Free Radical Reactions Radical Reactions Chapter 10 A





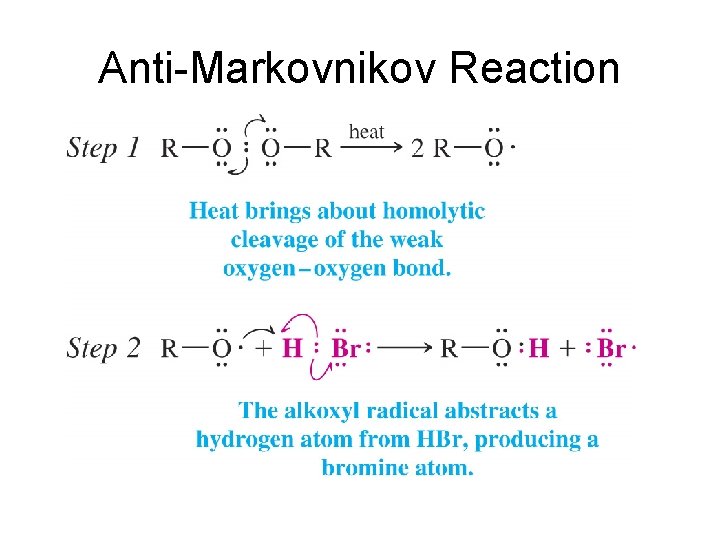


- Slides: 30

Free Radical Reactions

Radical Reactions Chapter 10 A. B. C. D. E. F. G. Description Causes of Free Radical Formation Importance in Biology, Medicine & Industry Mechanism Selectivity of Halogens Anti-Markovnikov Reaction Ozone Depletion & CFC’s

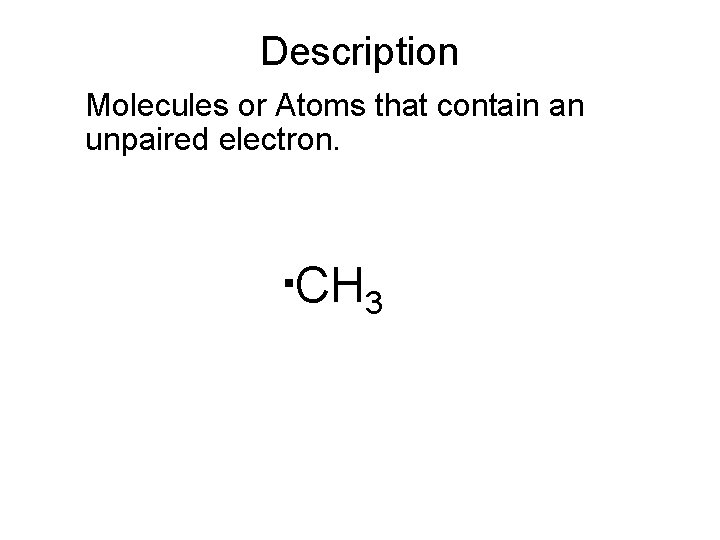
Description Molecules or Atoms that contain an unpaired electron. . CH 3

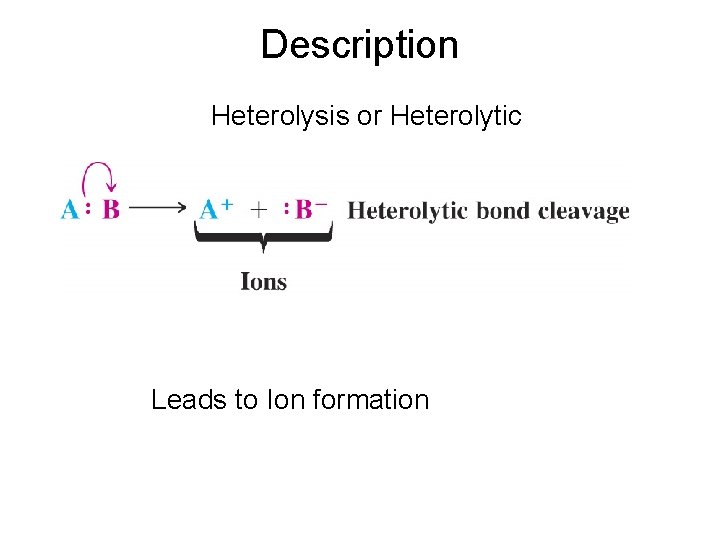
Description Heterolysis or Heterolytic Leads to Ion formation

