Free Radical Reaction Mechanism Asst Prof Miss Rupali






- Slides: 31

Free Radical Reaction Mechanism Asst. Prof. Miss. Rupali A. Chaudhari. Department of Chemistry KCES’s Post Graduate College of Science Technology and Research, Jalgaon

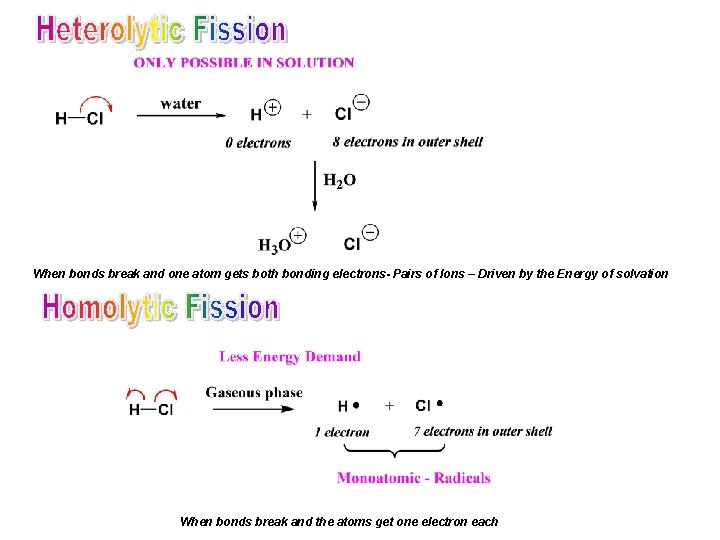
When bonds break and one atom gets both bonding electrons- Pairs of Ions – Driven by the Energy of solvation When bonds break and the atoms get one electron each

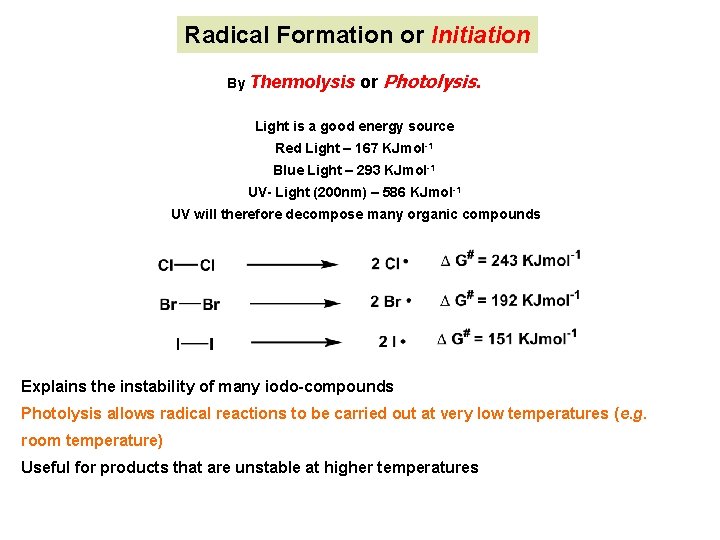
Radical Formation or Initiation By Thermolysis or Photolysis. Light is a good energy source Red Light – 167 KJmol-1 Blue Light – 293 KJmol-1 UV- Light (200 nm) – 586 KJmol-1 UV will therefore decompose many organic compounds Explains the instability of many iodo-compounds Photolysis allows radical reactions to be carried out at very low temperatures (e. g. room temperature) Useful for products that are unstable at higher temperatures

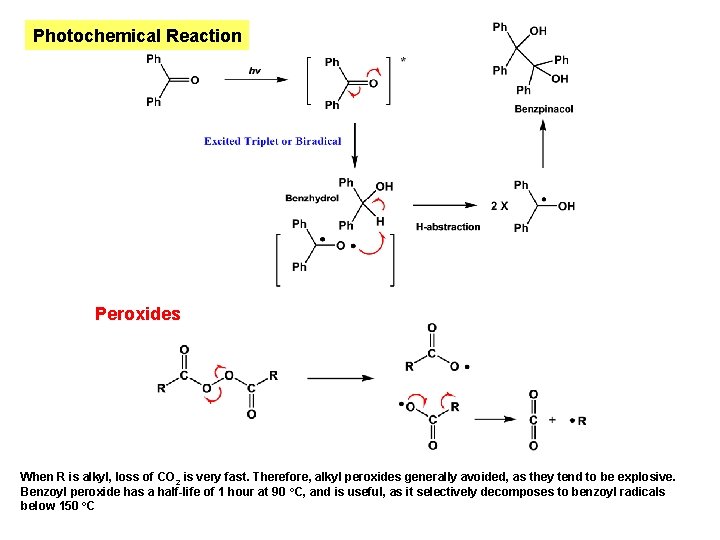
Photochemical Reaction Peroxides When R is alkyl, loss of CO 2 is very fast. Therefore, alkyl peroxides generally avoided, as they tend to be explosive. Benzoyl peroxide has a half-life of 1 hour at 90 o. C, and is useful, as it selectively decomposes to benzoyl radicals below 150 o. C

