Free Energy Changes Tro Chapter 18 Free Energy




- Slides: 14

Free Energy Changes Tro Chapter 18 – Free Energy and Thermodynamics 18. 8 Free Energy Changes in Chemical Reactions: Calculating DGorxn

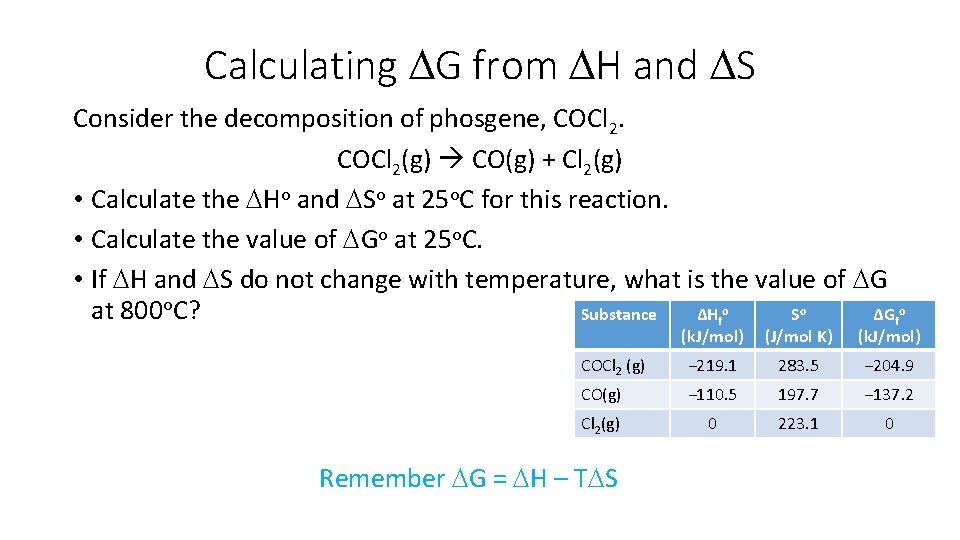
Calculating DG from DH and DS Consider the decomposition of phosgene, COCl 2(g) CO(g) + Cl 2(g) • Calculate the DHo and DSo at 25 o. C for this reaction. • Calculate the value of DGo at 25 o. C. • If DH and DS do not change with temperature, what is the value of DG at 800 o. C? Substance ΔHfo So ΔGfo (k. J/mol) (J/mol K) (k. J/mol) COCl 2 (g) − 219. 1 283. 5 − 204. 9 CO(g) − 110. 5 197. 7 − 137. 2 Cl 2(g) 0 223. 1 0 Remember DG = DH – TDS

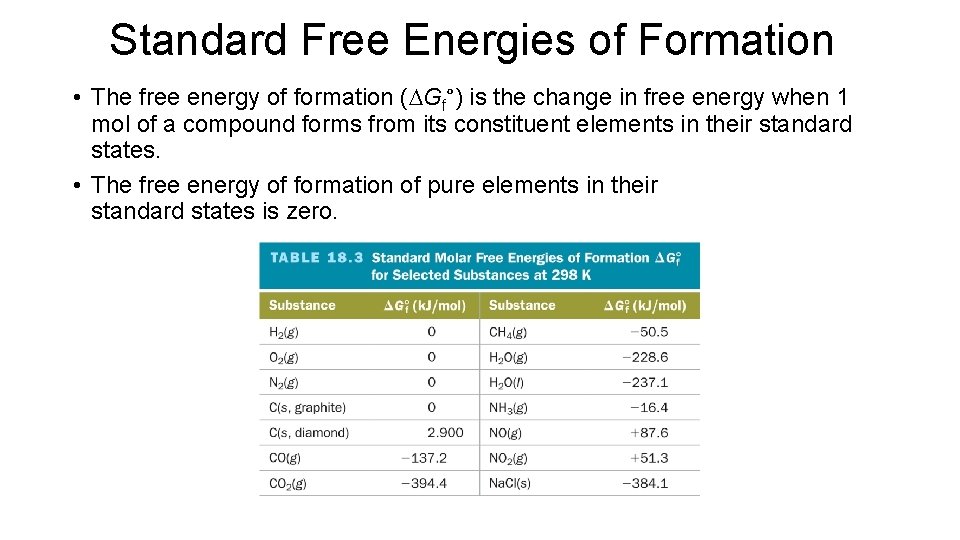
Standard Free Energies of Formation • The free energy of formation (DGf°) is the change in free energy when 1 mol of a compound forms from its constituent elements in their standard states. • The free energy of formation of pure elements in their standard states is zero.