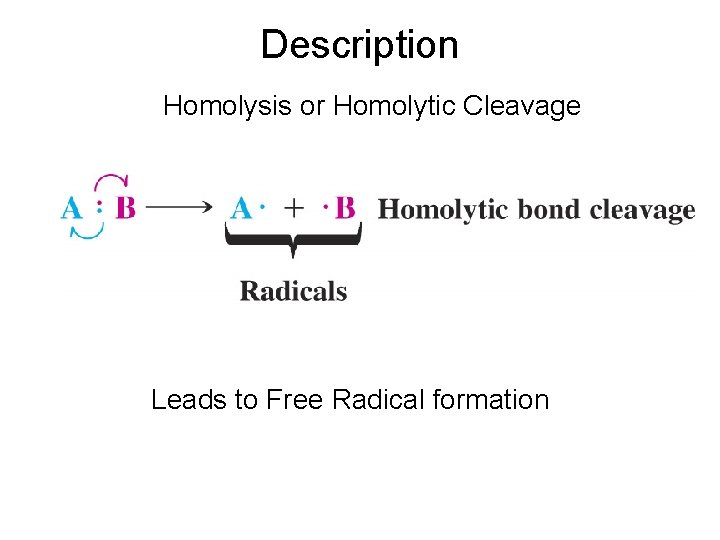
Description Homolysis or Homolytic Cleavage Leads to Free Radical formation

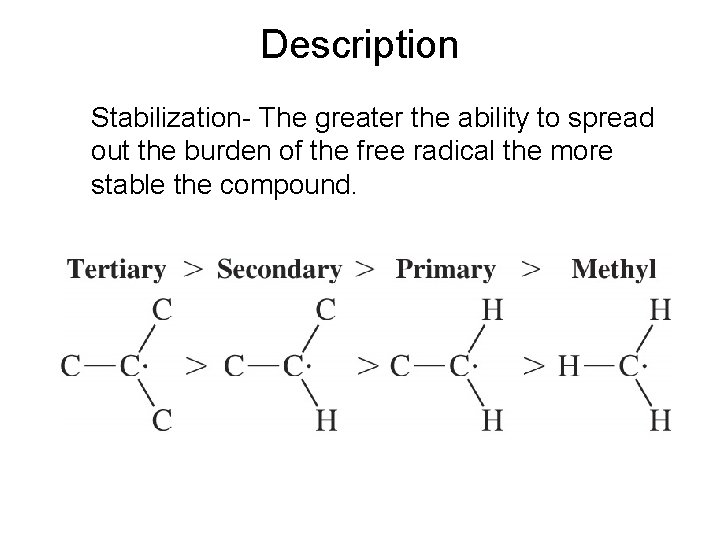
Description Stabilization- The greater the ability to spread out the burden of the free radical the more stable the compound.

Causes of Free Radical Formation • • • Metabolism Immune Response Combustion Radiation UV (Sunlight) Environmental Pollutants

Importance in Biology & Medicine • • Aging (Link 2) Cancer Emphysema Nitric Oxide – – Blood Pressure Regulation Blood Clotting Neurotransmission Immune Response • Metabolism • Wrinkles • Etc.