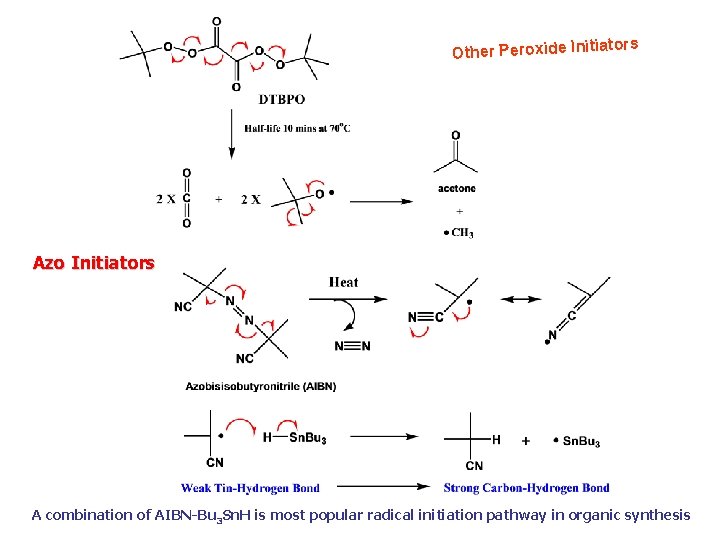
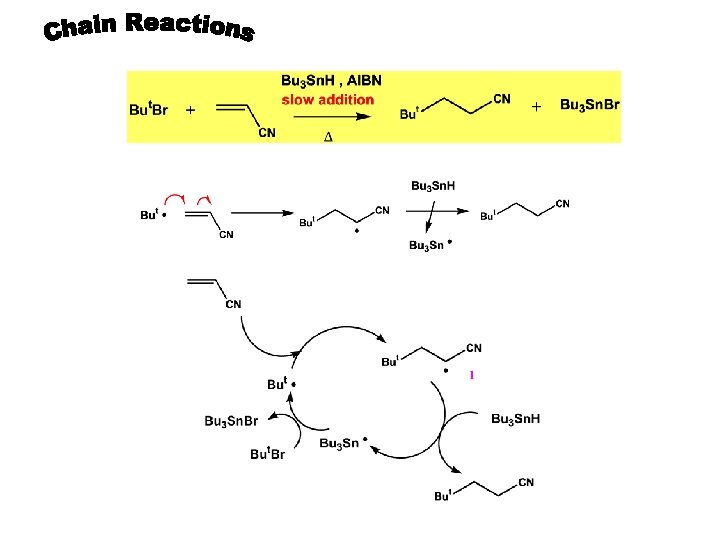
tors Other Peroxide Initia Azo Initiators A combination of AIBN-Bu 3 Sn. H is most popular radical initiation pathway in organic synthesis

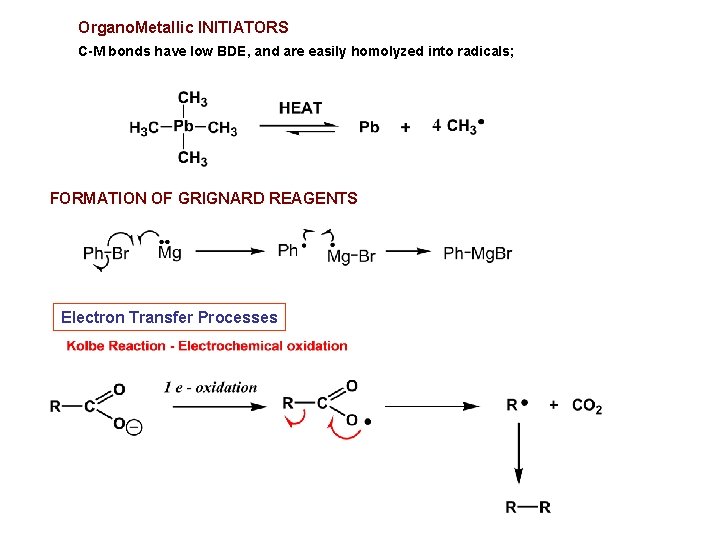
Organo. Metallic INITIATORS C-M bonds have low BDE, and are easily homolyzed into radicals; FORMATION OF GRIGNARD REAGENTS Electron Transfer Processes

