FRICTION CONDUCTION AND INDUCTION HOW DO YOU MEASURE














- Slides: 24

FRICTION, CONDUCTION AND INDUCTION

HOW DO YOU MEASURE “CHARGE” • The unit of measure for electrical charge is the Coulomb (C). In equations it is symbolized by a “q” • One Coulomb is equal to the charge of 6. 25 x 1018 electrons (-) or protons (+). • One electron, or proton, has a charge of 1. 60 x 10 -19 C.

COULOMB’S LAW • The strength of electric force increases with increasing electric charges. • The strength of electric force varies according to the distance between charged objects. • The electric force between two objects depends on the types of charges. Charles-Augustin de Coulomb (17361806) 3

HOW DO YOU CHARGE AN OBJECT? • There are three ways to charge an object: 1. Charge by Friction 2. Charge by Conduction 3. Charge by Induction

HOW DO YOU CHARGE AN OBJECT? • All objects begin neutral & can become positively or negatively charged. • A positively charged object has more positive charges than negative charges. • A negatively charged object has more negative charges than positive charges.

STATIC ELECTRICITY • When one object or material rubs over another, electrons are often transferred from one to the other. • If electrons are rubbed off, then the object or material will have a positive charge. • If electrons are gathered then the result is a negative charge. • As charges (electrons) build up, there is always a tendency for them to return to their original locations. All that is needed is a pathway for charges to use. 6

CHARGING OBJECTS • You can charge an object through: • Friction – the transfer of electrons from one object to the other by rubbing them together. • Conduction – by having two objects TOUCH each other and transfer electrons from one object to the next. • Induction – By inducing electrons to move from one object to the other.

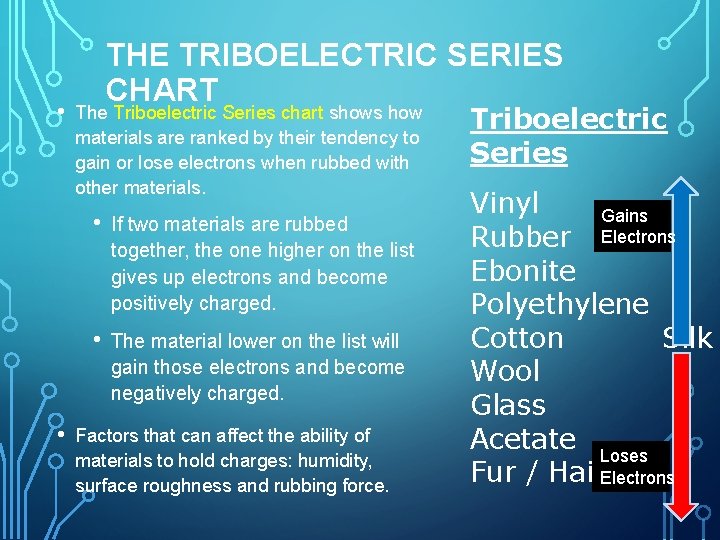
• • THE TRIBOELECTRIC SERIES CHART The Triboelectric Series chart shows how materials are ranked by their tendency to gain or lose electrons when rubbed with other materials. • If two materials are rubbed together, the one higher on the list gives up electrons and become positively charged. • The material lower on the list will gain those electrons and become negatively charged. Factors that can affect the ability of materials to hold charges: humidity, surface roughness and rubbing force. Triboelectric Series Vinyl Gains Rubber Electrons Ebonite Polyethylene Cotton Silk Wool Glass Acetate Loses 8 Fur / Hair. Electrons

ELECTRICITY IN CLOUDS

FRICTION • Electrostatic series is a list that ranks objects ability to take negative charges. Rubber Ebonite Polyethylene Cotton Silk Wool Glass Acetate Fur / Hair Items at top take negatives Items at bottom lose negatives

EXAMPLE #1 Items at top take negatives Your cat rubs against a rubber balloon. What will be the charge on the balloon? Your cat’s fur? Rubber balloon becomes negative Rubber Ebonite Polyethylene Cotton Silk Wool Glass Acetate Fur / / Hair Fur Hair Negatives Cat’s fur becomes positive

EXAMPLE #2 Items at top take negatives In a lab, you take a piece of neutral wool & neutral polyethylene & rub them together. What will be their charges? Rubber Ebonite Polyethylene Cotton Silk Wool Glass Negative Acetate Fur / Hair Polyethylene balloon becomes negative Wool becomes positive