Importance in Biology & Medicine • Antioxidants - absorb free radicals – – – Vitamin E Vitamin C Beta-carotene Selenium Lycopene Melatonin (Link 2) Source: http: //www. biochem. wisc. edu/biochem 510/slides/20 antiox 1. pdf

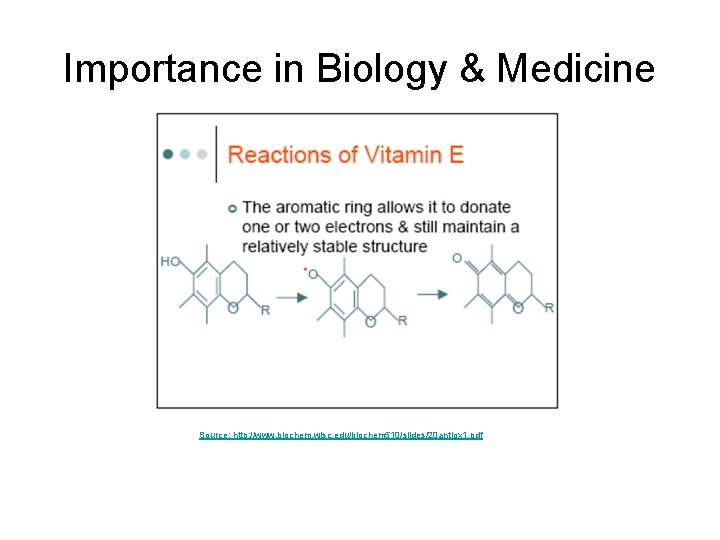
Importance in Biology & Medicine Source: http: //www. biochem. wisc. edu/biochem 510/slides/20 antiox 1. pdf

Importance in Industry • Polymers (Plastics) – Polyethylene – Telfon – Polystyrene • Combustion • Cracking

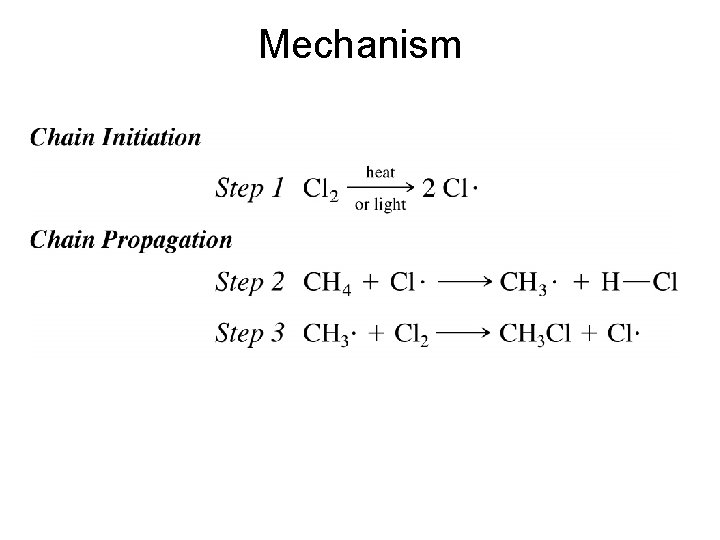
Mechanism Three major Steps • Chain Initiation Step • Chain Propagation Steps • Chain Termination Steps (Initiation) (Propagation) (Termination)