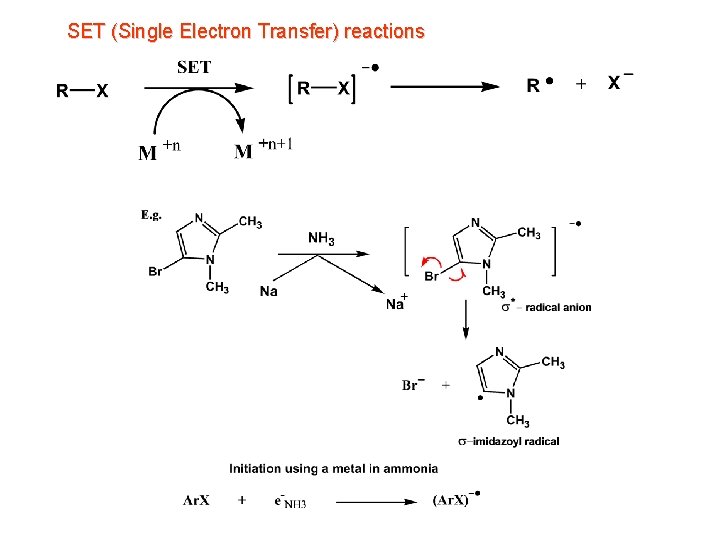
SET (Single Electron Transfer) reactions

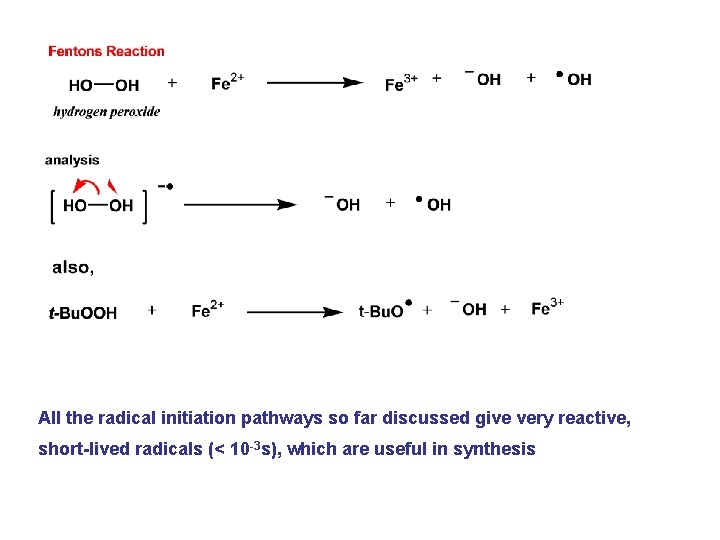
All the radical initiation pathways so far discussed give very reactive, short-lived radicals (< 10 -3 s), which are useful in synthesis

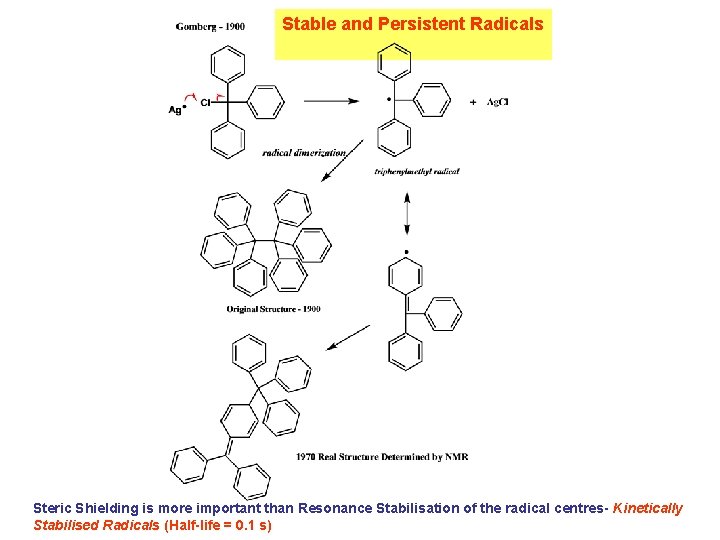
Stable and Persistent Radicals Steric Shielding is more important than Resonance Stabilisation of the radical centres- Kinetically Stabilised Radicals (Half-life = 0. 1 s)

