Framework Silicates Tectosilicates FRAMEWORK SILICATES More than three





















- Slides: 68

Framework Silicates (Tectosilicates)

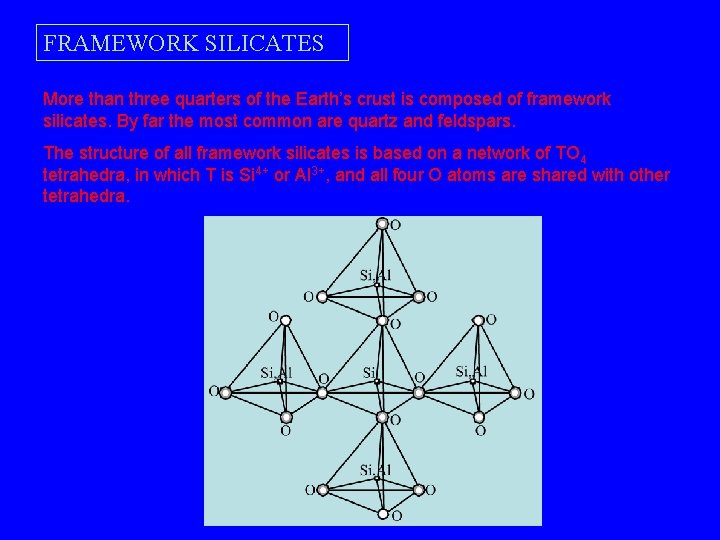
FRAMEWORK SILICATES More than three quarters of the Earth’s crust is composed of framework silicates. By far the most common are quartz and feldspars. The structure of all framework silicates is based on a network of TO 4 tetrahedra, in which T is Si 4+ or Al 3+, and all four O atoms are shared with other tetrahedra.

Si. O 2 3 -D frameworks of tetrahedra: fully polymerized Tectosilicates quartz and the silica minerals feldspars feldspathoids zeolites other tectosilicates

The fact that all O 2 - ions are shared, together with the repulsion of the highly charged cations, means that the structure of the framework silicates is more open than the other silicates. This has two consequences: (i) large cations can fit in the open structure of the framework silicates e. g. Ca 2+, Na+, K+. (ii) lower density than the other silicates e. g. quartz has density 2. 65, olivine has density 3. 3, even though Mg has a lower atomic mass than Si. Low density means stable at relatively low pressures i. e. minerals of the earth crust

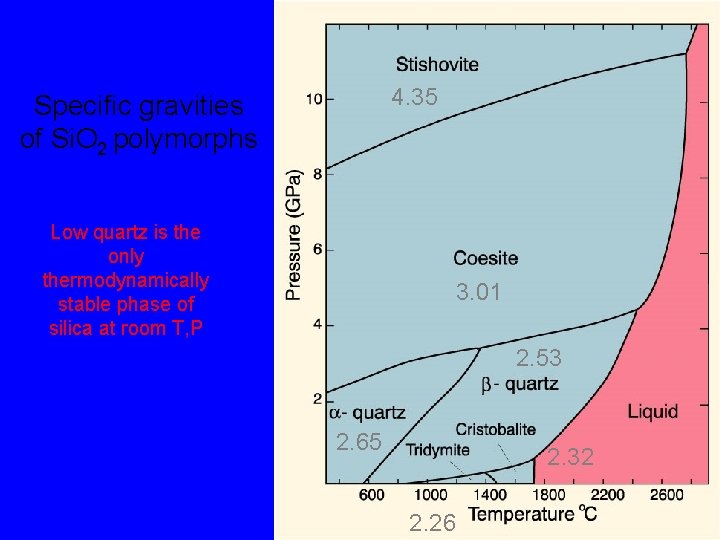
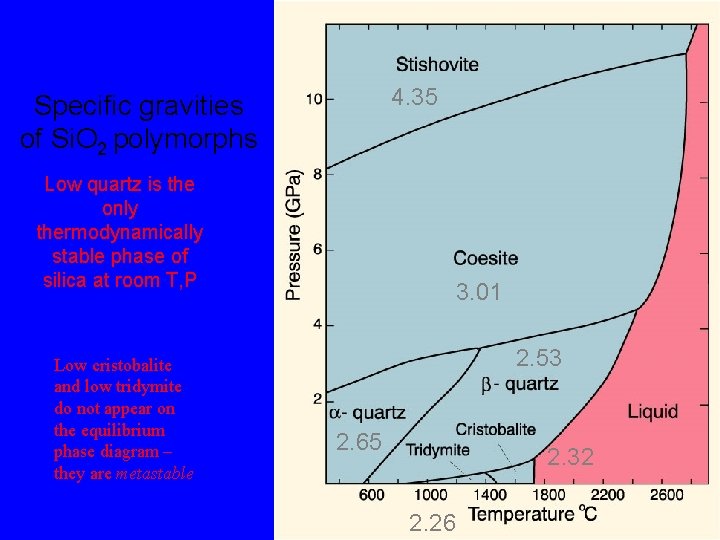
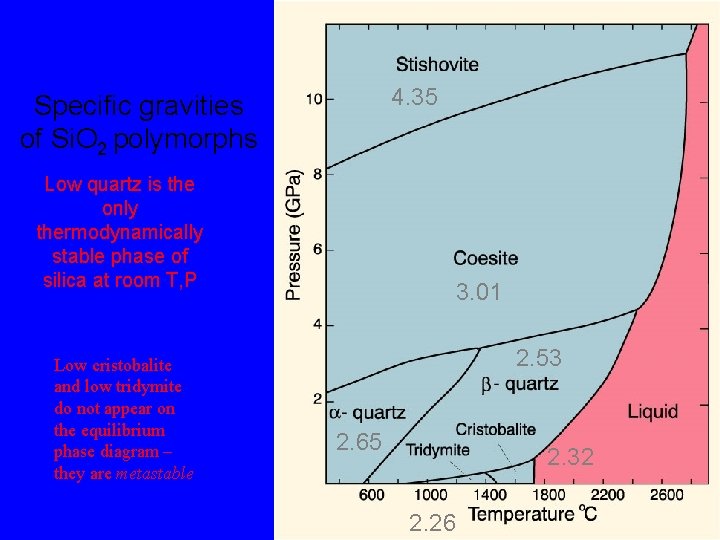
4. 35 Specific gravities of Si. O 2 polymorphs Low quartz is the only thermodynamically stable phase of silica at room T, P 3. 01 2. 53 2. 65 2. 32 2. 26

The silica group minerals, Si. O 2 By far the most common silica mineral is quartz. It is the only thermodynamically stable phase of silica at room T, P

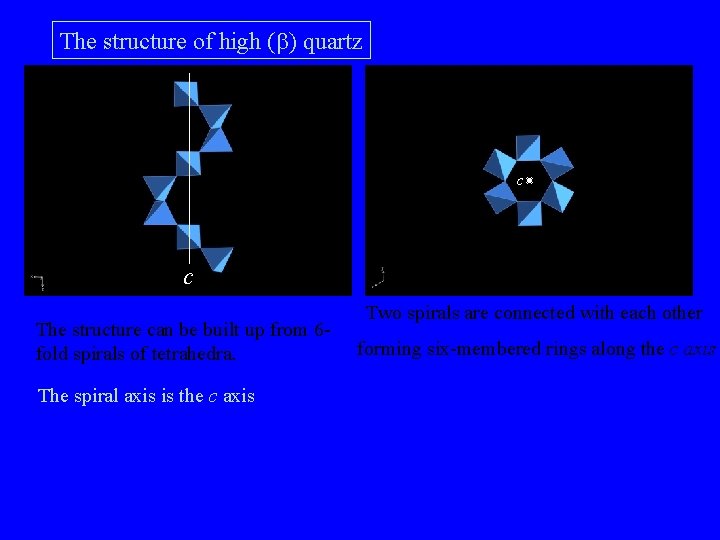
The structure of high (b) quartz c c The structure can be built up from 6 fold spirals of tetrahedra. The spiral axis is the c axis Two spirals are connected with each other forming six-membered rings along the c axis