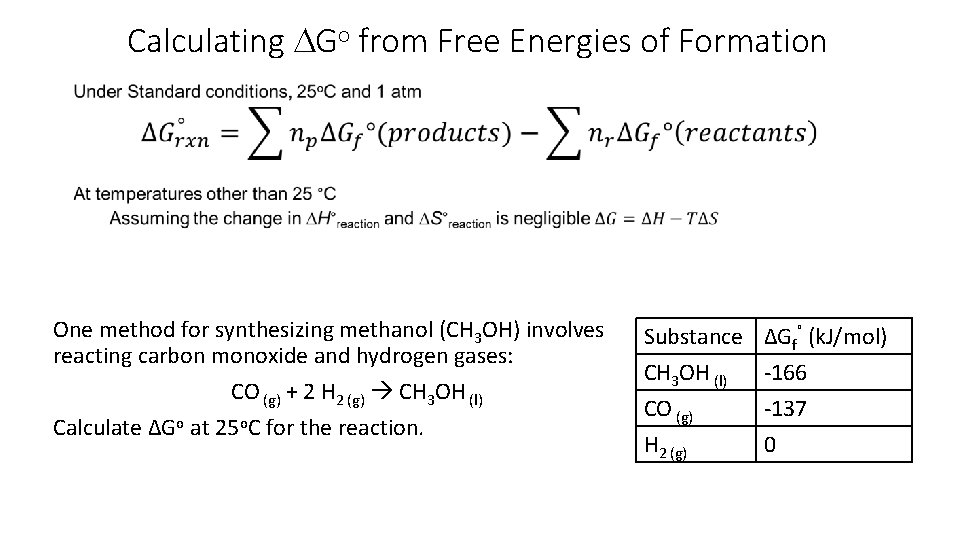
Calculating DGo from Free Energies of Formation One method for synthesizing methanol (CH 3 OH) involves reacting carbon monoxide and hydrogen gases: CO (g) + 2 H 2 (g) CH 3 OH (l) Calculate ΔGo at 25 o. C for the reaction. Substance CH 3 OH (l) CO (g) H 2 (g) ΔGf˚ (k. J/mol) -166 -137 0

