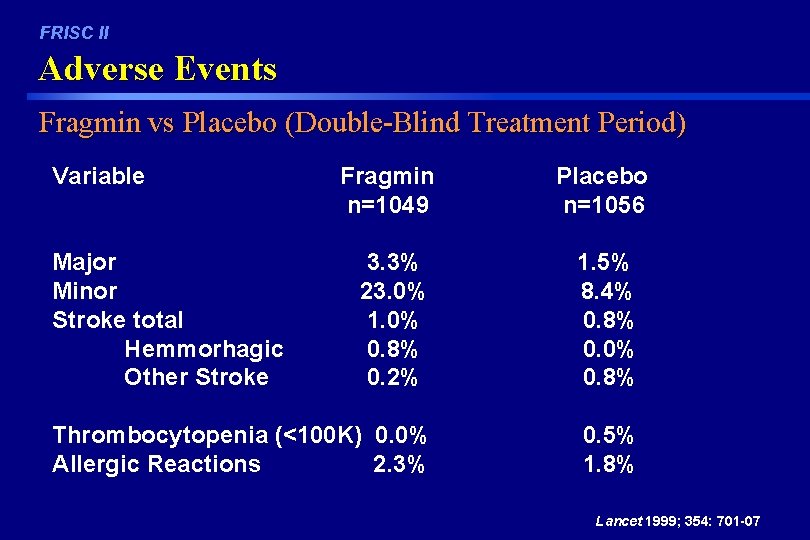
FRISC II Adverse Events Fragmin vs Placebo (Double-Blind Treatment Period) Variable Fragmin n=1049 Placebo n=1056 3. 3% 23. 0% 1. 0% 0. 8% 0. 2% 1. 5% 8. 4% 0. 8% 0. 0% 0. 8% Thrombocytopenia (<100 K) 0. 0% Allergic Reactions 2. 3% 0. 5% 1. 8% Major Minor Stroke total Hemmorhagic Other Stroke Lancet 1999; 354: 701 -07

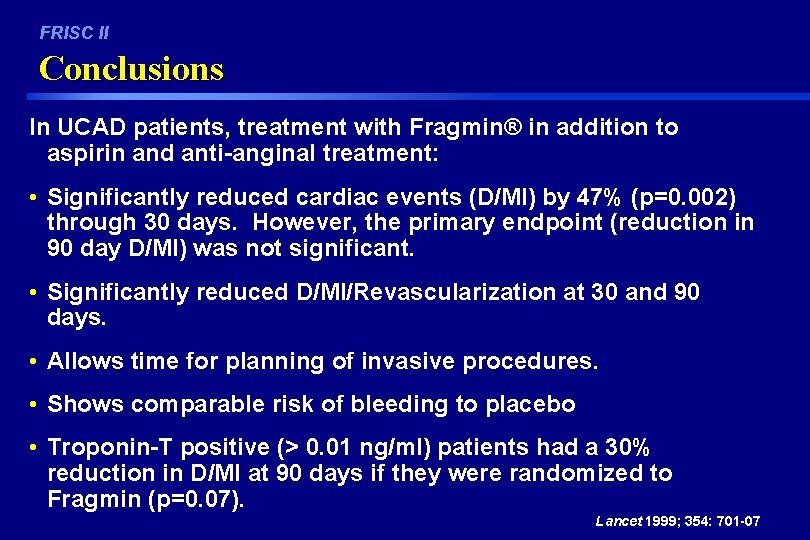
FRISC II Conclusions In UCAD patients, treatment with Fragmin® in addition to aspirin and anti-anginal treatment: • Significantly reduced cardiac events (D/MI) by 47% (p=0. 002) through 30 days. However, the primary endpoint (reduction in 90 day D/MI) was not significant. • Significantly reduced D/MI/Revascularization at 30 and 90 days. • Allows time for planning of invasive procedures. • Shows comparable risk of bleeding to placebo • Troponin-T positive (> 0. 01 ng/ml) patients had a 30% reduction in D/MI at 90 days if they were randomized to Fragmin (p=0. 07). Lancet 1999; 354: 701 -07

FRISC II Fragmin® and Fast Revascularization during In. Stablity in Coronary artery disease