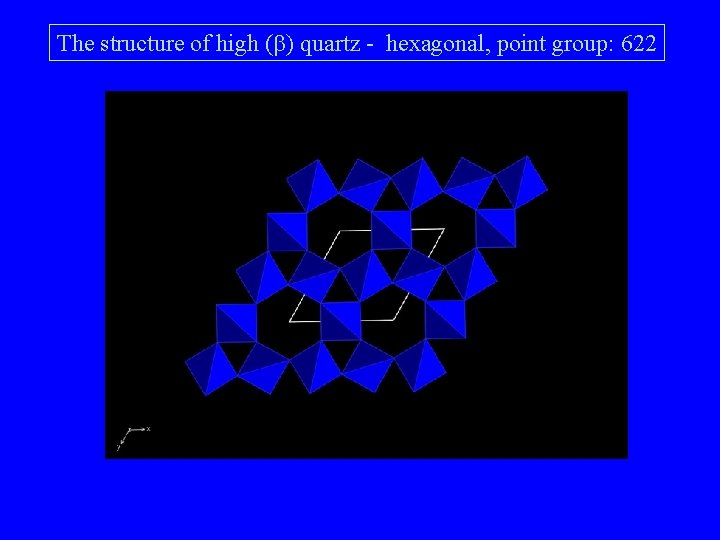
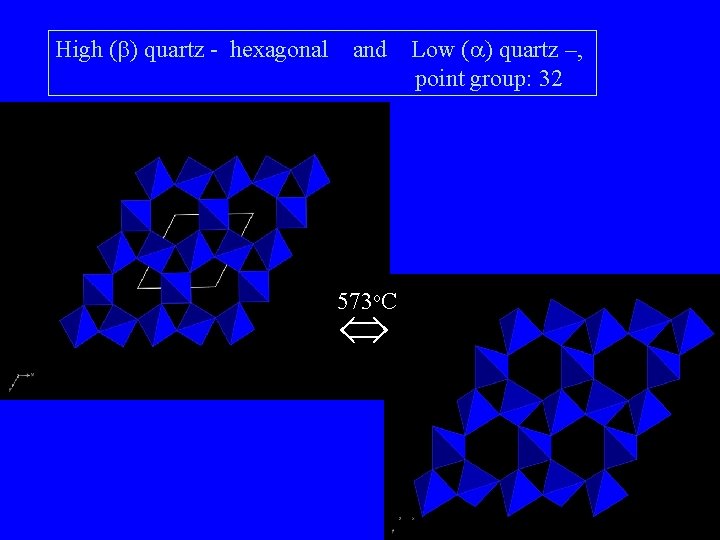
The structure of high (b) quartz - hexagonal, point group: 622

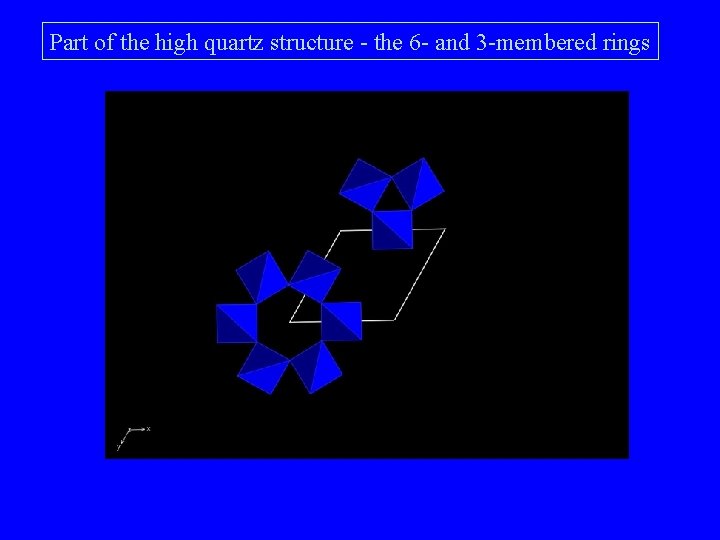
Part of the high quartz structure - the 6 - and 3 -membered rings

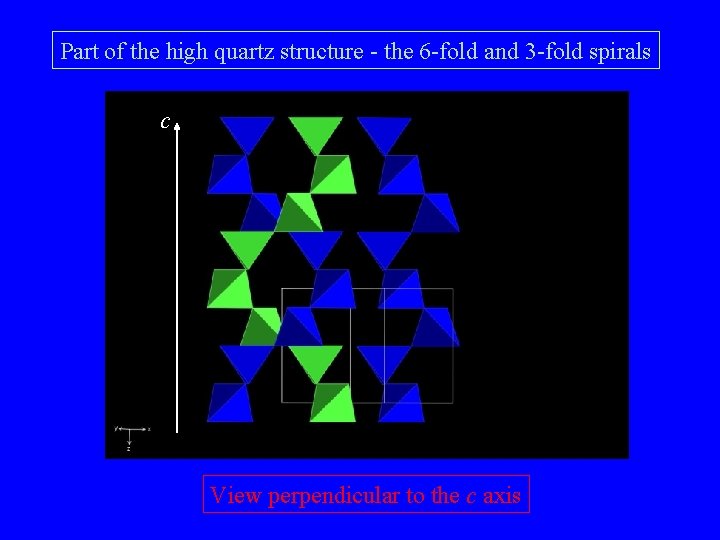
Part of the high quartz structure - the 6 -fold and 3 -fold spirals c View perpendicular to the c axis

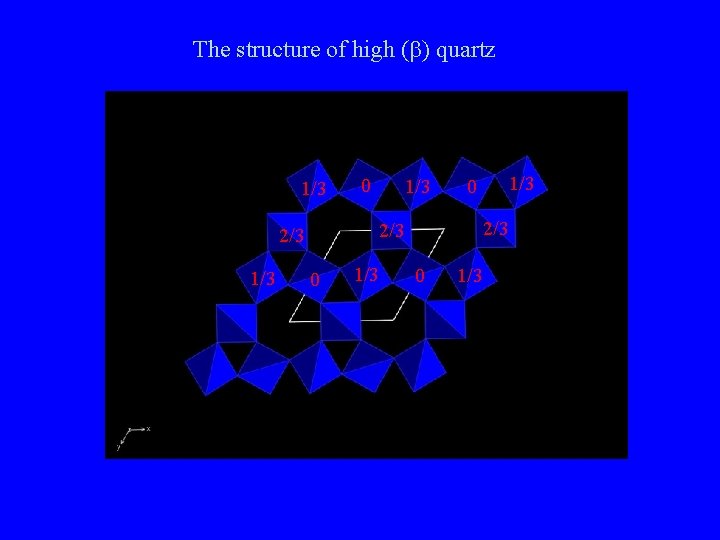
The structure of high (b) quartz 1/3 1/3 0 2/3 2/3 1/3 0 0 1/3