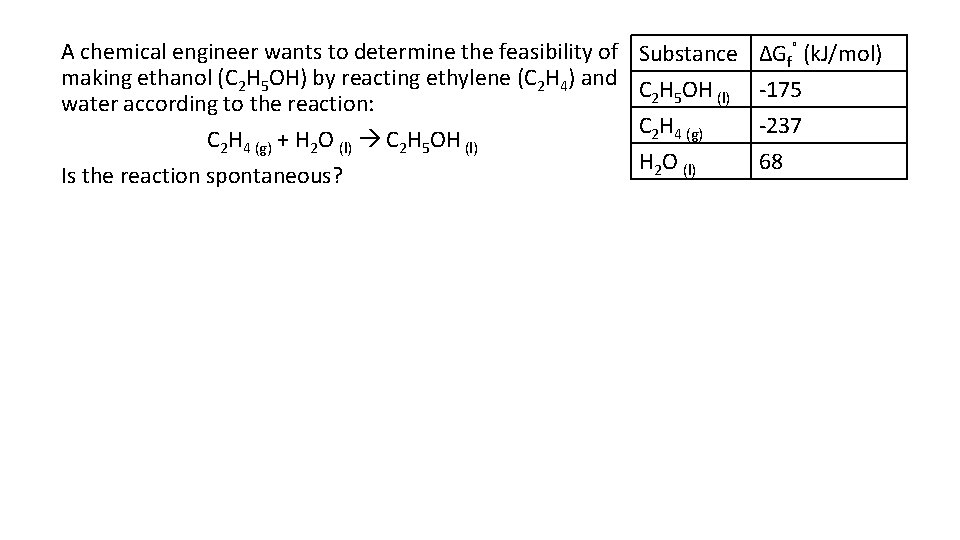
A chemical engineer wants to determine the feasibility of making ethanol (C 2 H 5 OH) by reacting ethylene (C 2 H 4) and water according to the reaction: C 2 H 4 (g) + H 2 O (l) C 2 H 5 OH (l) Is the reaction spontaneous? Substance C 2 H 5 OH (l) C 2 H 4 (g) H 2 O (l) ΔGf˚ (k. J/mol) -175 -237 68

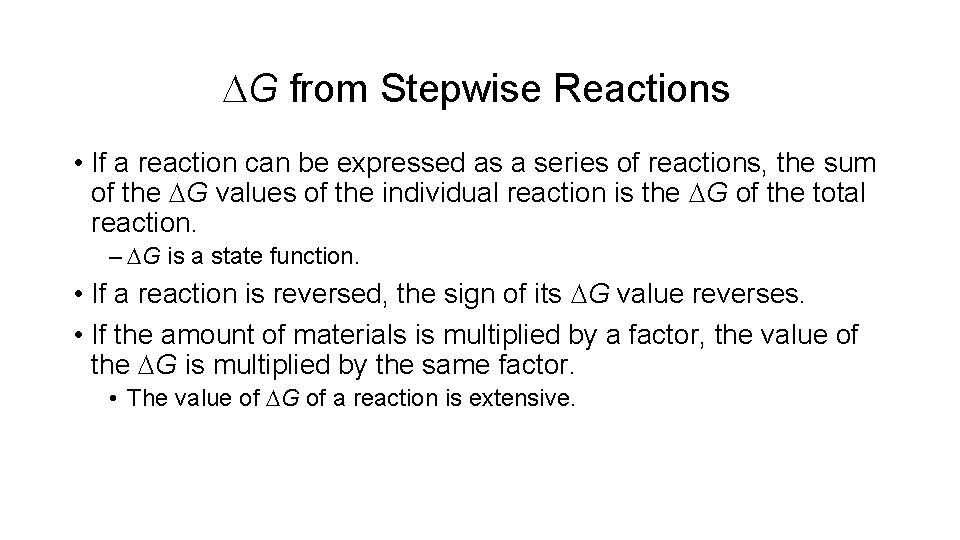
DG from Stepwise Reactions • If a reaction can be expressed as a series of reactions, the sum of the DG values of the individual reaction is the DG of the total reaction. – DG is a state function. • If a reaction is reversed, the sign of its DG value reverses. • If the amount of materials is multiplied by a factor, the value of the DG is multiplied by the same factor. • The value of DG of a reaction is extensive.