EXAMPLE #3 • In a lab, you rub a piece of cotton & ebonite together. Then you rub a piece of silk & wool together. • You then bring the charged piece of cotton & the charged piece of silk together. What will happen? Rubber Ebonite Polyethylene Cotton + Silk Wool + Glass Acetate Fur / Hair Cotton is + Silk is - They would ATTRACT

You rub your hair with a balloon. Explain using words & pictures, why your hair “sticks up”. 1 st Hair & balloon are both neutral 2 nd Rubber balloon takes negative charges from the hair. So, balloon becomes negatively charged & hair becomes positively charged 3 rd Since hair is positive & like charges repel, hair sticks up!!! _ +_ _ + + _

CHARGING BY CONDUCTION • An object can be charged by touching it with another object that already has a charge. The resulting object will then have the same charge but weaker in strength than the original object.

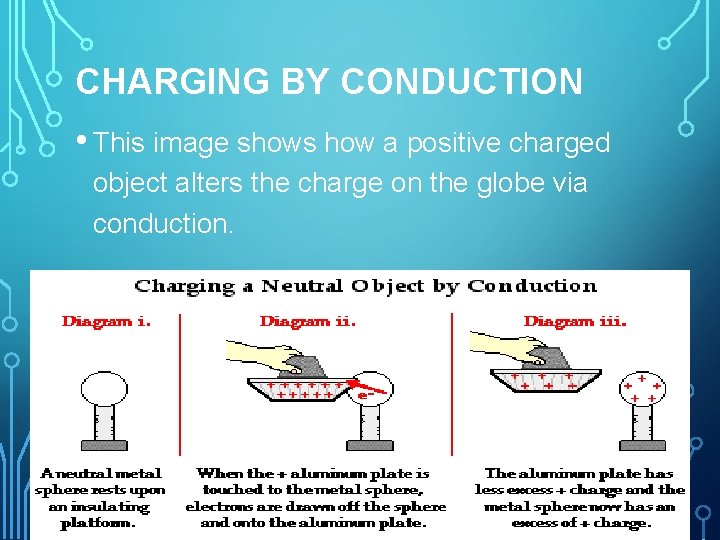
CHARGING BY CONDUCTION • This image shows how a positive charged object alters the charge on the globe via conduction.

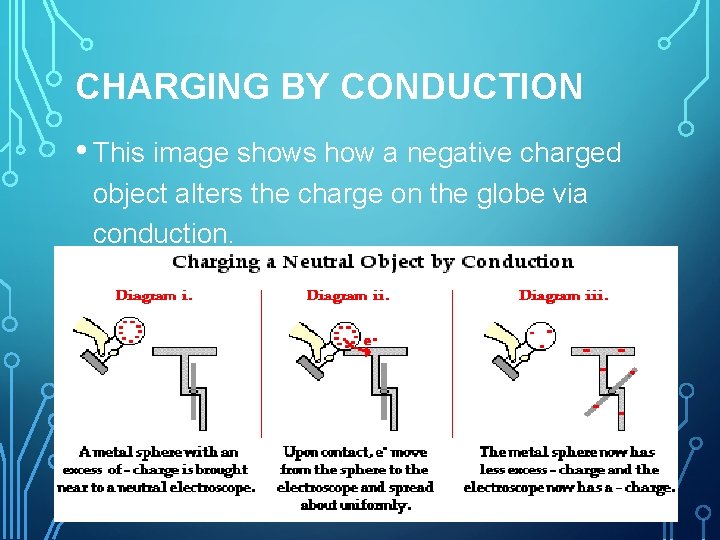
CHARGING BY CONDUCTION • This image shows how a negative charged object alters the charge on the globe via conduction.

CHARGING BY INDUCTION • Objects do not touch - one object is charged, one is neutral. • Proximity of the charged object causes (induces) the charges in the neutral object to separate.