High (b) quartz - hexagonal and 573 o. C Low ( ) quartz –, point group: 32

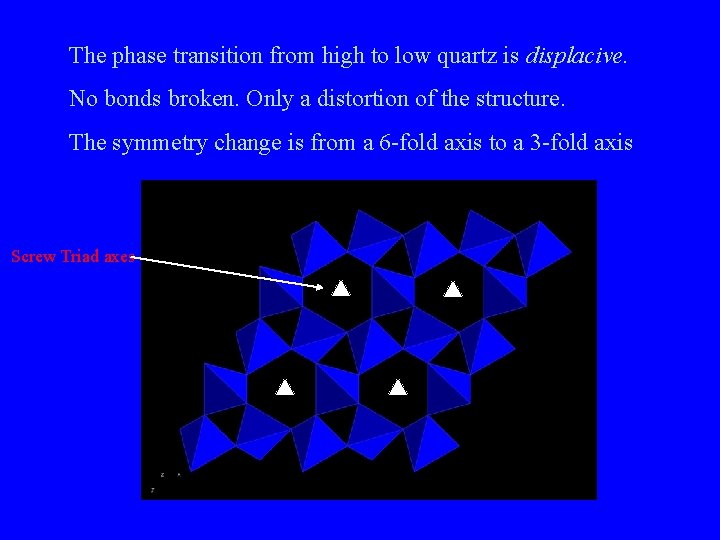
The phase transition from high to low quartz is displacive. No bonds broken. Only a distortion of the structure. The symmetry change is from a 6 -fold axis to a 3 -fold axis Screw Triad axes

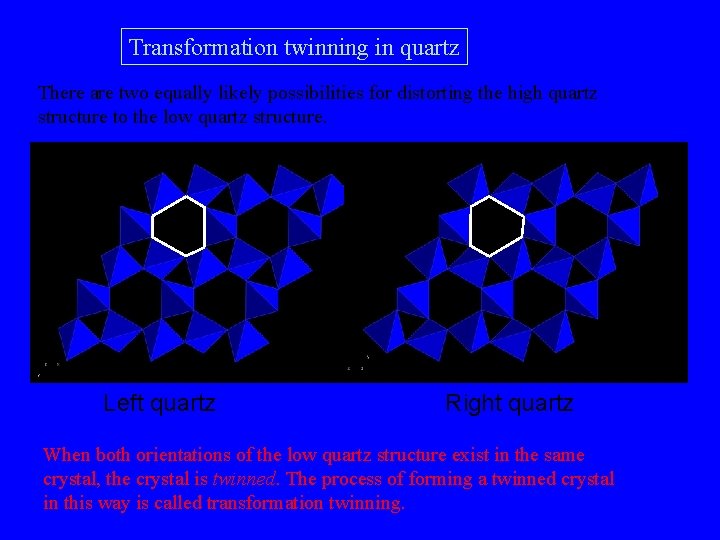
Transformation twinning in quartz There are two equally likely possibilities for distorting the high quartz structure to the low quartz structure. Left quartz Right quartz When both orientations of the low quartz structure exist in the same crystal, the crystal is twinned. The process of forming a twinned crystal in this way is called transformation twinning.

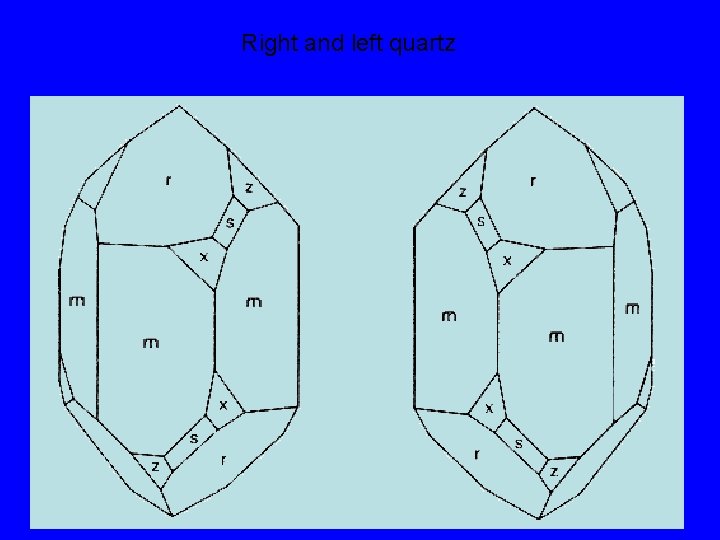
Right and left quartz

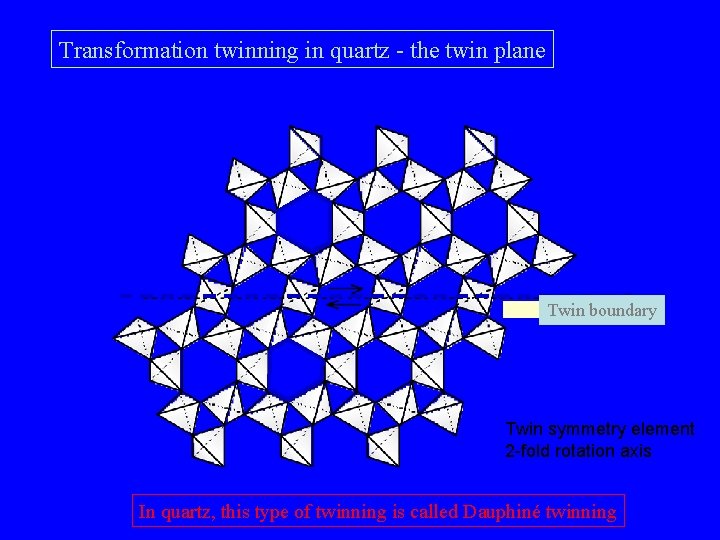
Transformation twinning in quartz - the twin plane Twin boundary Twin symmetry element 2 -fold rotation axis In quartz, this type of twinning is called Dauphiné twinning

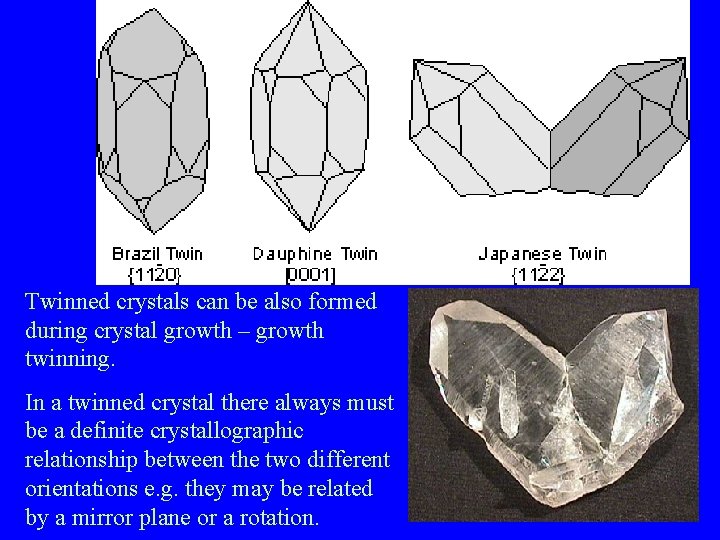
Twinned crystals can be also formed during crystal growth – growth twinning. In a twinned crystal there always must be a definite crystallographic relationship between the two different orientations e. g. they may be related by a mirror plane or a rotation.