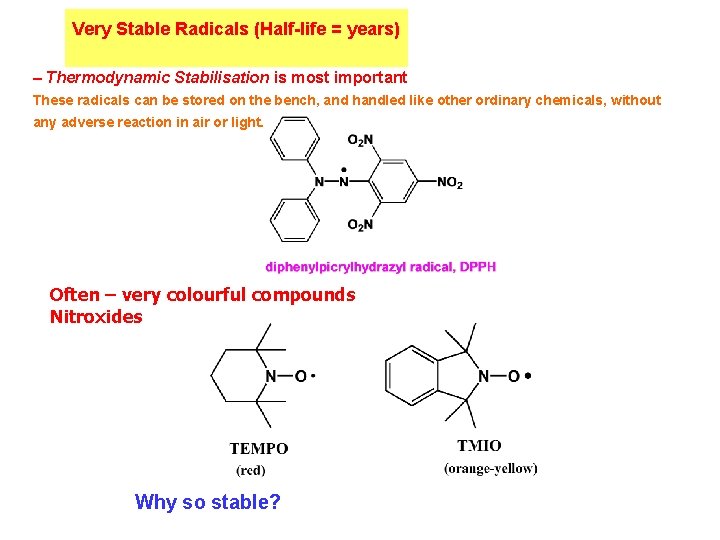
Very Stable Radicals (Half-life = years) – Thermodynamic Stabilisation is most important These radicals can be stored on the bench, and handled like other ordinary chemicals, without any adverse reaction in air or light. Often – very colourful compounds Nitroxides Why so stable?

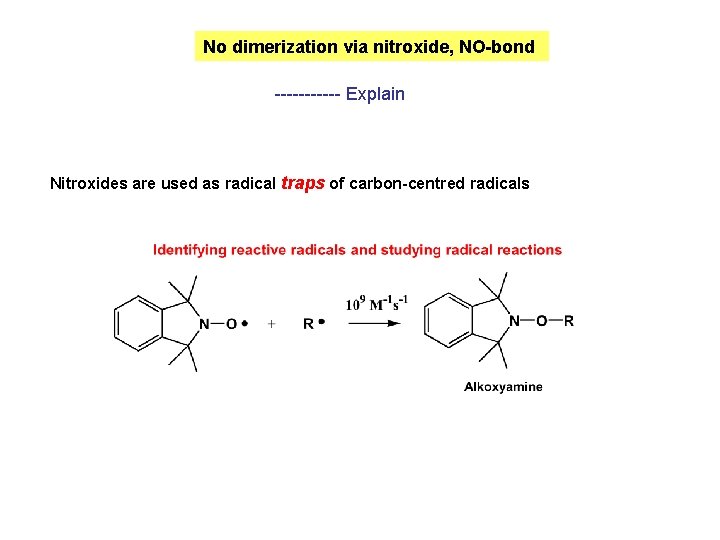
No dimerization via nitroxide, NO-bond ------ Explain Nitroxides are used as radical traps of carbon-centred radicals

Configuration or Geometry of Radicals Normally, configurational isomers are only obtained by breaking covalent bonds, this is not the case with radicals With radicals, bond rotation determines the geometry and hybridisation of molecules.