Mechanism

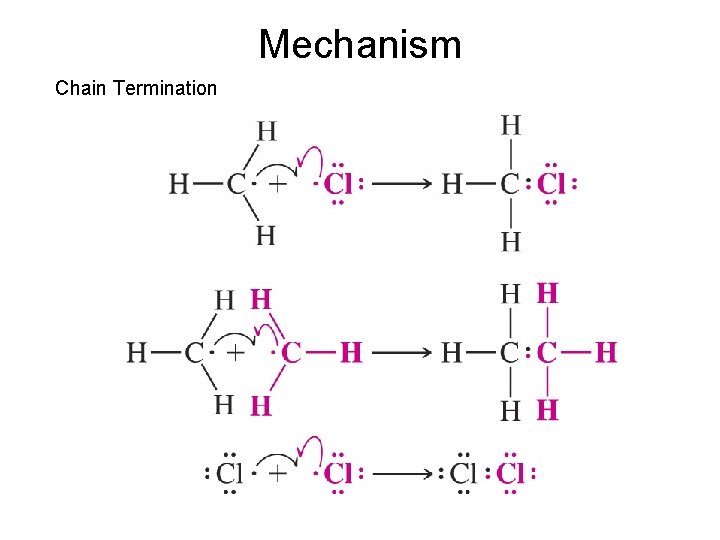
Mechanism Chain Termination

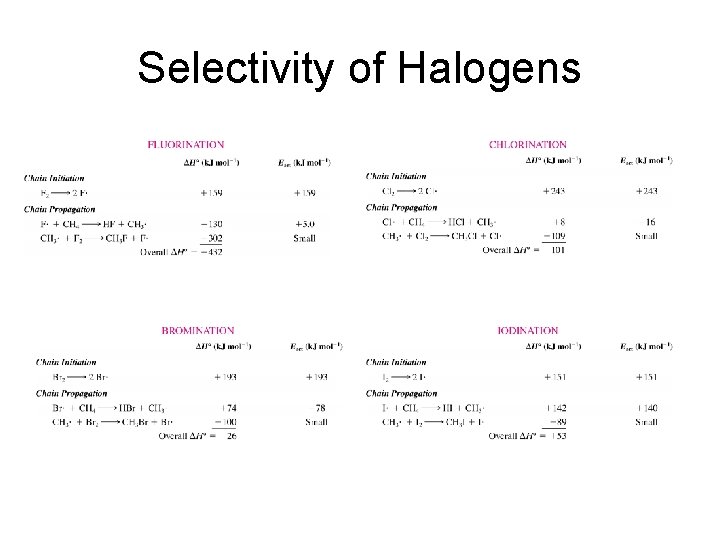
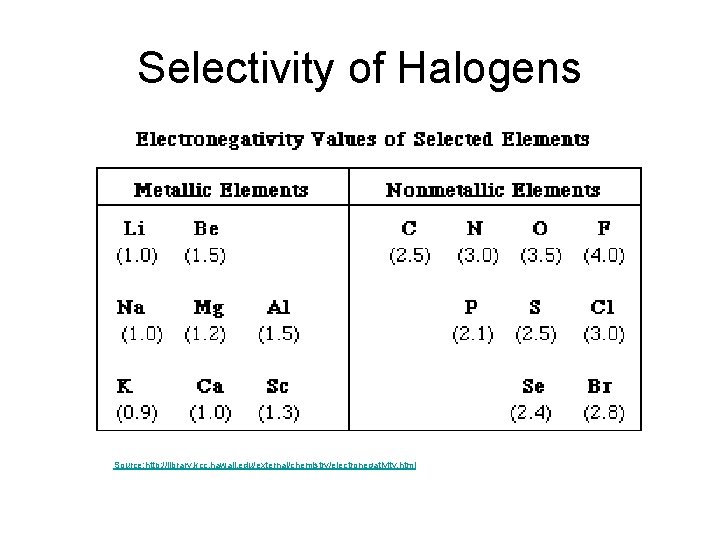