Citrine Amethyst

Rose quartz Smokey quartz Milky quartz

Aventurine quartz Rutilated quartz

Other natural low temperature forms of Si. O 2 1. Agate is made from very fine fibrous crystals of quartz. Agate grows from Si-rich solutions in the shallow Earth’s crust.

Chalcedony is the fibrous form of quartz Petrified wood Onyx

Jasper

4. 35 Specific gravities of Si. O 2 polymorphs Low quartz is the only thermodynamically stable phase of silica at room T, P Low cristobalite and low tridymite do not appear on the equilibrium phase diagram – they are metastable 3. 01 2. 53 2. 65 2. 32 2. 26

High tridymite

Cristobalite always shows very fine cracks because the high low transformation involves a volume decrease of ~3% Glass Cristobalite with fine cracks This is an example of cristobalite in a silica ceramic brick - optical micrograph

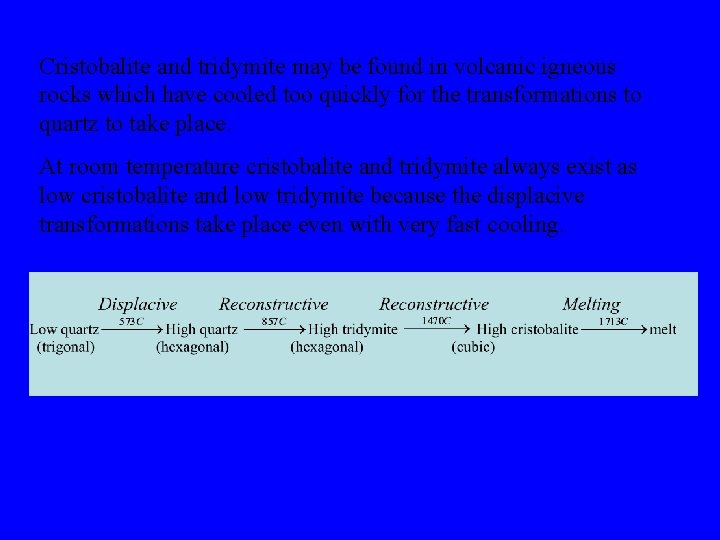
Cristobalite and tridymite may be found in volcanic igneous rocks which have cooled too quickly for the transformations to quartz to take place. At room temperature cristobalite and tridymite always exist as low cristobalite and low tridymite because the displacive transformations take place even with very fast cooling.

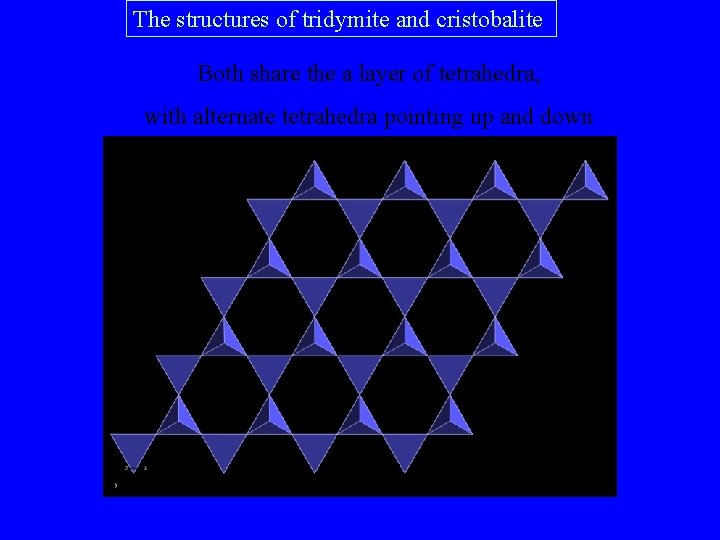
The structures of tridymite and cristobalite Both share the a layer of tetrahedra, with alternate tetrahedra pointing up and down

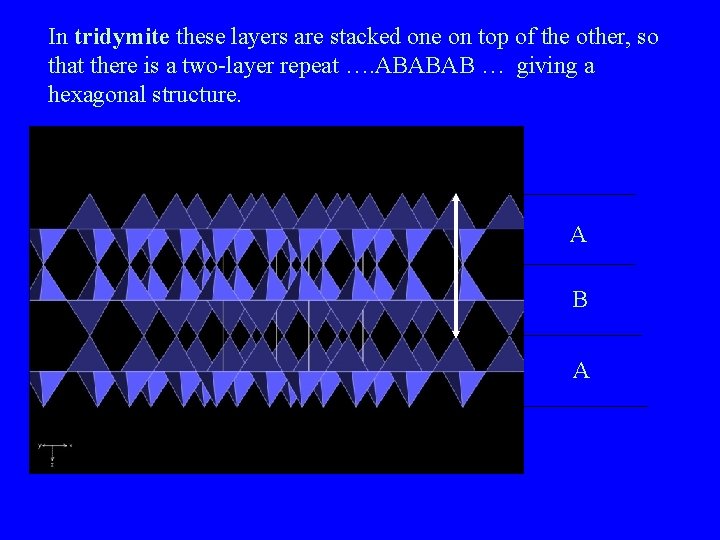
In tridymite these layers are stacked one on top of the other, so that there is a two-layer repeat …. ABABAB … giving a hexagonal structure. A B A

The transformations from cristobalite - tridymite - quartz on cooling • These transformations are reconstructive and involve breaking strong Si-O bonds • Unless cooling is very slow, these transformations will not take place • When cristobalite cools down to about 200 o. C it undergoes a displacive transformation from cubic high cristobalite to tetragonal low cristobalite (i. e. a distortion of the structure which lowers the symmetry • The same is also the case for tridymite. If it fails to transform to quartz, then at around 200 o. C there is a high - low tridymite transition ( a distortion from hexagonal to orthorhombic symmetry)

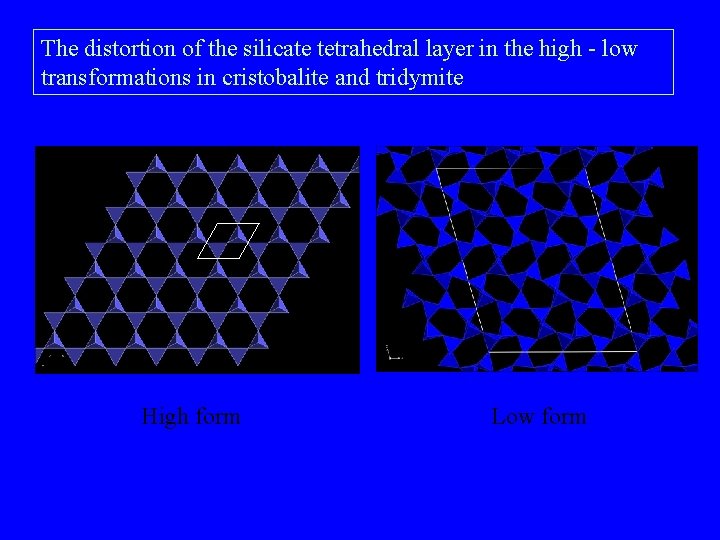
The distortion of the silicate tetrahedral layer in the high - low transformations in cristobalite and tridymite High form Low form

4. 35 Specific gravities of Si. O 2 polymorphs Low quartz is the only thermodynamically stable phase of silica at room T, P Low cristobalite and low tridymite do not appear on the equilibrium phase diagram – they are metastable 3. 01 2. 53 2. 65 2. 32 2. 26