Similarly, ESR spectroscopy is usually used to determine such features

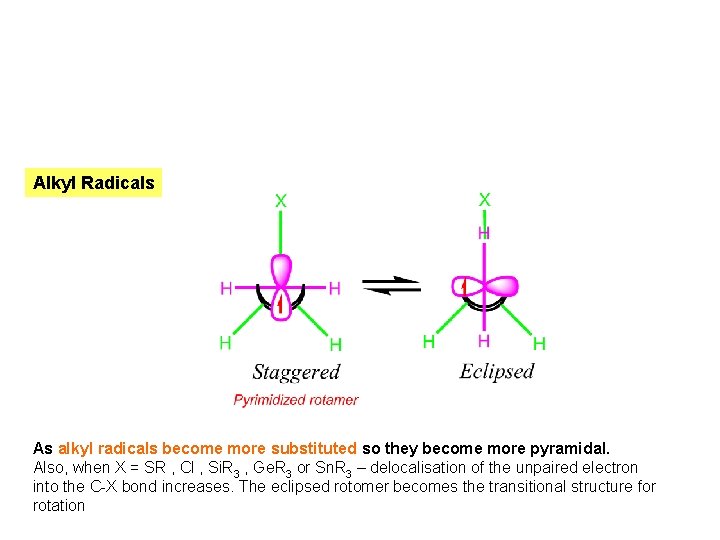
Alkyl Radicals As alkyl radicals become more substituted so they become more pyramidal. Also, when X = SR , Cl , Si. R 3 , Ge. R 3 or Sn. R 3 – delocalisation of the unpaired electron into the C-X bond increases. The eclipsed rotomer becomes the transitional structure for rotation

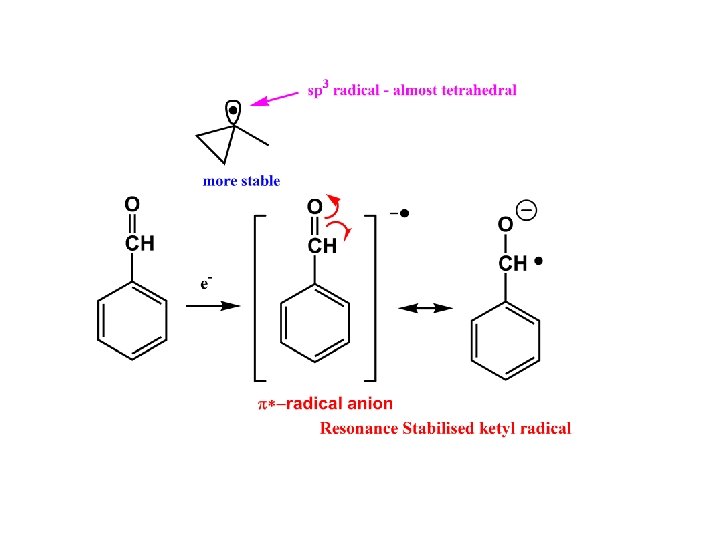
a/ Thermodynamic Stability Is quantified in terms of the enthalpy of dissociation of R-H into R and H The main factors which determine stability are Conjugation, Hyperconjugation, Hybridisation and Captodative effects

1. Conjugation or Mesomerism This is the primary reason for the existence of stable radicals (see notes on nitroxides and DPPH)

2. Hybridisation p-Radical is more stable than s-Radical. As the p- character of a radical increases so does its thermodynamic stabilisation


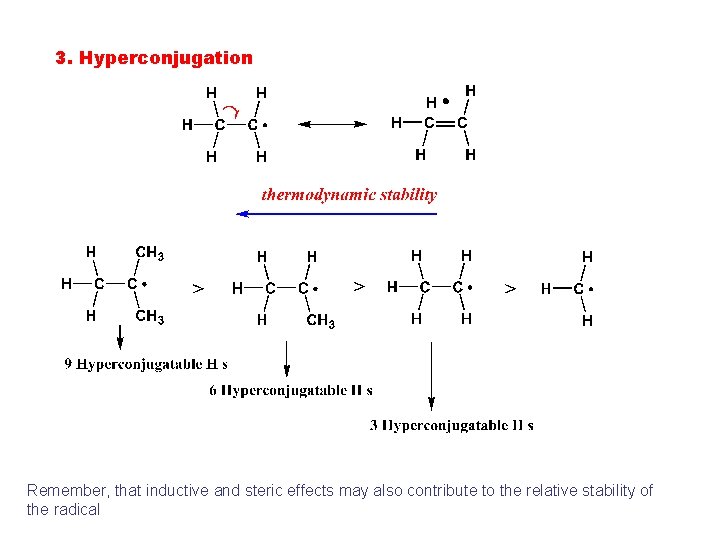
3. Hyperconjugation Remember, that inductive and steric effects may also contribute to the relative stability of the radical