FRISC II Trial Design • Compare an early invasive with a non-invasive treatment strategy in patients with unstable coronary artery disease – Enrolled 2457 patients with unstable coronary artery disease – Patients recruited from June, 1996 - May 1998 – 58 Scandinavian Centers – Randomized – Double Blind – Placebo Controlled – Intention to Treat Analysis Lancet 1999; 354: 708 -15

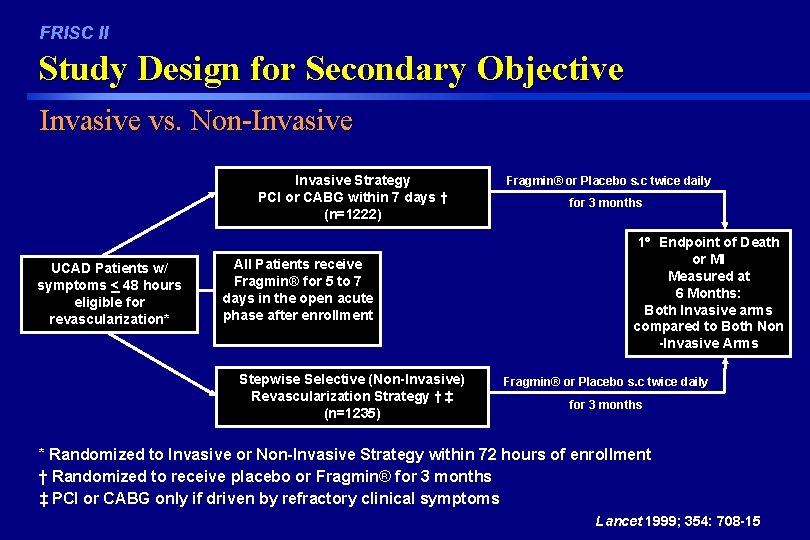
FRISC II Study Design for Secondary Objective Invasive vs. Non-Invasive Strategy PCI or CABG within 7 days † (n=1222) UCAD Patients w/ symptoms < 48 hours eligible for revascularization* All Patients receive Fragmin® for 5 to 7 days in the open acute phase after enrollment Stepwise Selective (Non-Invasive) Revascularization Strategy † ‡ (n=1235) Fragmin® or Placebo s. c twice daily for 3 months 1 Endpoint of Death or MI Measured at 6 Months: Both Invasive arms compared to Both Non -Invasive Arms Fragmin® or Placebo s. c twice daily for 3 months * Randomized to Invasive or Non-Invasive Strategy within 72 hours of enrollment † Randomized to receive placebo or Fragmin® for 3 months ‡ PCI or CABG only if driven by refractory clinical symptoms Lancet 1999; 354: 708 -15

FRISC II Secondary Objectives Invasive vs. Non-Invasive In patients eligible for an early invasive strategy: – Compare a direct invasive approach with early coronary angiography and revascularization (invasive) vs. a stepwise selective approach with coronary angiography and revascularization only if driven by refractory clinical symptoms (non-invasive) concerning: • death or MI – (1 Endpoint for this phase is D/MI at 6 months) • revascularization • bleeding Lancet 1999; 354: 708 -15

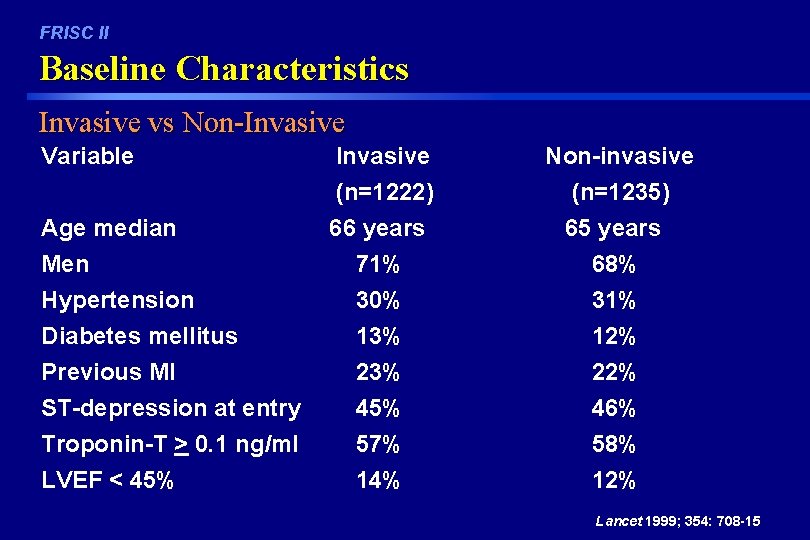
FRISC II Baseline Characteristics Invasive vs Non-Invasive Variable Invasive (n=1222) Non-invasive (n=1235) Age median Men Hypertension Diabetes mellitus Previous MI ST-depression at entry 66 years 71% 30% 13% 23% 45% 65 years 68% 31% 12% 22% 46% Troponin-T > 0. 1 ng/ml 57% 58% LVEF < 45% 14% 12% Lancet 1999; 354: 708 -15