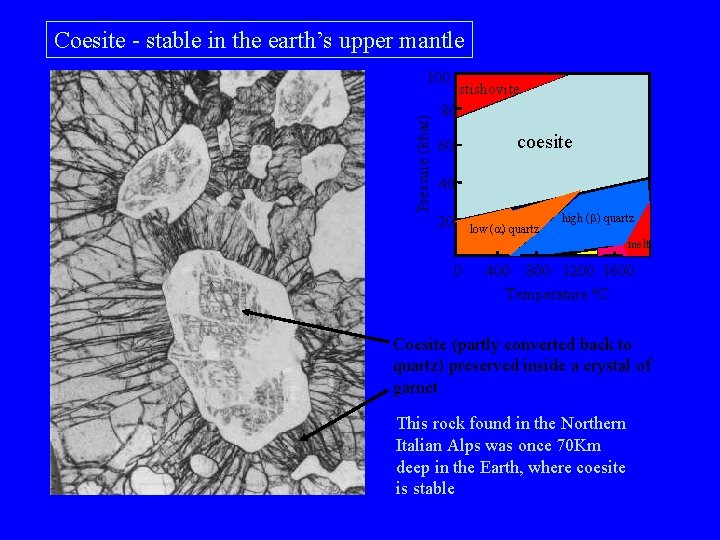
Coesite - stable in the earth’s upper mantle Pressure (kbar) 100 stishovite 80 60 coesite 40 20 low ( ) quartz high (b) quartz melt 0 400 800 1200 1600 Temperature o. C Coesite (partly converted back to quartz) preserved inside a crystal of garnet This rock found in the Northern Italian Alps was once 70 Km deep in the Earth, where coesite is stable

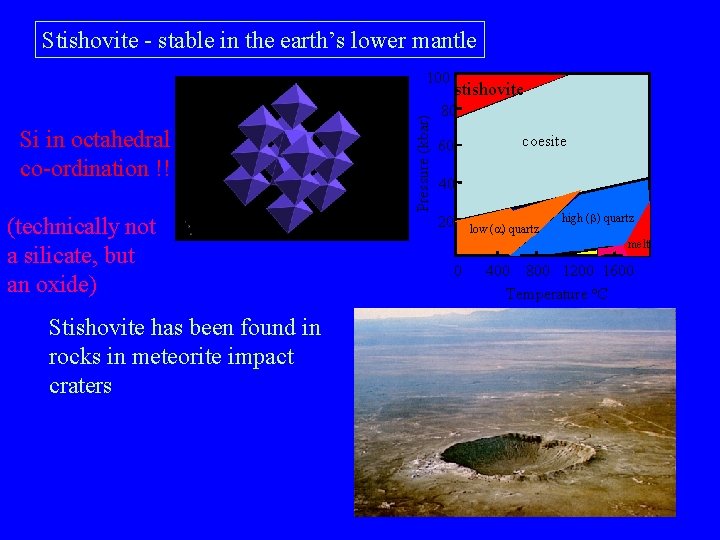
Stishovite - stable in the earth’s lower mantle Si in octahedral co-ordination !! (technically not a silicate, but an oxide) Stishovite has been found in rocks in meteorite impact craters Pressure (kbar) 100 stishovite 80 60 coesite 40 20 low ( ) quartz high (b) quartz melt 0 400 800 1200 1600 Temperature o. C

Other natural low temperature forms of Si. O 2 2. Opal is an amorphous form of silica formed from supersaturated Si-rich solutions.

Where do the colours in opal come from? Electron micrographs showing small spheres of amorphous Si. O 2, which scatter the light to produce the colours.

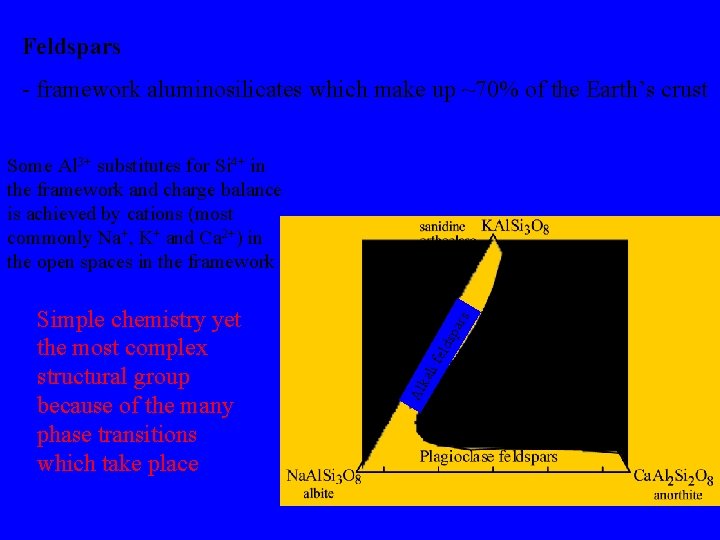
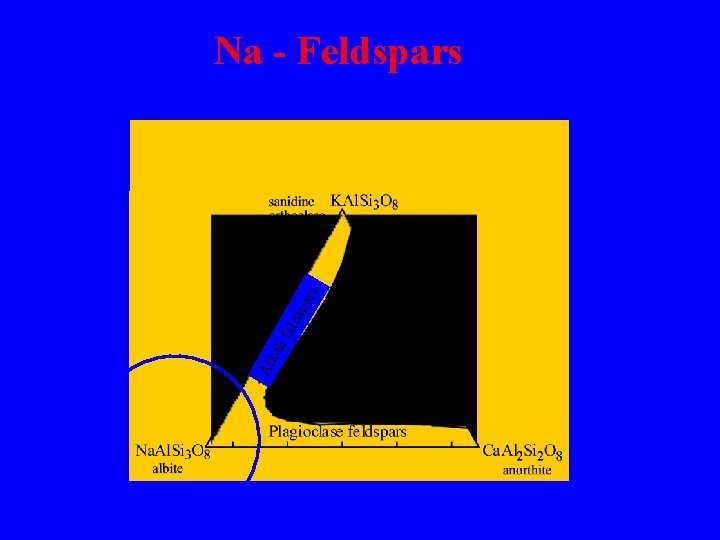
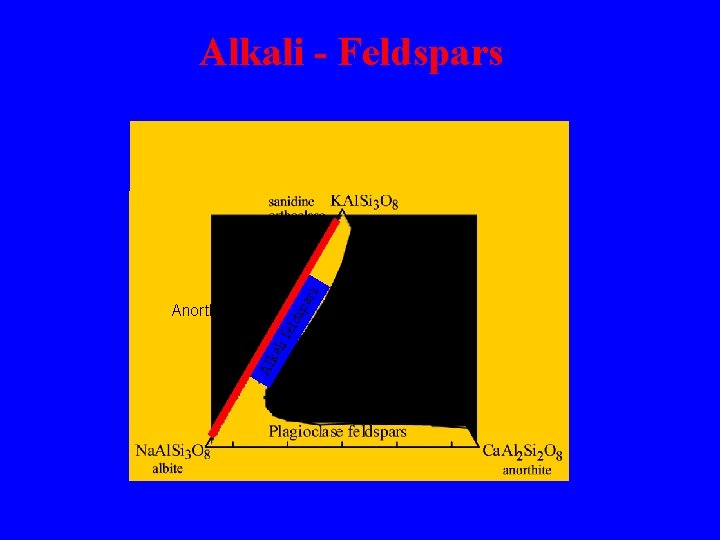
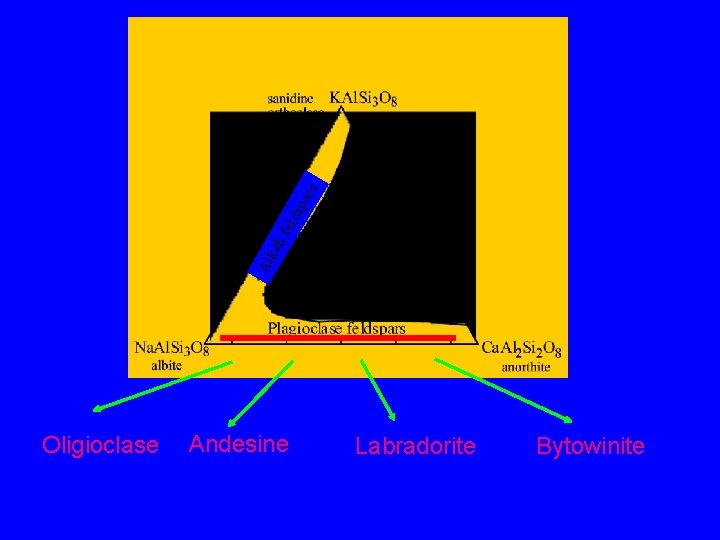
Feldspars - framework aluminosilicates which make up ~70% of the Earth’s crust par i fe lds kal Al Simple chemistry yet the most complex structural group because of the many phase transitions which take place s Some Al 3+ substitutes for Si 4+ in the framework and charge balance is achieved by cations (most commonly Na+, K+ and Ca 2+) in the open spaces in the framework Fields of composition of the common feldspar minerals

