Frederick A Bettelheim William H Brown Mary K

Frederick A. Bettelheim William H. Brown Mary K. Campbell Shawn O. Farrell www. cengage. com/chemistry/bettelheim Chapter 19 Carboxylic Anhydrides, Esters, and Amides William H. Brown • Beloit College
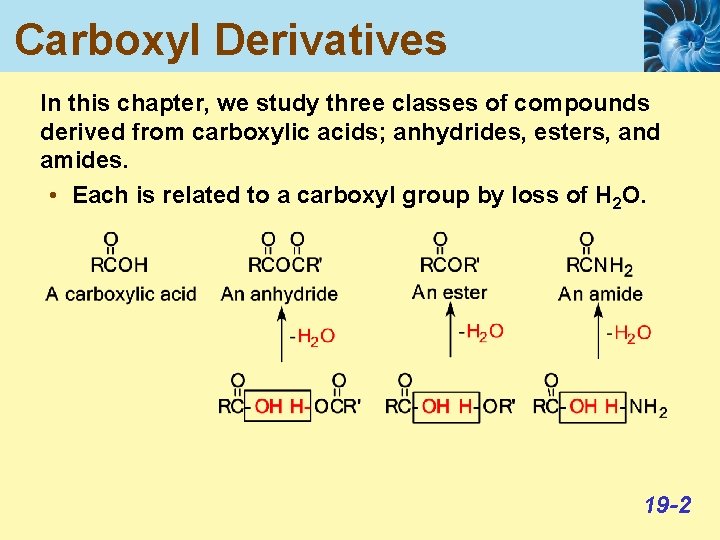
Carboxyl Derivatives In this chapter, we study three classes of compounds derived from carboxylic acids; anhydrides, esters, and amides. • Each is related to a carboxyl group by loss of H 2 O. 19 -2
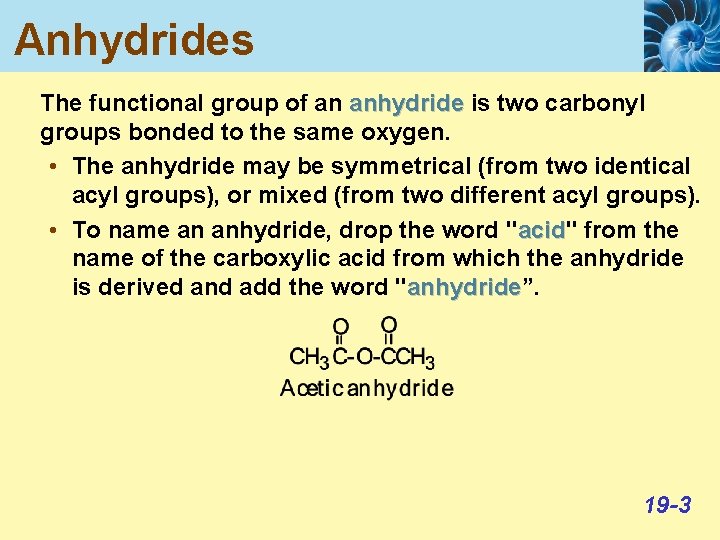
Anhydrides The functional group of an anhydride is two carbonyl groups bonded to the same oxygen. • The anhydride may be symmetrical (from two identical acyl groups), or mixed (from two different acyl groups). • To name an anhydride, drop the word "acid" acid from the name of the carboxylic acid from which the anhydride is derived and add the word "anhydride”. anhydride 19 -3
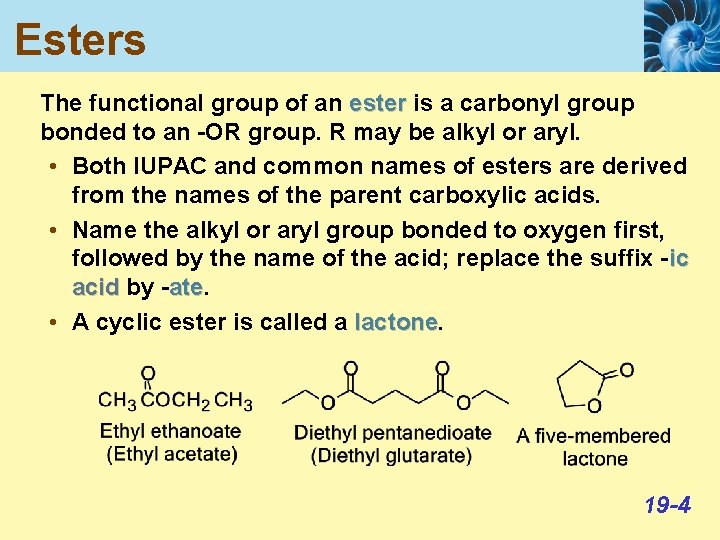