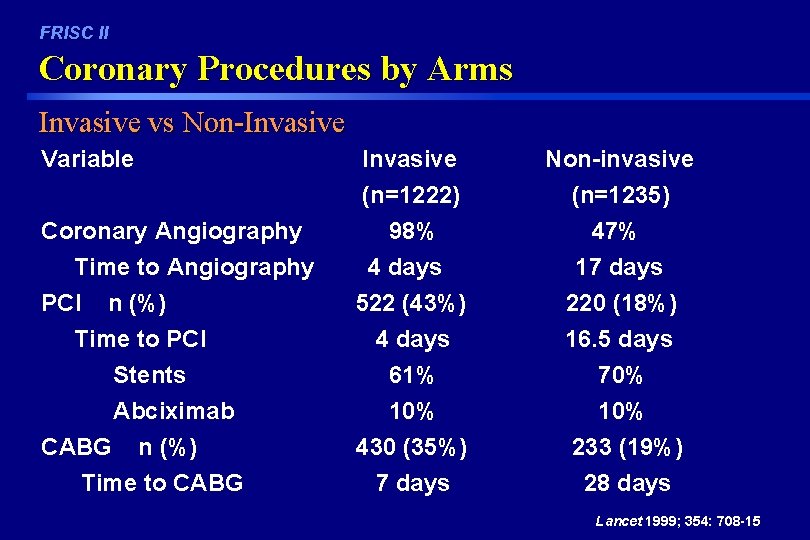
FRISC II Coronary Procedures by Arms Invasive vs Non-Invasive Variable Invasive (n=1222) Non-invasive (n=1235) Coronary Angiography Time to Angiography PCI n (%) Time to PCI Stents Abciximab 98% 4 days 522 (43%) 4 days 61% 10% 47% 17 days 220 (18%) 16. 5 days 70% 10% CABG n (%) 430 (35%) 233 (19%) Time to CABG 7 days 28 days Lancet 1999; 354: 708 -15

FRISC II Important Exclusion Criteria Invasive vs Non-Invasive • Increased risk of bleeding • Thrombolysis indication or administered within the last 24 hours • PTCA within the last 6 months • Waiting for coronary angiogram/revascularization • Contraindication to early revascularization – Previous open heart surgery – Advanced age (> 75 years) – Other severe disease Lancet 1999; 354: 708 -15

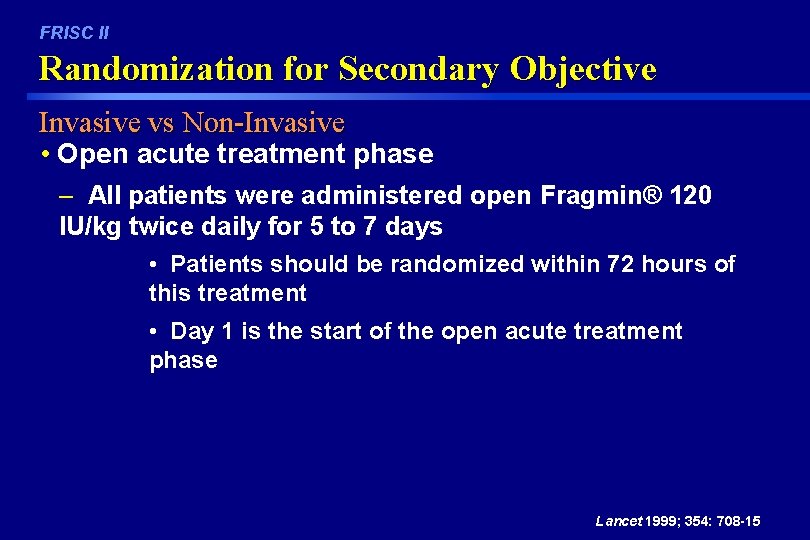
FRISC II Randomization for Secondary Objective Invasive vs Non-Invasive • Open acute treatment phase – All patients were administered open Fragmin® 120 IU/kg twice daily for 5 to 7 days • Patients should be randomized within 72 hours of this treatment • Day 1 is the start of the open acute treatment phase Lancet 1999; 354: 708 -15