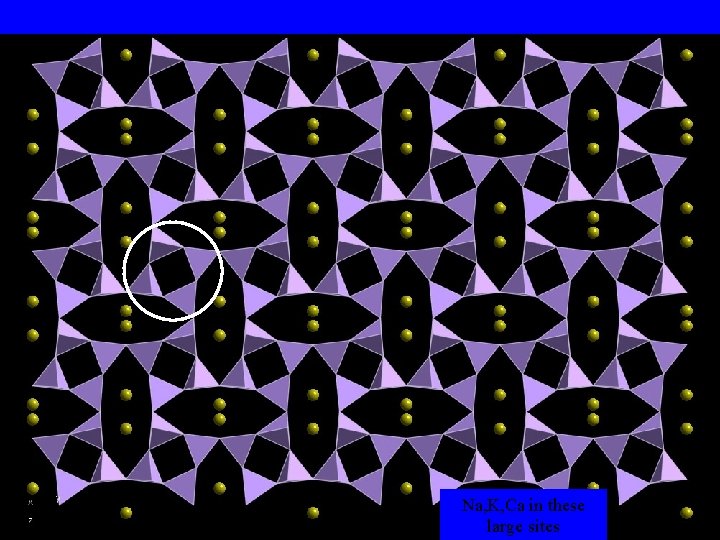
Na, K, Ca in these large sites

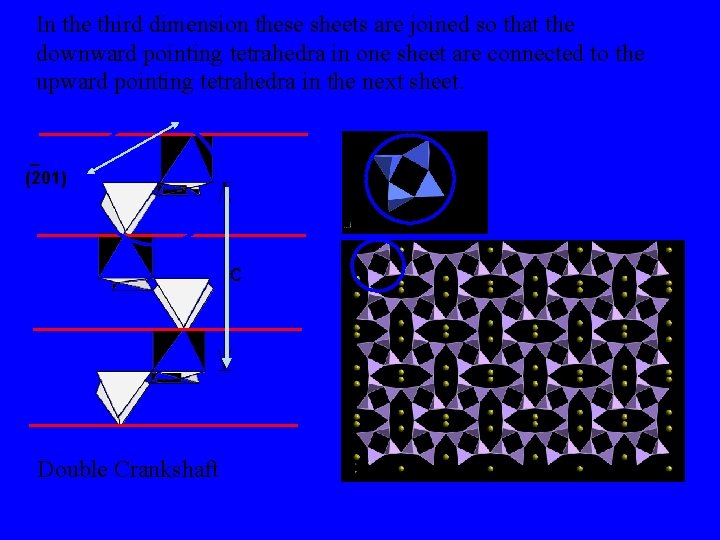
In the third dimension these sheets are joined so that the downward pointing tetrahedra in one sheet are connected to the upward pointing tetrahedra in the next sheet. _ (201) c Double Crankshaft

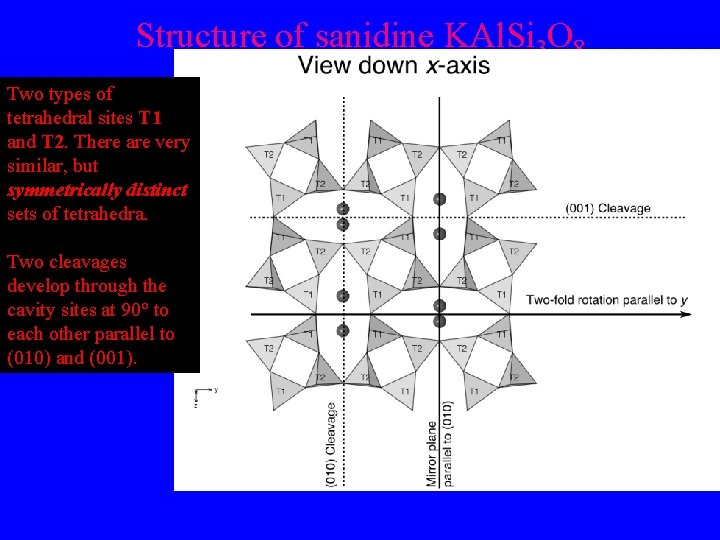
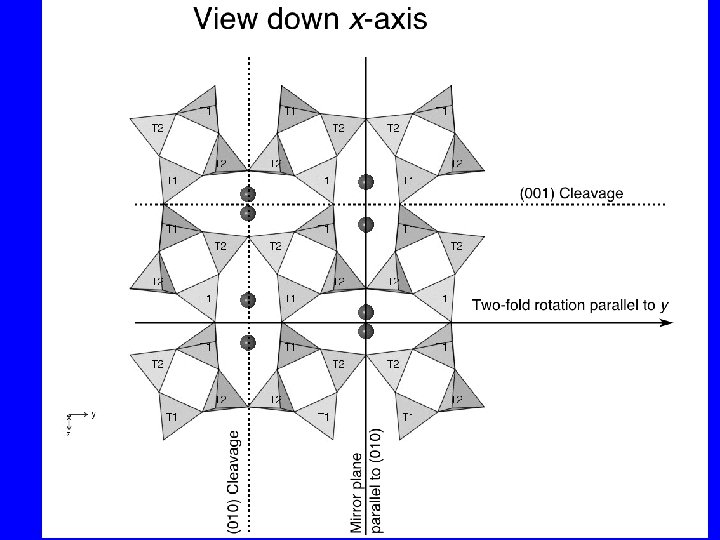
Structure of sanidine KAl. Si 3 O 8 Two types of tetrahedral sites T 1 and T 2. There are very similar, but symmetrically distinct sets of tetrahedra. Two cleavages develop through the cavity sites at 90° to each other parallel to (010) and (001).