Esters The functional group of an ester is a carbonyl group bonded to an -OR group. R may be alkyl or aryl. • Both IUPAC and common names of esters are derived from the names of the parent carboxylic acids. • Name the alkyl or aryl group bonded to oxygen first, followed by the name of the acid; replace the suffix -ic acid by -ate. ate • A cyclic ester is called a lactone 19 -4

Amides The functional group of an amide is a carbonyl group bonded to a nitrogen atom. • To name an amide, drop the suffix -oic acid from the IUPAC name of the parent acid, or -ic acid from its common name, and add -amide • If the amide nitrogen is also bonded to an alkyl or aryl group, name the group and show its location on nitrogen by N- ; two alkyl or aryl groups by N, N-di-. 19 -5
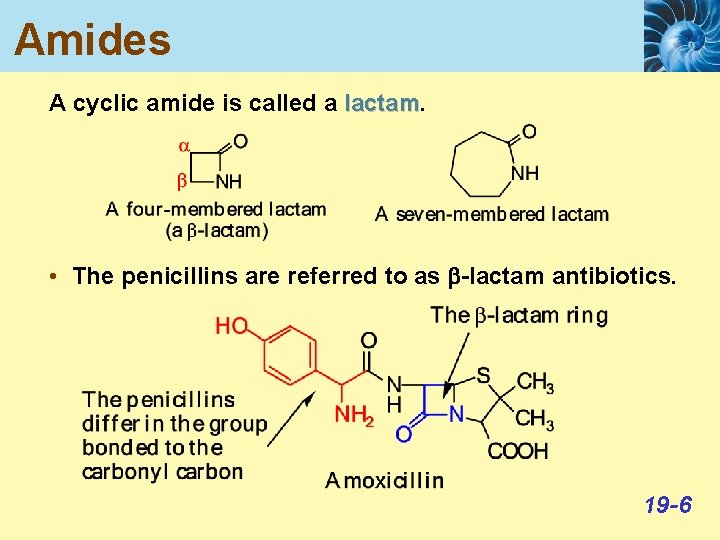
Amides A cyclic amide is called a lactam • The penicillins are referred to as b-lactam antibiotics. 19 -6
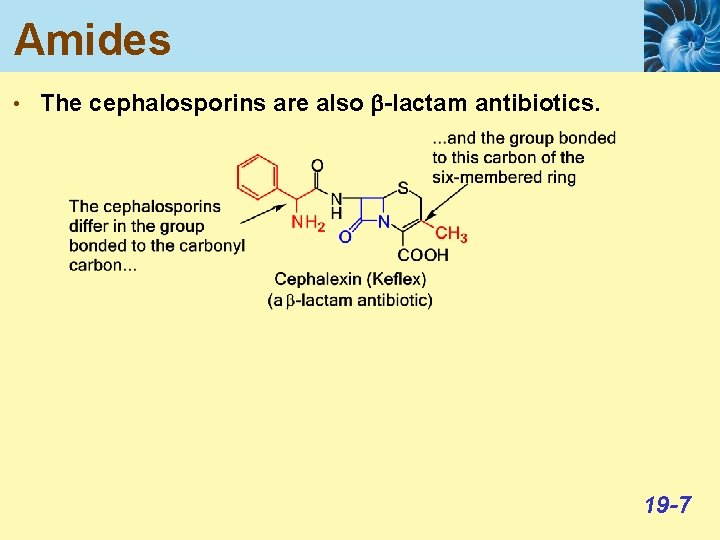
Amides • The cephalosporins are also b-lactam antibiotics. 19 -7
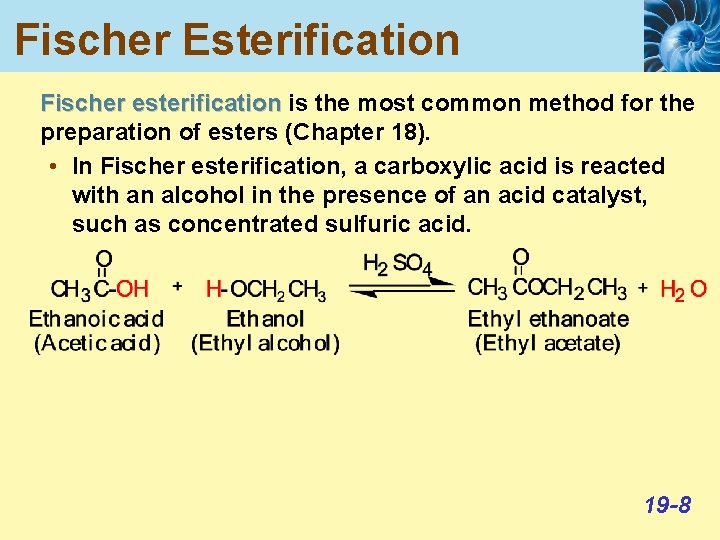