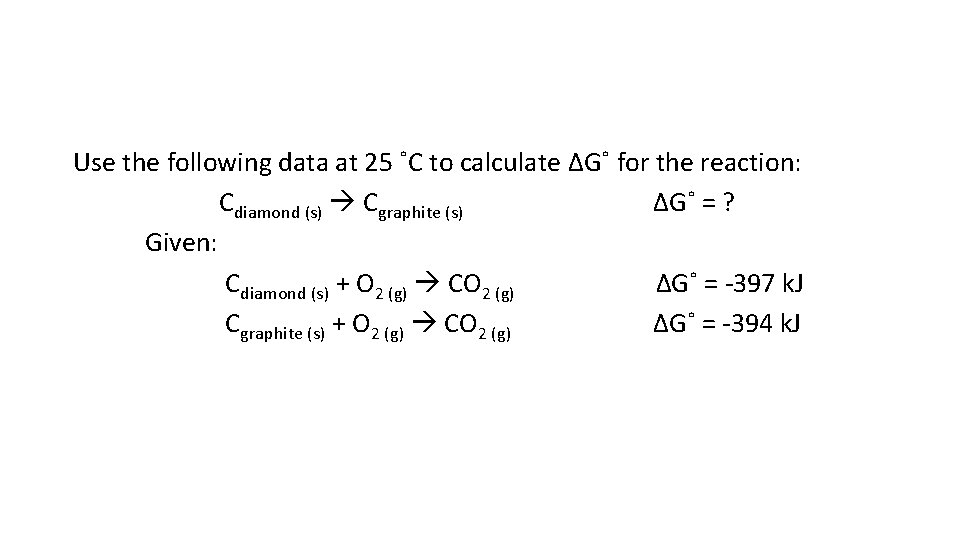
Use the following data at 25 ˚C to calculate ΔG˚ for the reaction: Cdiamond (s) Cgraphite (s) ΔG˚ = ? Given: Cdiamond (s) + O 2 (g) CO 2 (g) ΔG˚ = -397 k. J Cgraphite (s) + O 2 (g) CO 2 (g) ΔG˚ = -394 k. J

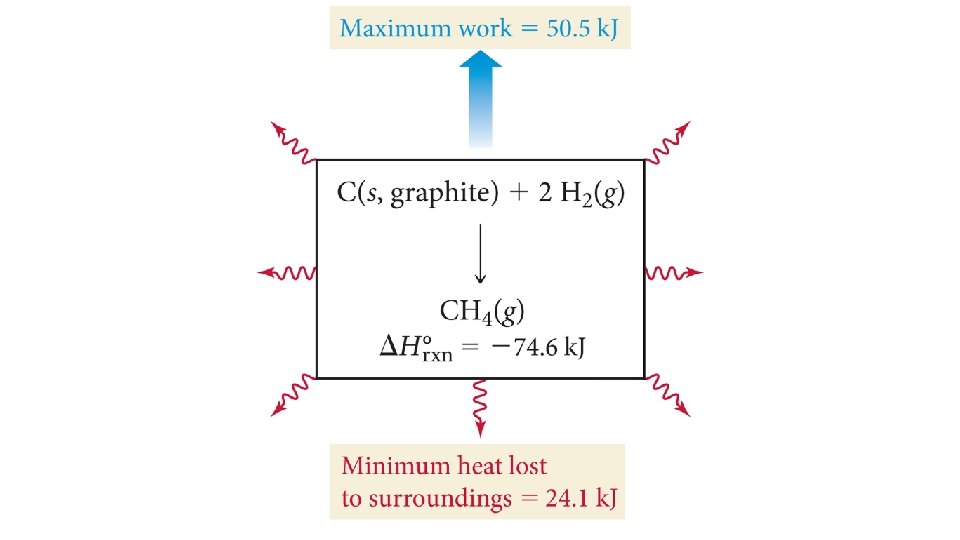
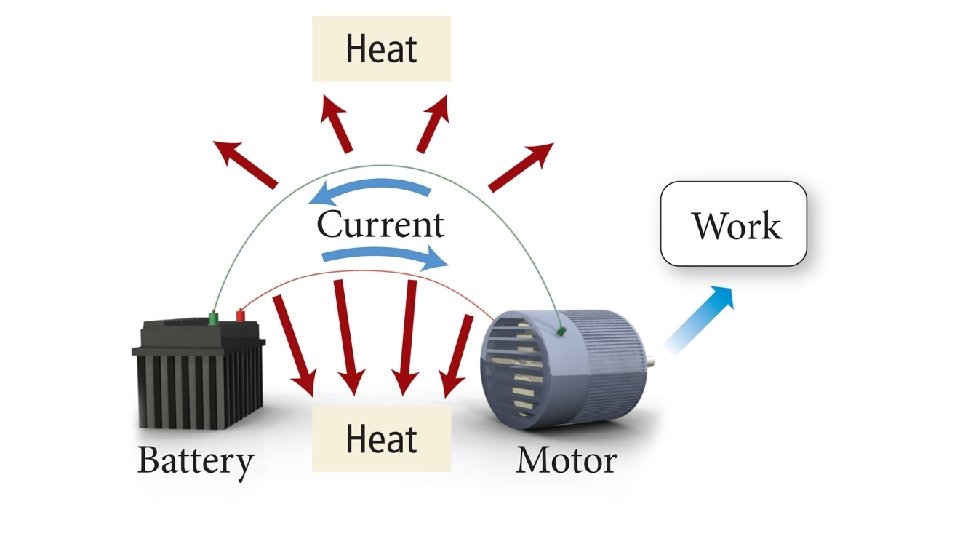
Why Free Energy Is “Free” • The free energy is the maximum amount of energy available to do work. • For many exothermic reactions, some of the heat released due to the enthalpy change goes into increasing the entropy of the surroundings, so it is not available to do work. • And even some of this free energy is generally lost to heating up the surroundings.