Phase transitions in the feldspars There are three types of behaviour which take place in the feldspar structure on cooling: At high temperatures: (i) at high temperatures the feldspar structure is expanded and can contain Na, K and Ca in the large M-sites. (ii) at high temperatures the Al and Si are randomly distributed in the Tsites (iii) at high temperatures there are extensive solid solutions in the alkali feldspars and in the plagioclase feldspars. In this ideal high-T state, feldspars are monoclinic.

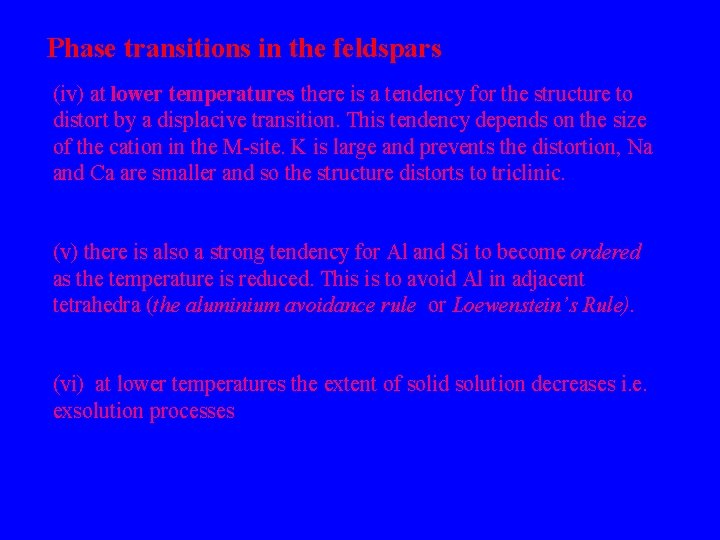
Phase transitions in the feldspars (iv) at lower temperatures there is a tendency for the structure to distort by a displacive transition. This tendency depends on the size of the cation in the M-site. K is large and prevents the distortion, Na and Ca are smaller and so the structure distorts to triclinic. (v) there is also a strong tendency for Al and Si to become ordered as the temperature is reduced. This is to avoid Al in adjacent tetrahedra (the aluminium avoidance rule or Loewenstein’s Rule). (vi) at lower temperatures the extent of solid solution decreases i. e. exsolution processes


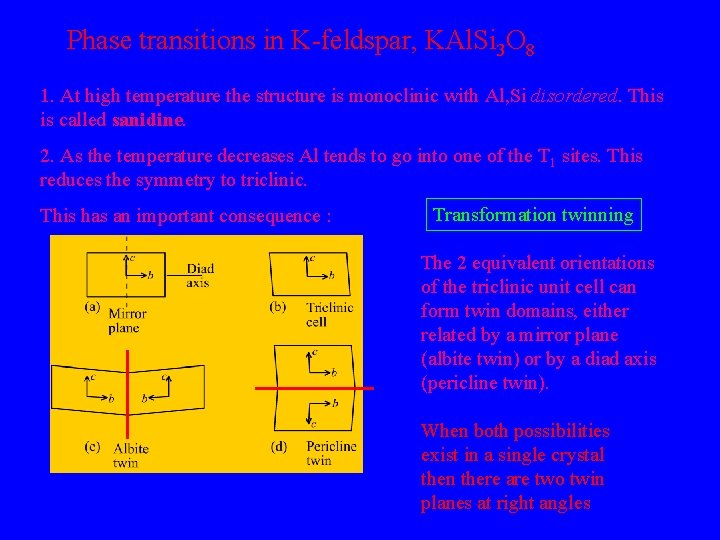
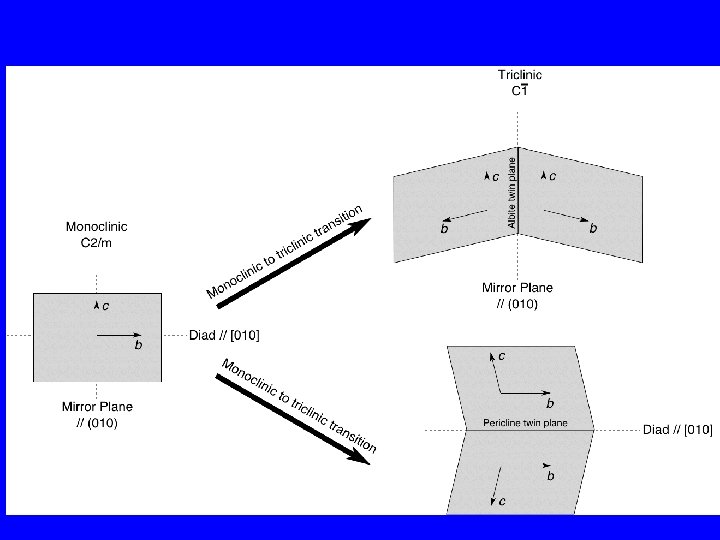
Phase transitions in K-feldspar, KAl. Si 3 O 8 1. At high temperature the structure is monoclinic with Al, Si disordered. This is called sanidine. 2. As the temperature decreases Al tends to go into one of the T 1 sites. This reduces the symmetry to triclinic. This has an important consequence : Transformation twinning The 2 equivalent orientations of the triclinic unit cell can form twin domains, either related by a mirror plane (albite twin) or by a diad axis (pericline twin). When both possibilities exist in a single crystal then there are two twin planes at right angles

Transformation twinning in alkali feldspars

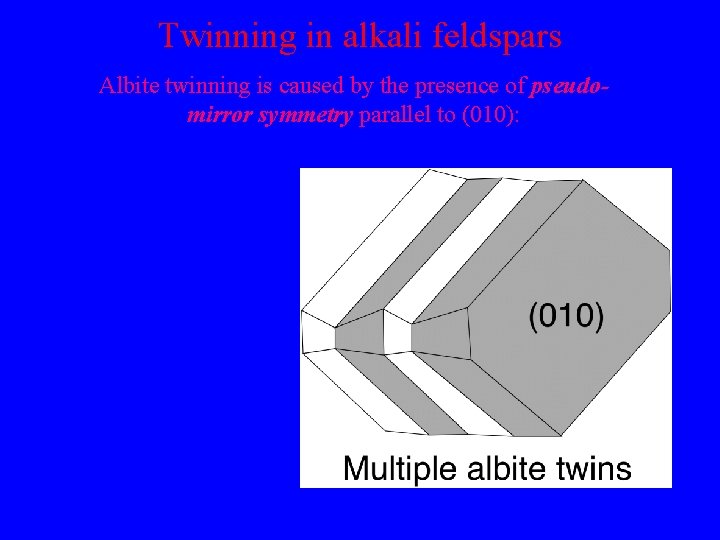
Twinning in alkali feldspars Albite twinning is caused by the presence of pseudomirror symmetry parallel to (010): Twin plane is // (010) Components are related by reflection in (010) Polysynthetic twinning

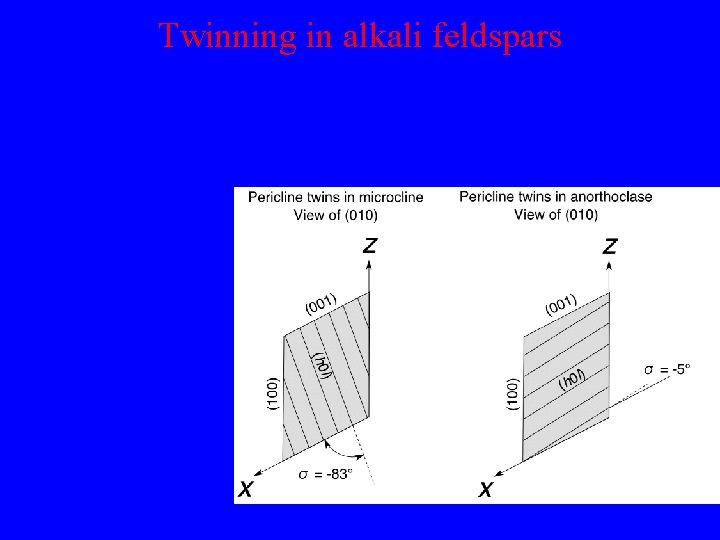
Twinning in alkali feldspars Pericline twinning is caused by the presence of pseudodiad symmetry parallel to y axis: Twin plane is parallel to (h 0 l). Exact orientation depends on precise values of lattice parameters and varies greatly in different types of feldspar.