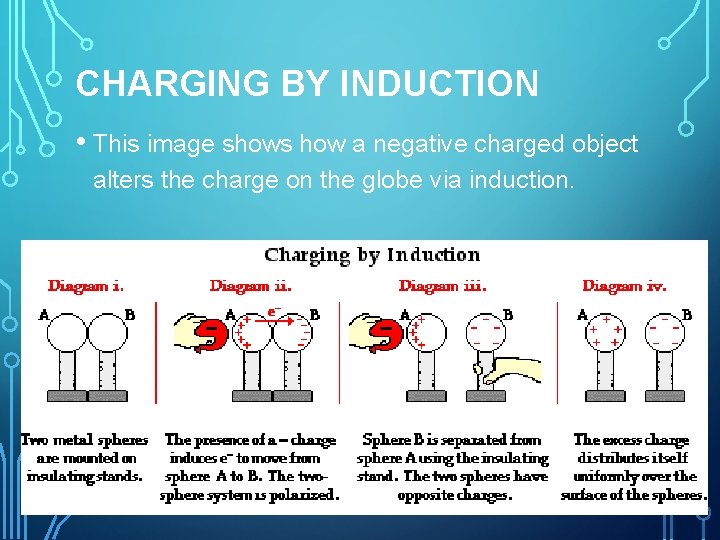
CHARGING BY INDUCTION • This image shows how a negative charged object alters the charge on the globe via induction.

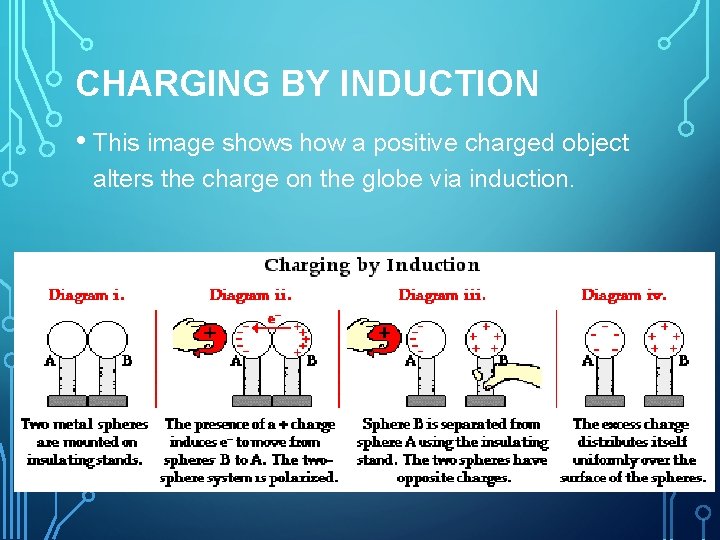
CHARGING BY INDUCTION • This image shows how a positive charged object alters the charge on the globe via induction.

INSULATORS AND CONDUCTORS • Insulator – is a substance in which the electrons are so tightly bound to the atoms making up the material that they are not free to move to a neighbouring atom. I. E. they cannot conduct electricity. • Conductors – allows electrons to flor freely from one atom to another as the atom has the capability of allowing electrons to move freely.

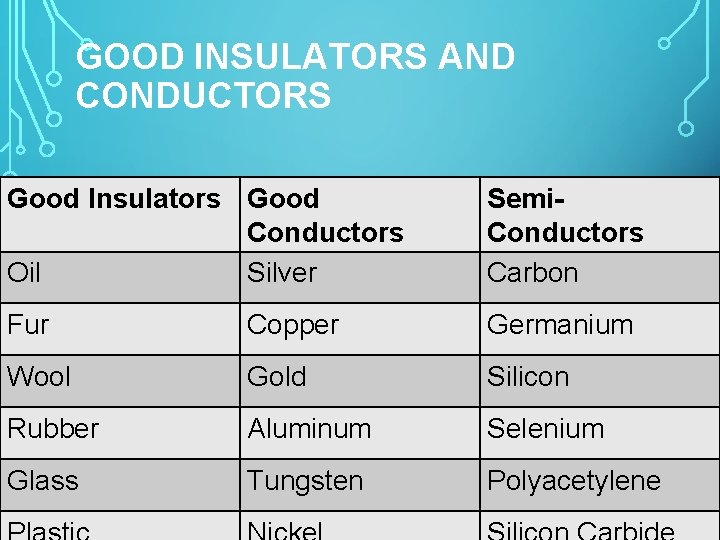
GOOD INSULATORS AND CONDUCTORS Good Insulators Good Conductors Oil Silver Semi. Conductors Carbon Fur Copper Germanium Wool Gold Silicon Rubber Aluminum Selenium Glass Tungsten Polyacetylene

KEY POINTS TO REMEMBER • Two types of charges – positive (+) & negative (-). • “Opposite Charges Attract” and “Like Charges Repel. ” • The electrostatic series is a list that identifies what objects will take negative charges from another object. • Only negative charges move. • Three methods to charge an object: friction, conduction, induction. These three methods are what cause static electricity.

ASSIGNMENT • Page 281 Q’s – 1 – 7 and 10 • Page 284 Q’s – 1, 2, and 7