Free Energy and Reversible Reactions • The change in free energy is a theoretical limit as to the amount of work that can be done. • If the reaction achieves its theoretical limit, it is a reversible reaction.

Real Reactions • In a real reaction, some (if not most) of the free energy is “lost” as heat. • Therefore, real reactions are irreversible.

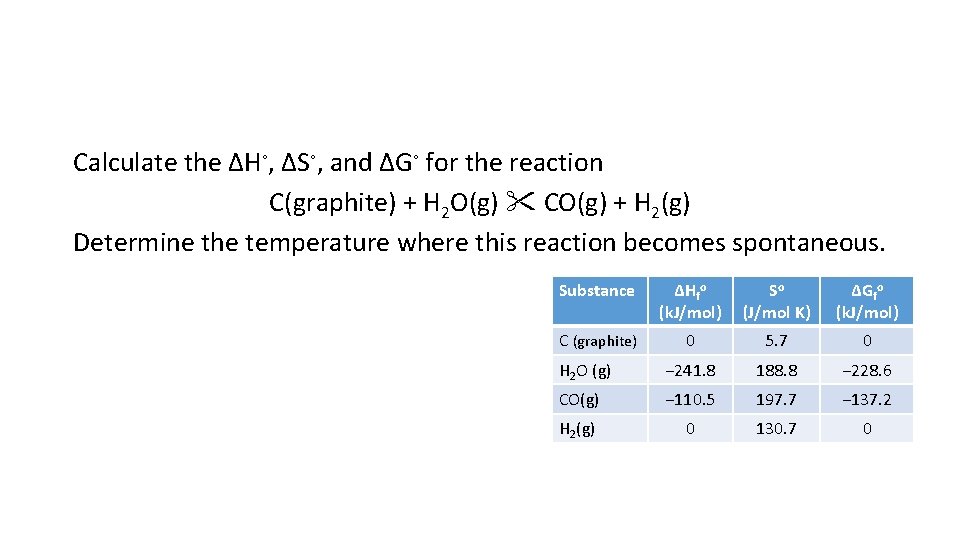
Calculate the ΔH◦, ΔS◦, and ΔG◦ for the reaction C(graphite) + H 2 O(g) CO(g) + H 2(g) Determine the temperature where this reaction becomes spontaneous. Substance ΔHfo (k. J/mol) So (J/mol K) ΔGfo (k. J/mol) C (graphite) 0 5. 7 0 H 2 O (g) − 241. 8 188. 8 − 228. 6 CO(g) − 110. 5 197. 7 − 137. 2 H 2(g) 0 130. 7 0