Fischer Esterification Fischer esterification is the most common method for the preparation of esters (Chapter 18). • In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, such as concentrated sulfuric acid. 19 -8
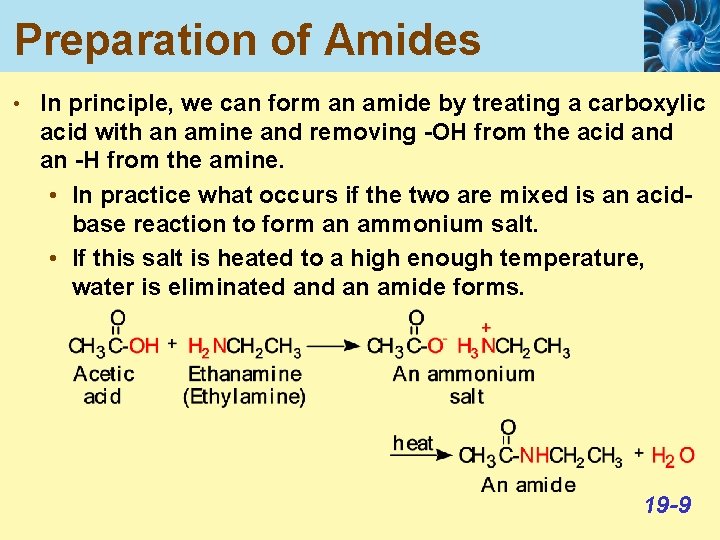
Preparation of Amides • In principle, we can form an amide by treating a carboxylic acid with an amine and removing -OH from the acid an -H from the amine. • In practice what occurs if the two are mixed is an acidbase reaction to form an ammonium salt. • If this salt is heated to a high enough temperature, water is eliminated an amide forms. 19 -9
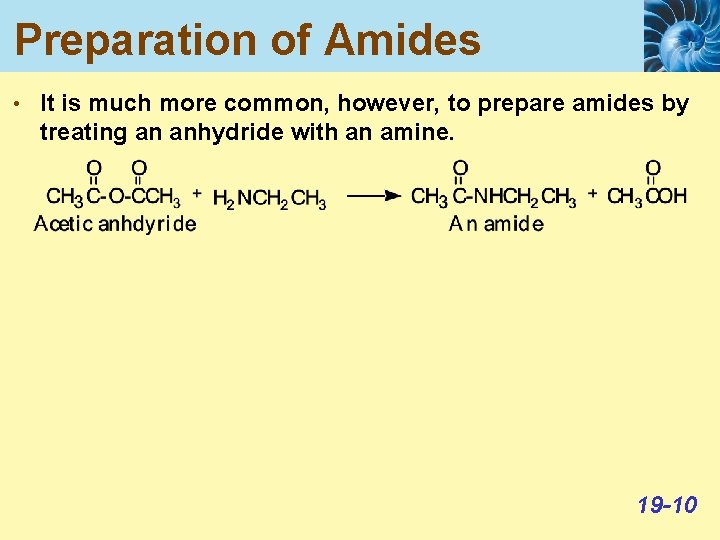
Preparation of Amides • It is much more common, however, to prepare amides by treating an anhydride with an amine. 19 -10
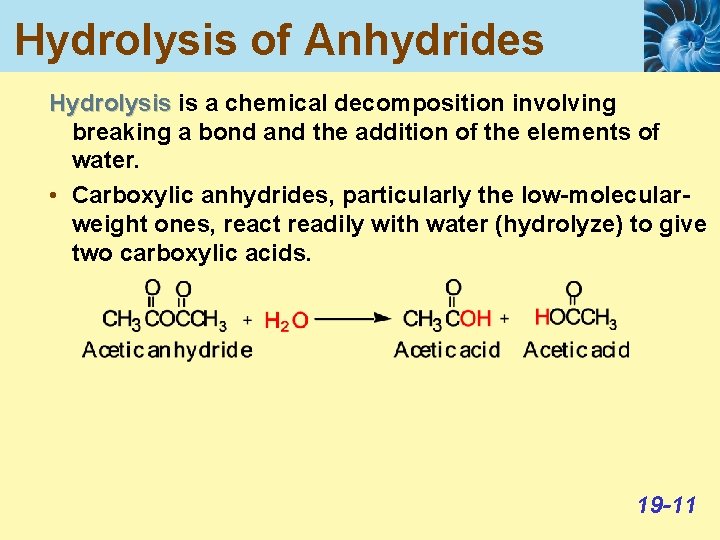
Hydrolysis of Anhydrides Hydrolysis is a chemical decomposition involving breaking a bond and the addition of the elements of water. • Carboxylic anhydrides, particularly the low-molecularweight ones, react readily with water (hydrolyze) to give two carboxylic acids. 19 -11