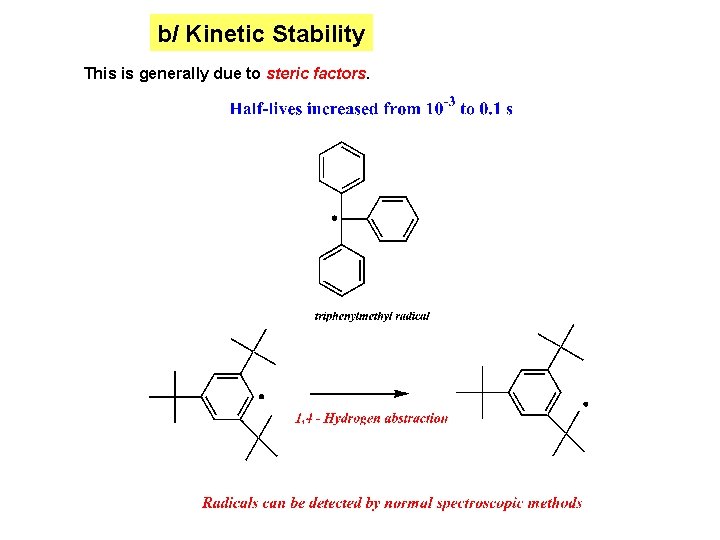
b/ Kinetic Stability This is generally due to steric factors.

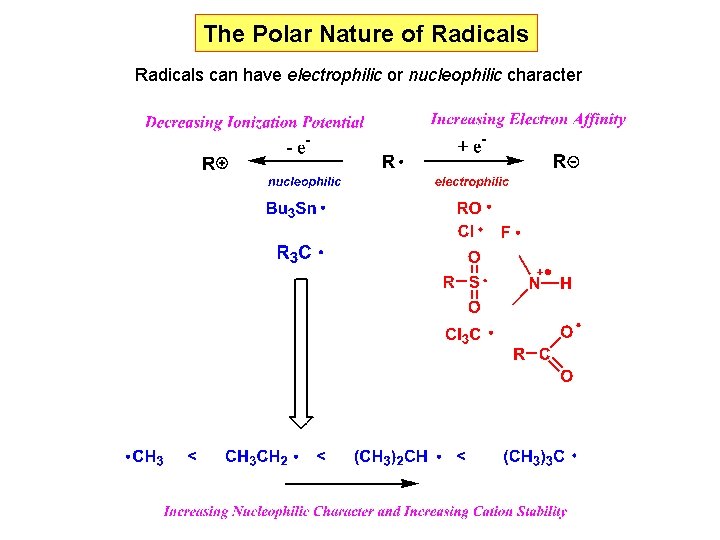
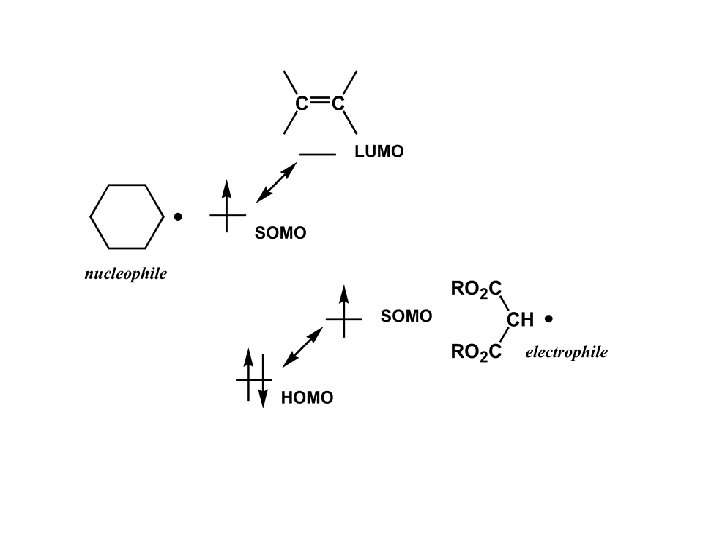
The Polar Nature of Radicals can have electrophilic or nucleophilic character