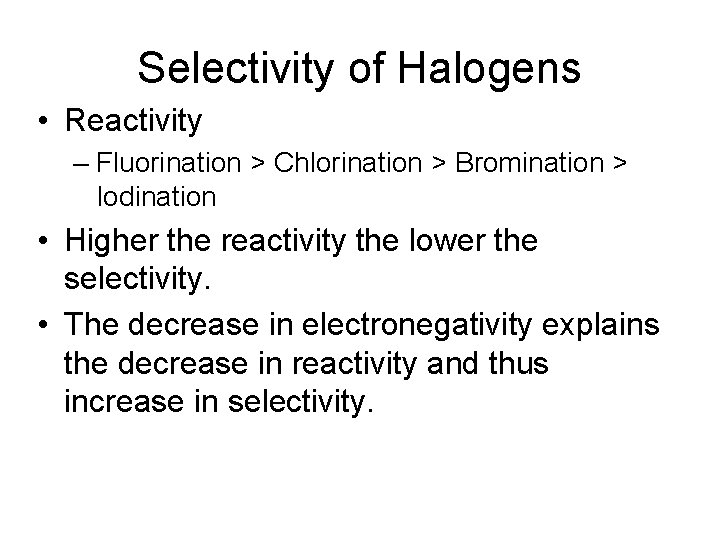
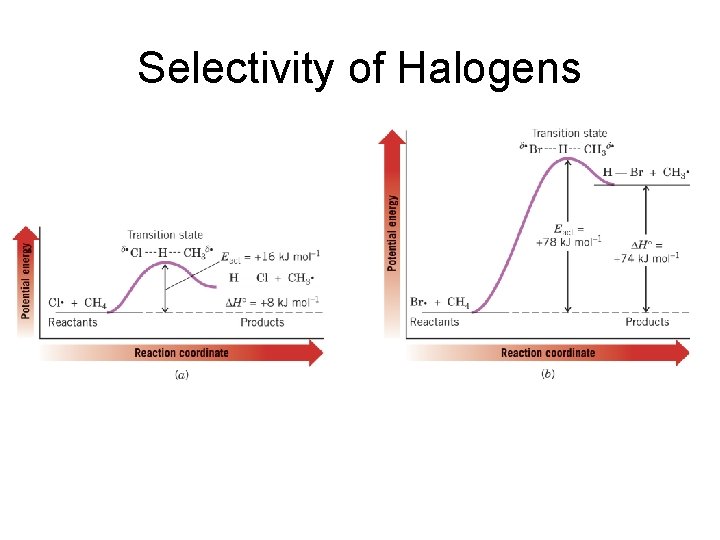
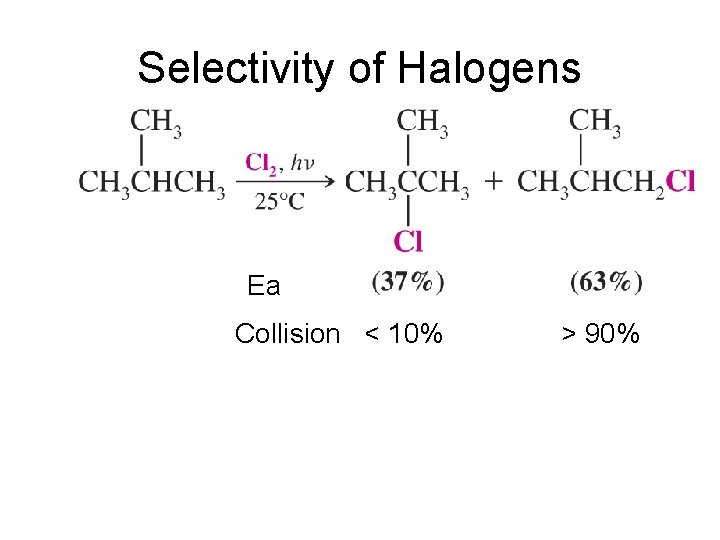
Selectivity of Halogens