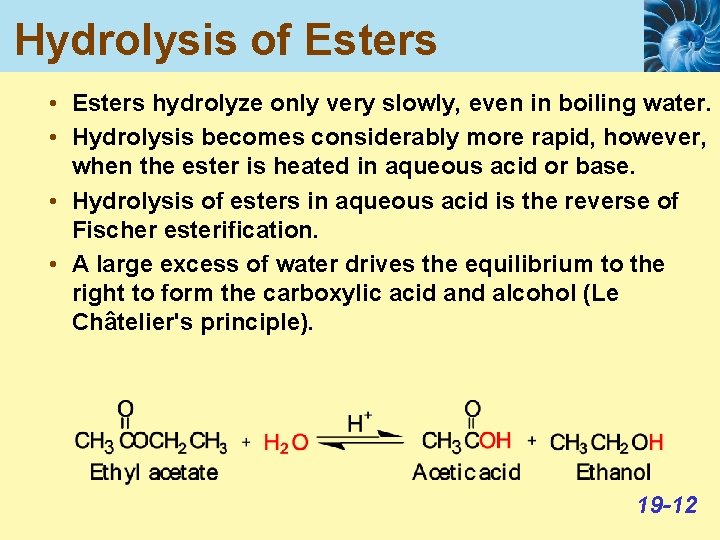
Hydrolysis of Esters • Esters hydrolyze only very slowly, even in boiling water. • Hydrolysis becomes considerably more rapid, however, when the ester is heated in aqueous acid or base. • Hydrolysis of esters in aqueous acid is the reverse of Fischer esterification. • A large excess of water drives the equilibrium to the right to form the carboxylic acid and alcohol (Le Châtelier's principle). 19 -12
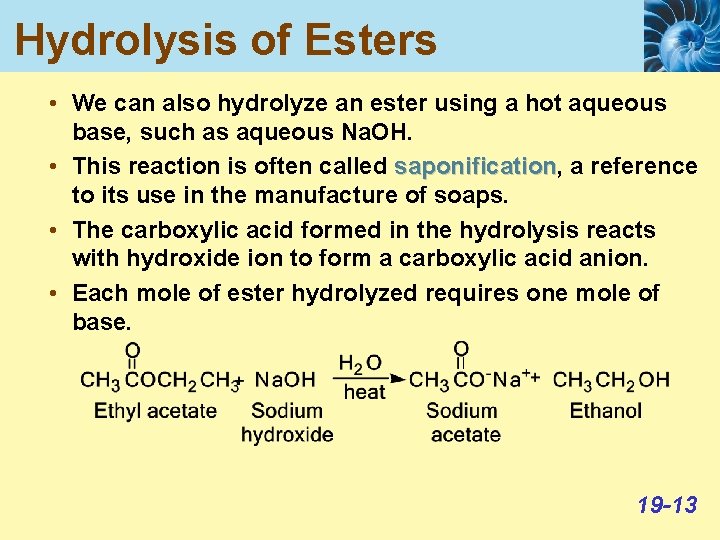
Hydrolysis of Esters • We can also hydrolyze an ester using a hot aqueous base, such as aqueous Na. OH. • This reaction is often called saponification, saponification a reference to its use in the manufacture of soaps. • The carboxylic acid formed in the hydrolysis reacts with hydroxide ion to form a carboxylic acid anion. • Each mole of ester hydrolyzed requires one mole of base. 19 -13
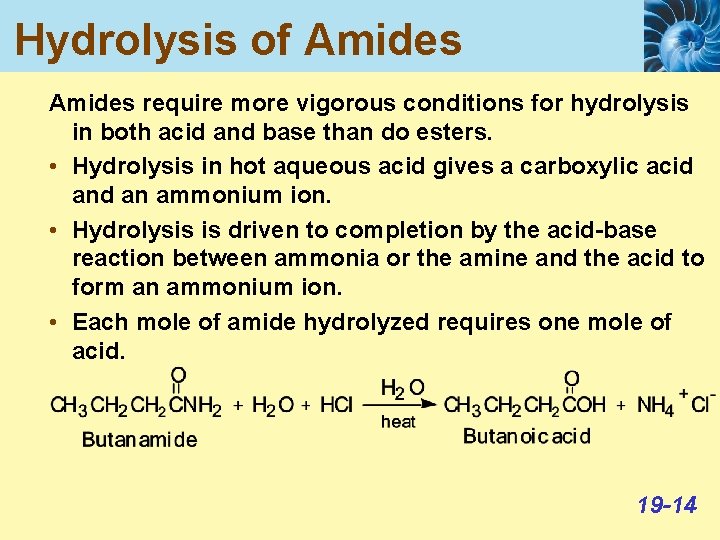