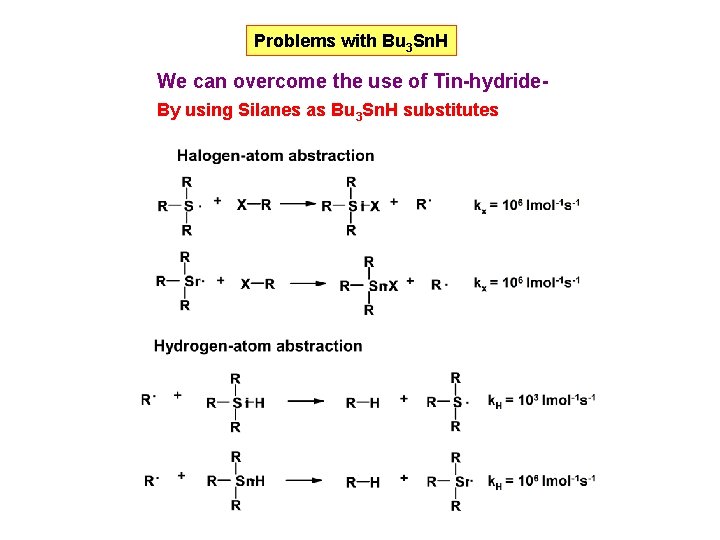
Problems with Bu 3 Sn. H We can overcome the use of Tin-hydride. By using Silanes as Bu 3 Sn. H substitutes

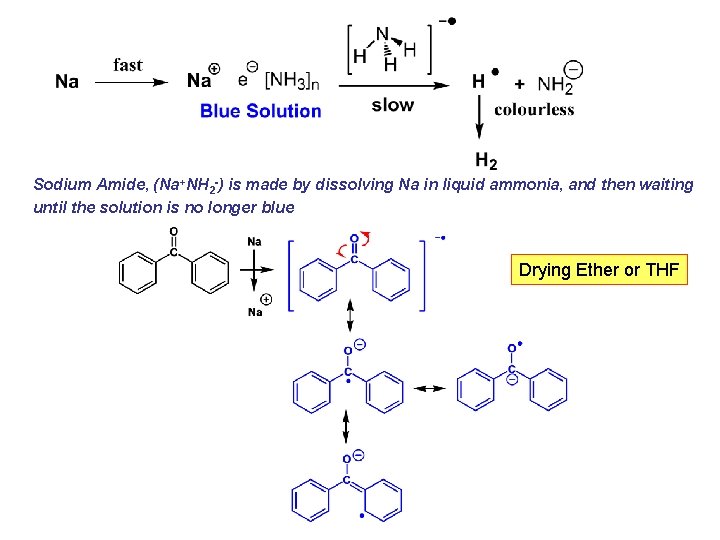
Sodium Amide, (Na+NH 2 -) is made by dissolving Na in liquid ammonia, and then waiting until the solution is no longer blue Drying Ether or THF

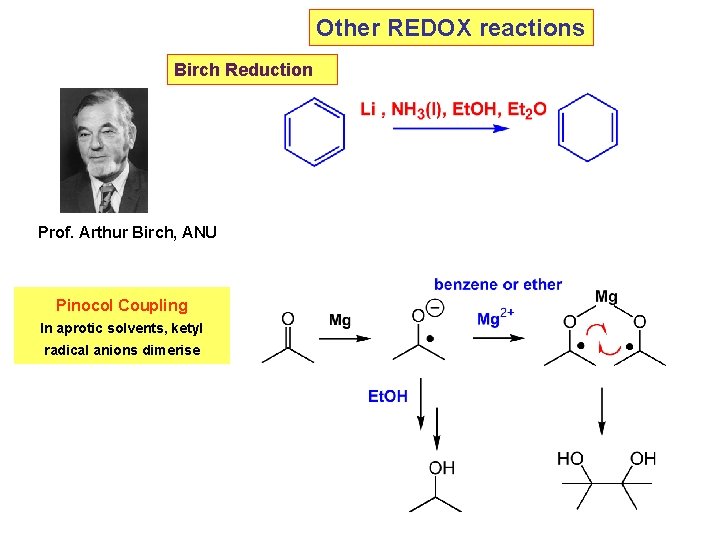
Other REDOX reactions Birch Reduction Prof. Arthur Birch, ANU Pinocol Coupling In aprotic solvents, ketyl radical anions dimerise