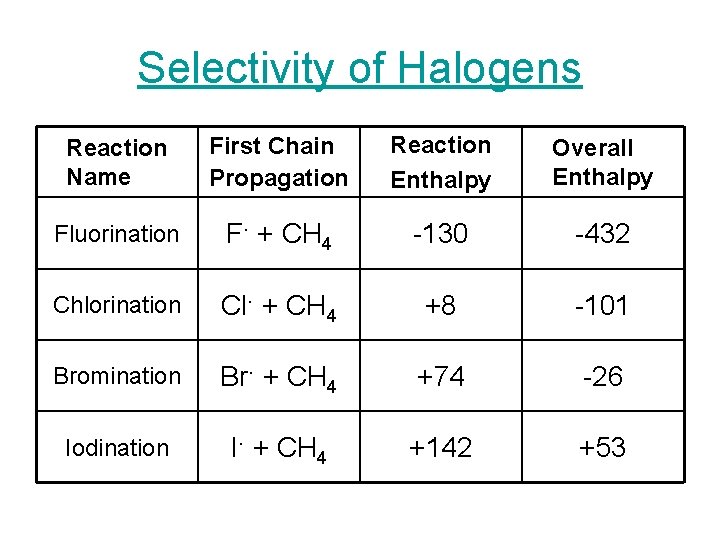
Selectivity of Halogens Reaction Name First Chain Propagation Reaction Enthalpy Overall Enthalpy Fluorination F. + CH 4 -130 -432 Chlorination Cl. + CH 4 +8 -101 Bromination Br. + CH 4 +74 -26 Iodination I. + CH 4 +142 +53