Hydrolysis of Amides require more vigorous conditions for hydrolysis in both acid and base than do esters. • Hydrolysis in hot aqueous acid gives a carboxylic acid an ammonium ion. • Hydrolysis is driven to completion by the acid-base reaction between ammonia or the amine and the acid to form an ammonium ion. • Each mole of amide hydrolyzed requires one mole of acid. 19 -14
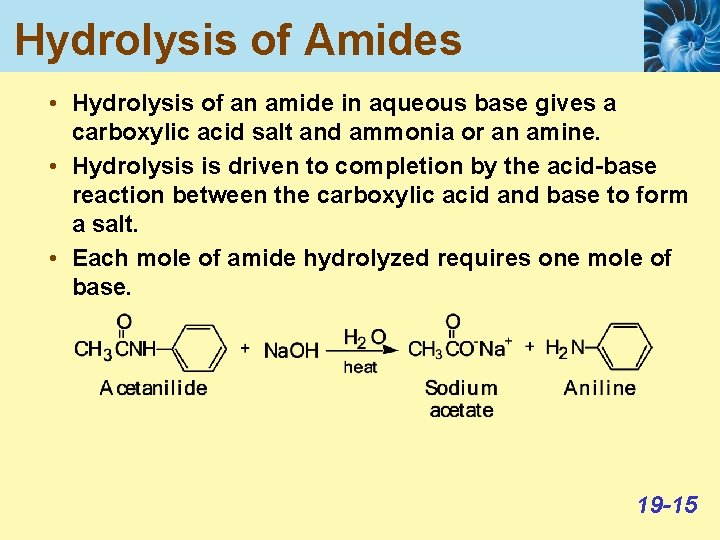
Hydrolysis of Amides • Hydrolysis of an amide in aqueous base gives a carboxylic acid salt and ammonia or an amine. • Hydrolysis is driven to completion by the acid-base reaction between the carboxylic acid and base to form a salt. • Each mole of amide hydrolyzed requires one mole of base. 19 -15
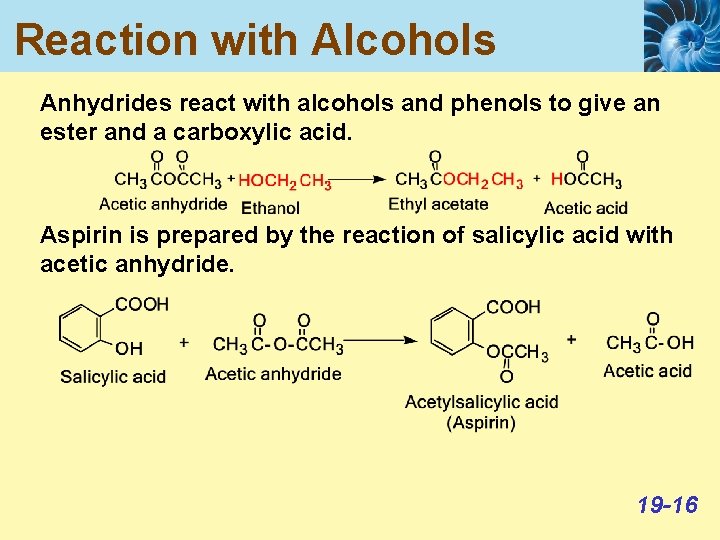
Reaction with Alcohols Anhydrides react with alcohols and phenols to give an ester and a carboxylic acid. Aspirin is prepared by the reaction of salicylic acid with acetic anhydride. 19 -16
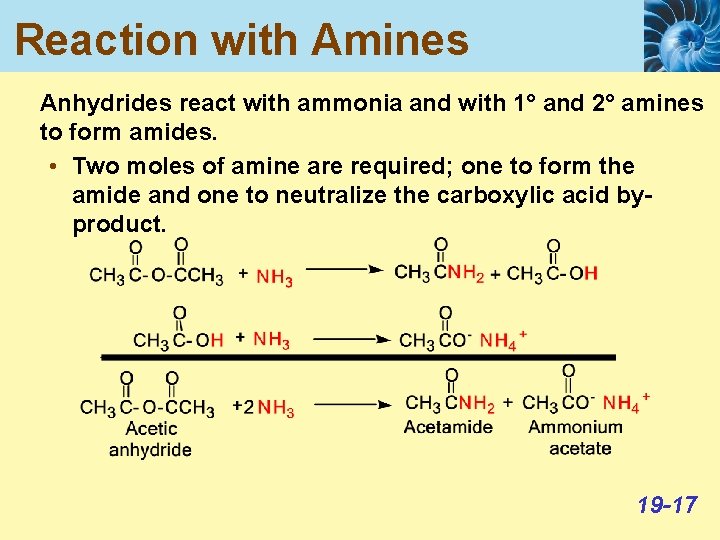