FRISC II Randomization for Secondary Objective (cont. ) Invasive vs Non-Invasive Patients without contra-indications to an early invasive strategy are randomized to either an: • Invasive direct strategy – Coronary angiogram/revascularization < 7 days in all patients – PCI/CABG at > 70% stenosis in major coronary arteries: • PTCA preferred for 1 -2 vessel disease • CABG preferred for 3 vessel or left main disease • Non-invasive selective strategy – Coronary angiography and revascularization only at : • recurring or incapacitating symptoms or • severe ischemia at exercise test Lancet 1999; 354: 708 -15

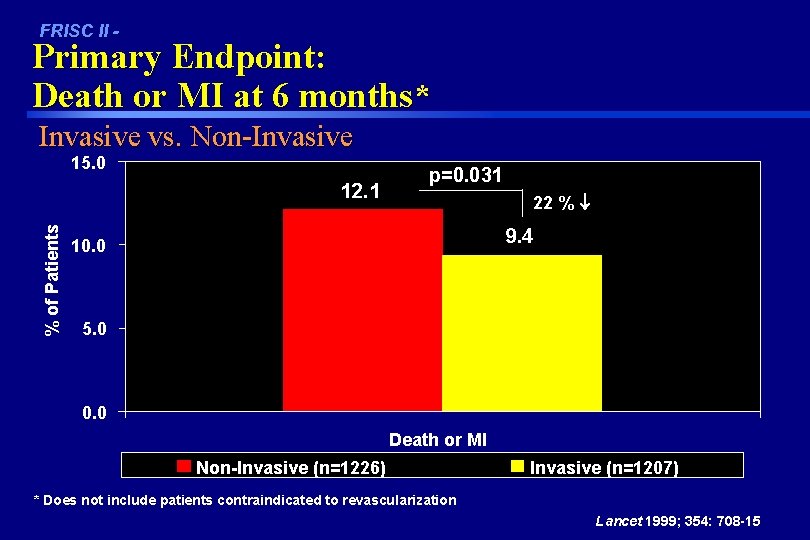
FRISC II - Primary Endpoint: Death or MI at 6 months* Invasive vs. Non-Invasive 15. 0 % of Patients 12. 1 p=0. 031 22 % 9. 4 10. 0 5. 0 0. 0 Death or MI Non-Invasive (n=1226) Invasive (n=1207) * Does not include patients contraindicated to revascularization Lancet 1999; 354: 708 -15

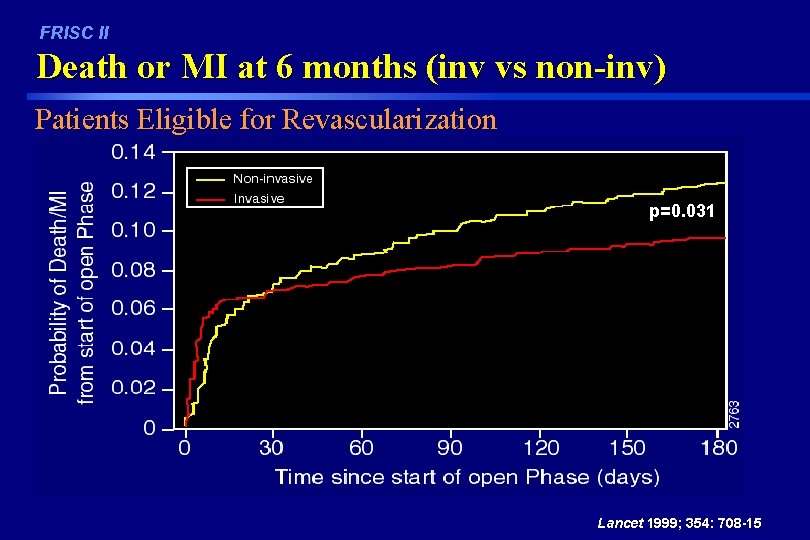
FRISC II Death or MI at 6 months (inv vs non-inv) Patients Eligible for Revascularization p=0. 031 Lancet 1999; 354: 708 -15