Selectivity of Halogens Source: http: //library. kcc. hawaii. edu/external/chemistry/electronegativity. html

Selectivity of Halogens • Reactivity – Fluorination > Chlorination > Bromination > Iodination • Higher the reactivity the lower the selectivity. • The decrease in electronegativity explains the decrease in reactivity and thus increase in selectivity.

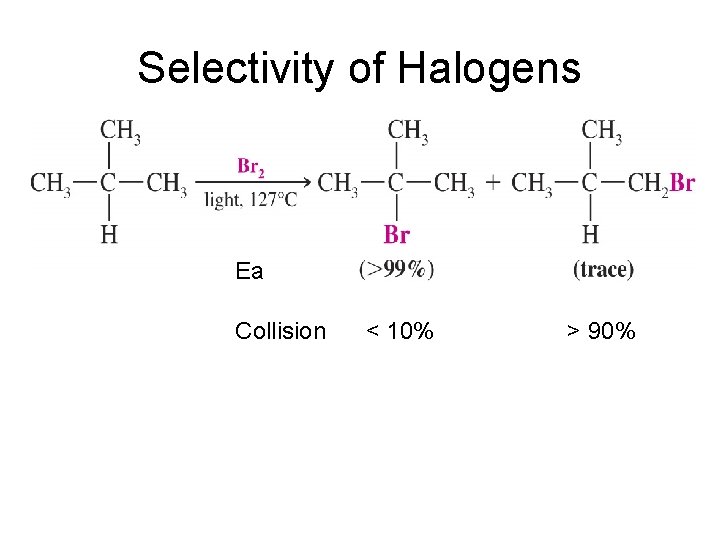
Selectivity of Halogens

Selectivity of Halogens Ea Collision < 10% > 90%

Selectivity of Halogens Ea Collision < 10% > 90%

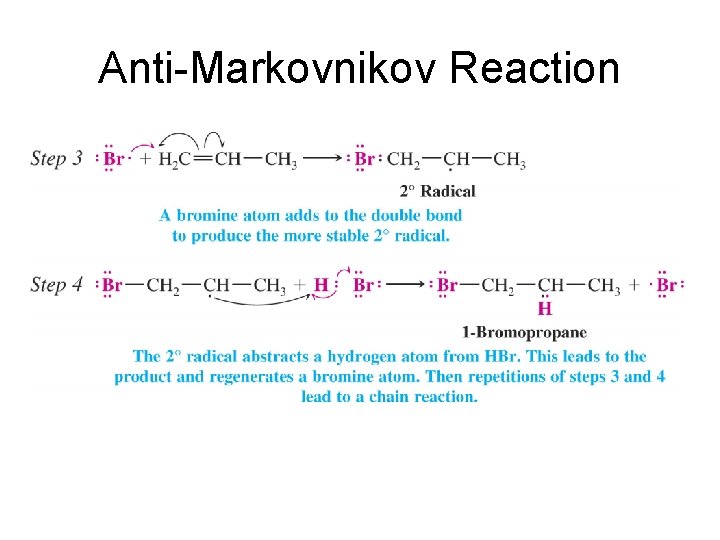
Anti-Markovnikov Reaction • Free Radical Reaction • Ea Driven Reaction
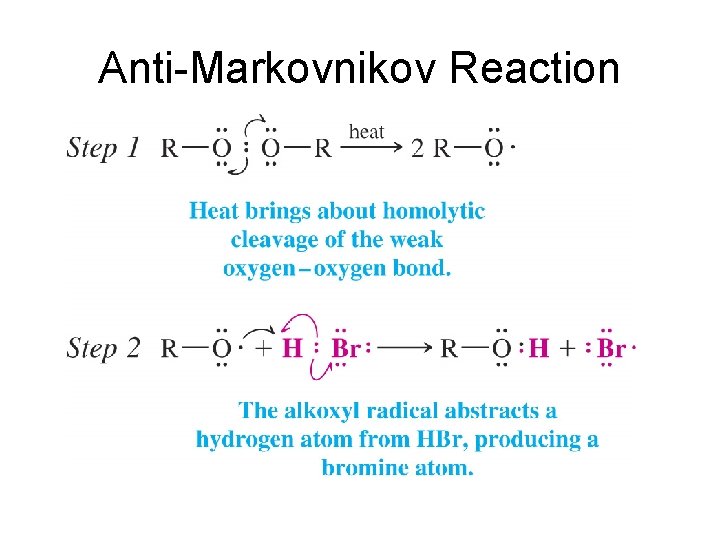
Anti-Markovnikov Reaction

Anti-Markovnikov Reaction

Markovnikov vs. Anti-Markovnikov • Both reactions are Ea driven • In Markovnikov addition the hydrogen adds first leading to the most stable carbocation. • In anti-Markovnikov addition the Bromine adds first leading to the most stable free radical.

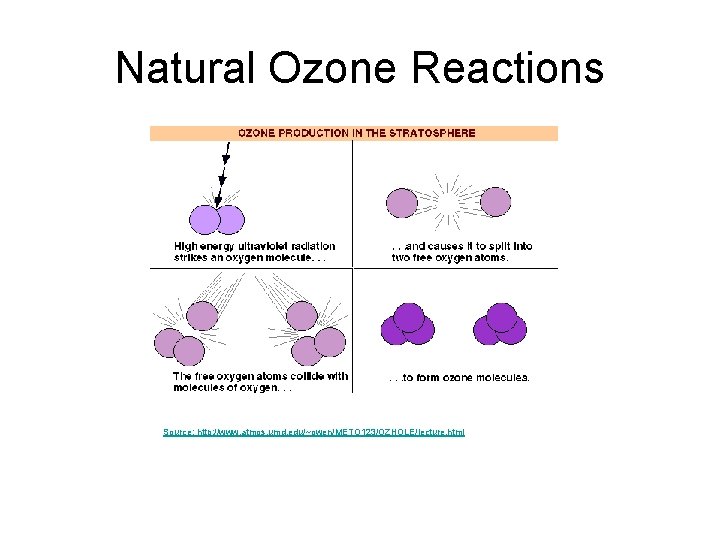
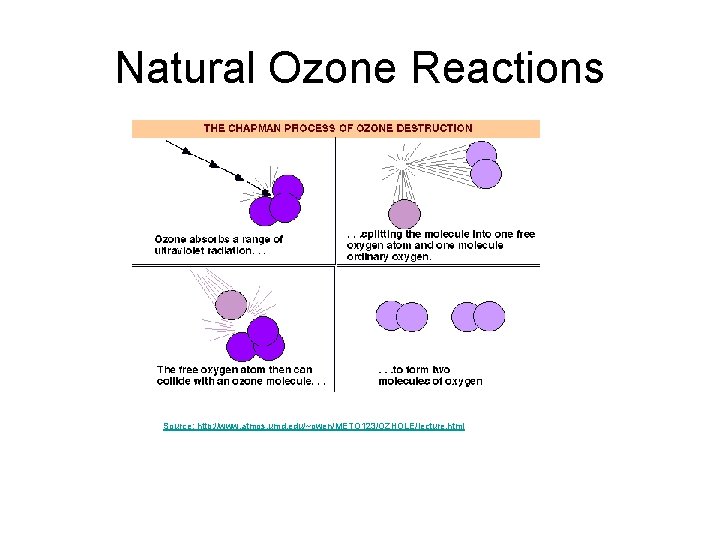
Natural Ozone Reactions Source: http: //www. atmos. umd. edu/~owen/METO 123/OZHOLE/lecture. html