Reaction with Amines Anhydrides react with ammonia and with 1° and 2° amines to form amides. • Two moles of amine are required; one to form the amide and one to neutralize the carboxylic acid byproduct. 19 -17
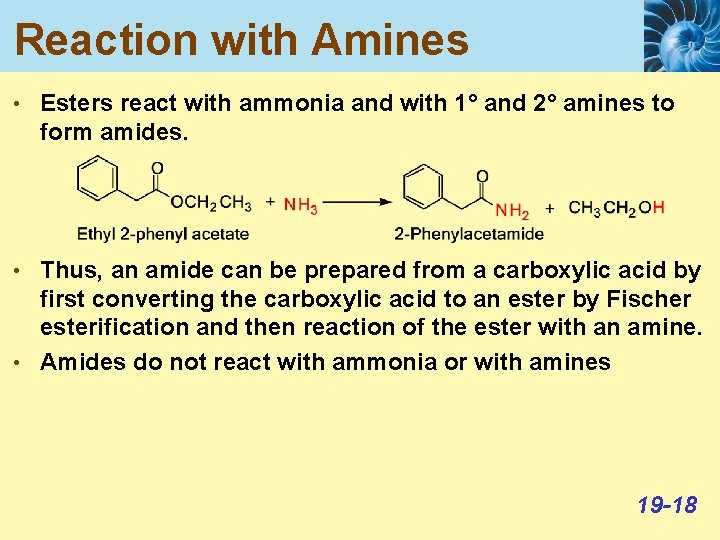
Reaction with Amines • Esters react with ammonia and with 1° and 2° amines to form amides. • Thus, an amide can be prepared from a carboxylic acid by first converting the carboxylic acid to an ester by Fischer esterification and then reaction of the ester with an amine. • Amides do not react with ammonia or with amines 19 -18
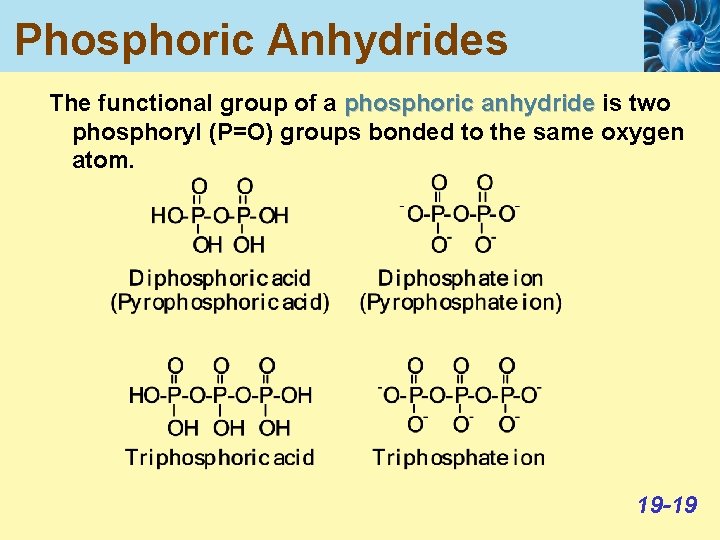
Phosphoric Anhydrides The functional group of a phosphoric anhydride is two phosphoryl (P=O) groups bonded to the same oxygen atom. 19 -19
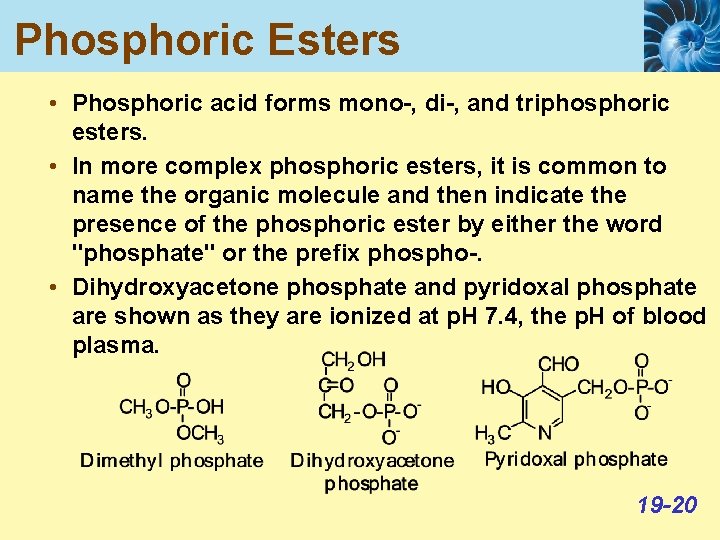
Phosphoric Esters • Phosphoric acid forms mono-, di-, and triphosphoric esters. • In more complex phosphoric esters, it is common to name the organic molecule and then indicate the presence of the phosphoric ester by either the word "phosphate" or the prefix phospho-. • Dihydroxyacetone phosphate and pyridoxal phosphate are shown as they are ionized at p. H 7. 4, the p. H of blood plasma. 19 -20
Step-Growth Polymerization Step-growth polymers are formed by reaction between two molecules, each of which contains two functional groups. Each new bond is created in a separate step. • in this section, we discuss three types of step-growth polymers; polyamides, polyesters, and polycarbonates. 19 -21

Polyamides Nylon-66 was the first purely synthetic fiber. • It is synthesized from two six-carbon monomers. 19 -22
Polyamides The polyaromatic amide known as Kevlar is made from an aromatic dicarboxylic acid an aromatic diamine. 19 -23

Polyesters The first polyester involved polymerization of this diester and diol. 19 -24

Polycarbonates Lexan, the most familiar polycarbonate, polycarbonate is formed by reaction between the disodium salt of bisphenol A and phosgene. 19 -25
Chapter 19 Carboxylic Anhydrides, Esters, and Amides End Chapter 19 19 -26
- Slides: 26