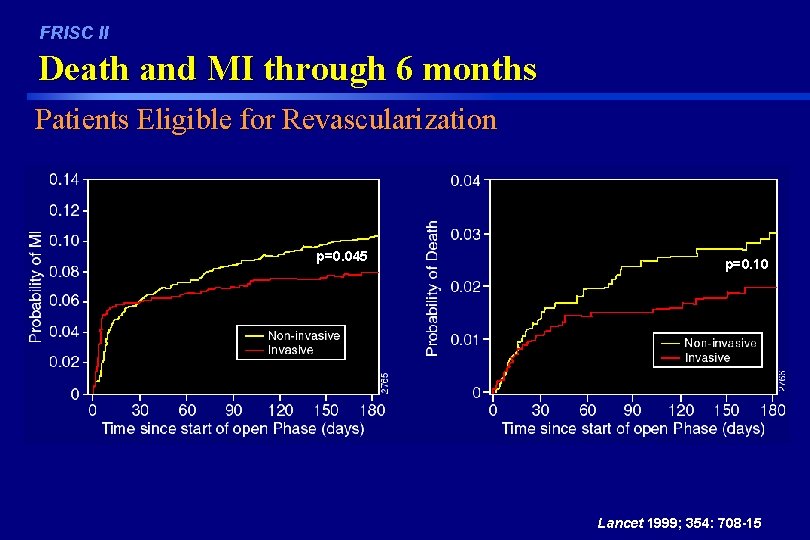
FRISC II Death and MI through 6 months Patients Eligible for Revascularization p=0. 045 p=0. 10 Lancet 1999; 354: 708 -15

FRISC II Death or MI at 6 months* * Does not include patients contraindicated to revascularization Lancet 1999; 354: 708 -15

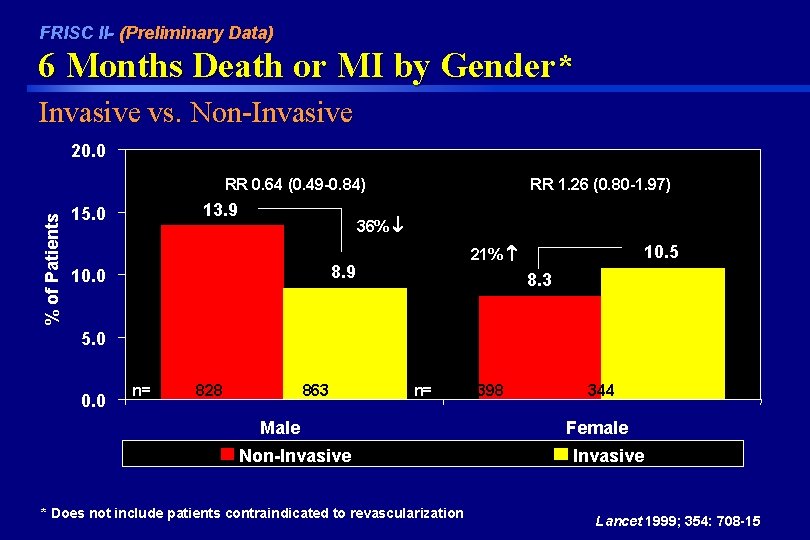
FRISC II- (Preliminary Data) 6 Months Death or MI by Gender* Invasive vs. Non-Invasive 20. 0 % of Patients RR 0. 64 (0. 49 -0. 84) 13. 9 15. 0 RR 1. 26 (0. 80 -1. 97) 36% 8. 9 10. 0 10. 5 21% 8. 3 5. 0 0. 0 n= 828 863 n= Male Non-Invasive * Does not include patients contraindicated to revascularization 398 344 Female Invasive Lancet 1999; 354: 708 -15