Natural Ozone Reactions Source: http: //www. atmos. umd. edu/~owen/METO 123/OZHOLE/lecture. html

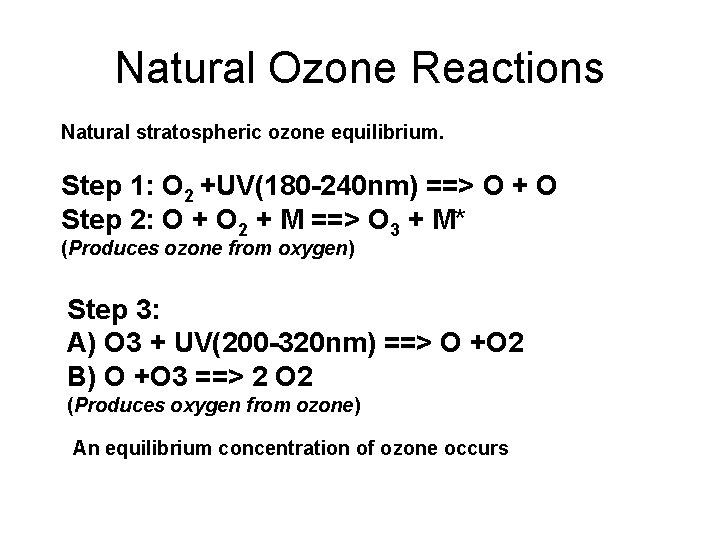
Natural Ozone Reactions Natural stratospheric ozone equilibrium. Step 1: O 2 +UV(180 -240 nm) ==> O + O Step 2: O + O 2 + M ==> O 3 + M* (Produces ozone from oxygen) Step 3: A) O 3 + UV(200 -320 nm) ==> O +O 2 B) O +O 3 ==> 2 O 2 (Produces oxygen from ozone) An equilibrium concentration of ozone occurs

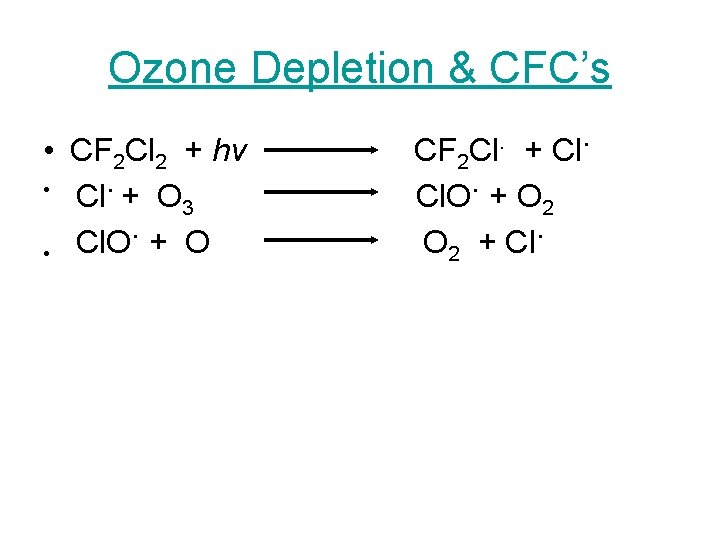
Ozone Depletion & CFC’s • CF 2 Cl 2 + hv • Cl. + O 3. • Cl. O + O Cl. . + Cl CF 2 Cl. O. + O 2 + Cl

Problems (p. 459, 460, 461) • • 10. 2 10. 4 10. 6 10. 8