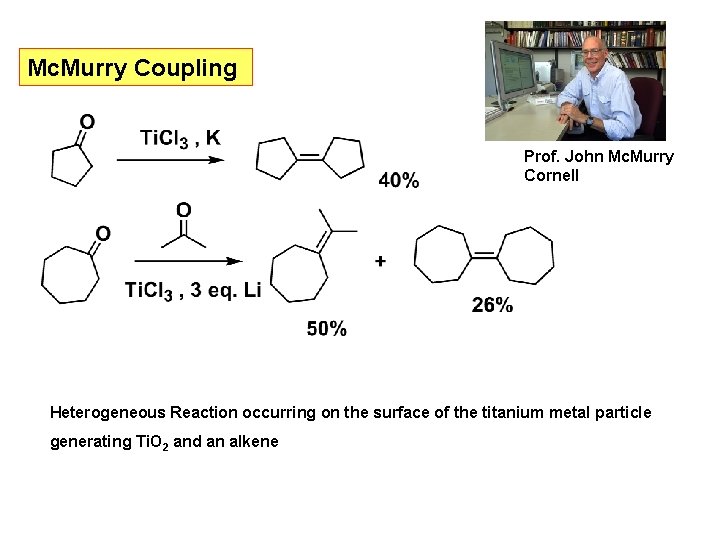
Mc. Murry Coupling Prof. John Mc. Murry Cornell Heterogeneous Reaction occurring on the surface of the titanium metal particle generating Ti. O 2 and an alkene

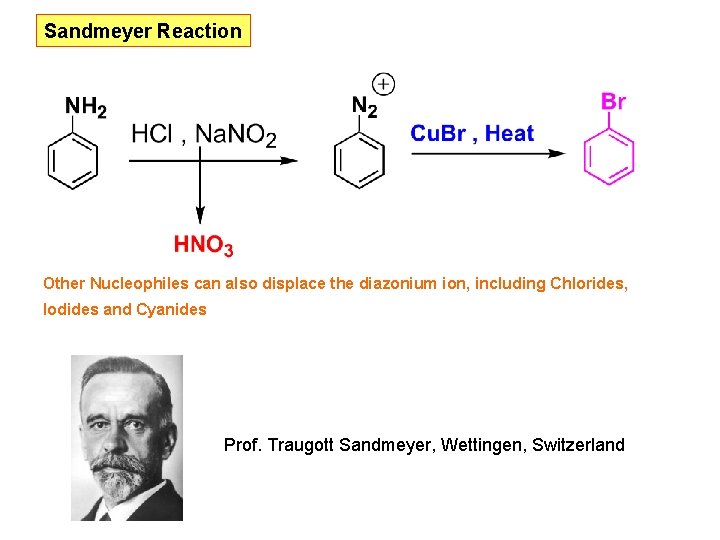
Sandmeyer Reaction Other Nucleophiles can also displace the diazonium ion, including Chlorides, Iodides and Cyanides Prof. Traugott Sandmeyer, Wettingen, Switzerland

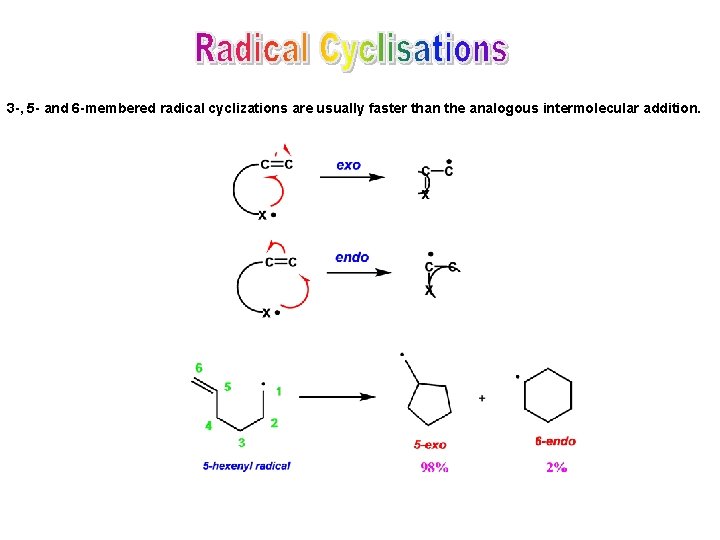
3 -, 5 - and 6 -membered radical cyclizations are usually faster than the analogous intermolecular addition.

The ‘Radical Clock’ is a standard fast reaction of known rate constant, which the rates of other competing radical or product radical reactions can be measured.