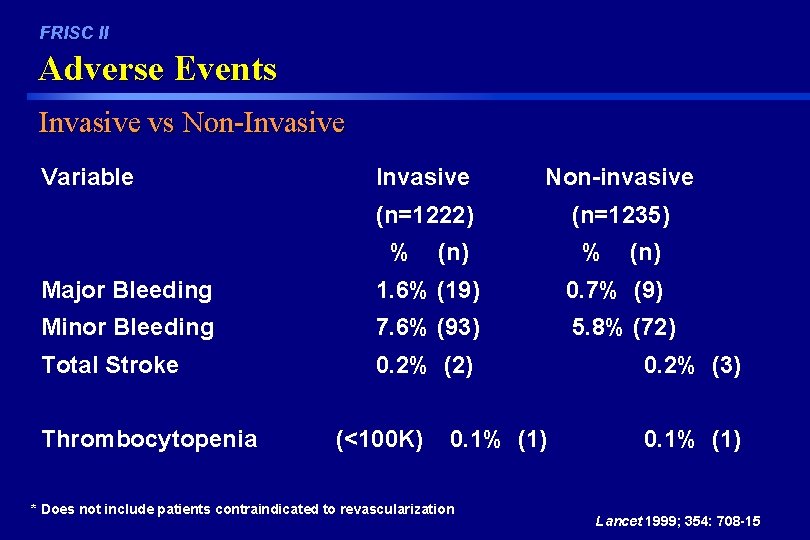
FRISC II Adverse Events Invasive vs Non-Invasive Variable Invasive Non-invasive (n=1222) (n=1235) % (n) Major Bleeding 1. 6% (19) 0. 7% (9) Minor Bleeding 7. 6% (93) 5. 8% (72) Total Stroke 0. 2% (2) Thrombocytopenia (<100 K) 0. 1% (1) * Does not include patients contraindicated to revascularization 0. 2% (3) 0. 1% (1) Lancet 1999; 354: 708 -15

FRISC II Conclusions Invasive vs Non-Invasive In unstable angina/non Q wave MI patients, the early invasive strategy: • Reduces the incidence of 6 month death or MI by 22% (p=0. 031) – reduced the incidence of 6 month death or MI in men by 36%; RR 0. 53 (0. 45 -0. 65) – increased the incidence of 6 month death or MI in women by 21%; RR 1. 26 (0. 80 -1. 97) • Reduces symptoms of angina • Reduces re-admissions and late procedures Lancet 1999; 354: 708 -15

FRISC II Conclusions In unstable angina/non Q wave MI patients • Early revascularization reduced the incidence of 6 month death or MI by 22% compared with a stepwise selective revascularization strategy: – Only 10% of all patients received abciximab. “The increasing use of abciximab in association with percutaneous coronary intervention and stenting will lower the rate of events related to percutaneous coronary interventions by 50%” – The superiority of an early invasive approach in reducing death or MI through 6 months was driven by a 36% reduction in men. Female patients had a non-significant increase in 6 month death or MI with an early invasive approach compared to a noninvasive strategy. Lancet 1999; 354: 708 -15

FRISC II Conclusions In unstable angina/non Q wave MI patients receiving a non-invasive treatment strategy: • Long-term anti-thrombotic treatment with Fragmin® reduces death or MI by 47% through 30 days which is useful if early interventional procedures are inappropriate. • Female patients may benefit from a non-invasive treatment strategy compared to an early invasive strategy. – In female patients treated with a non-invasive treatment strategy, long-term anti-thrombotic treatment with Fragmin® reduced death or MI by 23% through 90 days. • Troponin-T + (> 0. 1 g/ml) patients receiving a non-invasive treatment strategy had a trend in the reduction of death and MI through 90 days when treated with long-term Fragmin® compared to placebo (9. 3% vs. 6. 6%; p=0. 07).