Phase transitions in K-feldspar, KAl. Si 3 O 8 Fully Al, Si ordered K-feldspar is called microcline. Microcline has characteristic cross-hatched twinning, seen in a polarizing microscope : This characteristic microstructure is due to the existence of both albite and pericline twinning in the crystal which has transformed from the high temperature disordered monoclinic structure.

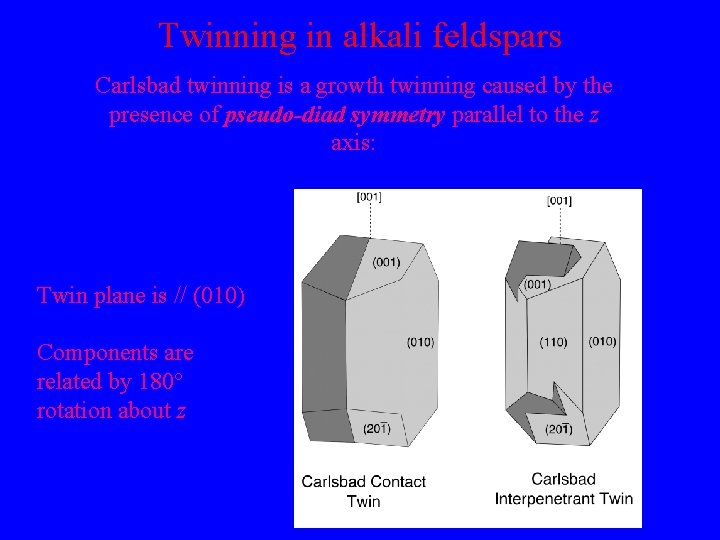
Twinning in alkali feldspars Carlsbad twinning is a growth twinning caused by the presence of pseudo-diad symmetry parallel to the z axis: Twin plane is // (010) Components are related by 180° rotation about z

Sanidine

Orthoclase Microcline

Adularia = colourless K-feldspar Amazonite = green microcline

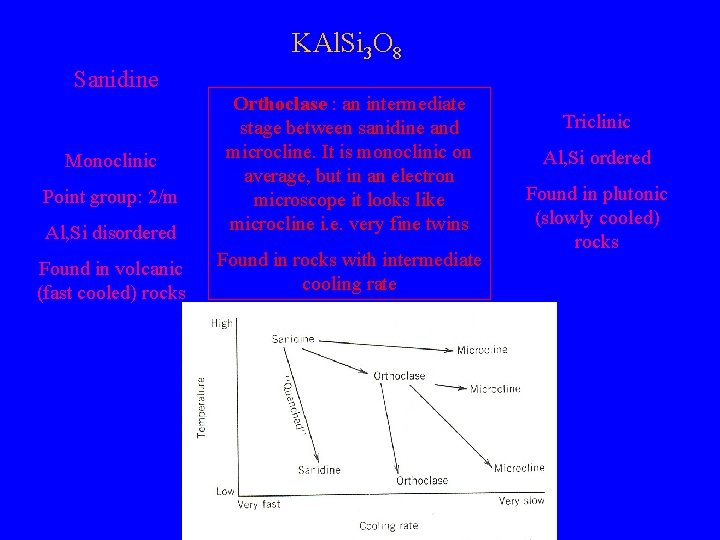
KAl. Si 3 O 8 Microcline Sanidine Al, Si disordered Orthoclase : an intermediate stage between sanidine and microcline. It is monoclinic on average, but in an electron microscope it looks like microcline i. e. very fine twins Found in volcanic (fast cooled) rocks Found in rocks with intermediate cooling rate Monoclinic Point group: 2/m Triclinic Al, Si ordered Found in plutonic (slowly cooled) rocks

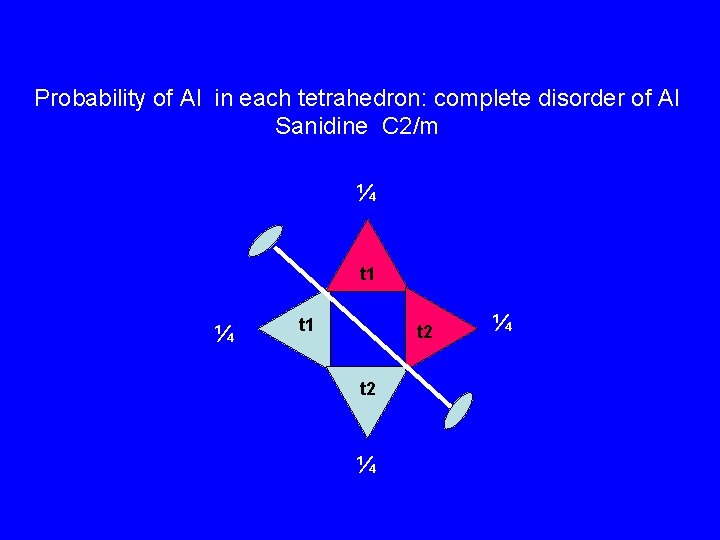
Probability of Al in each tetrahedron: complete disorder of Al Sanidine C 2/m ¼ t 1 t 2 ¼ ¼

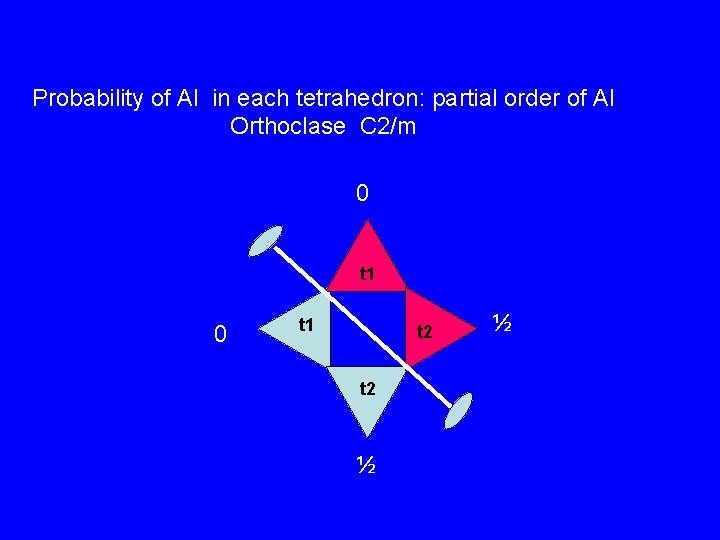
Probability of Al in each tetrahedron: partial order of Al Orthoclase C 2/m 0 t 1 t 2 ½ ½

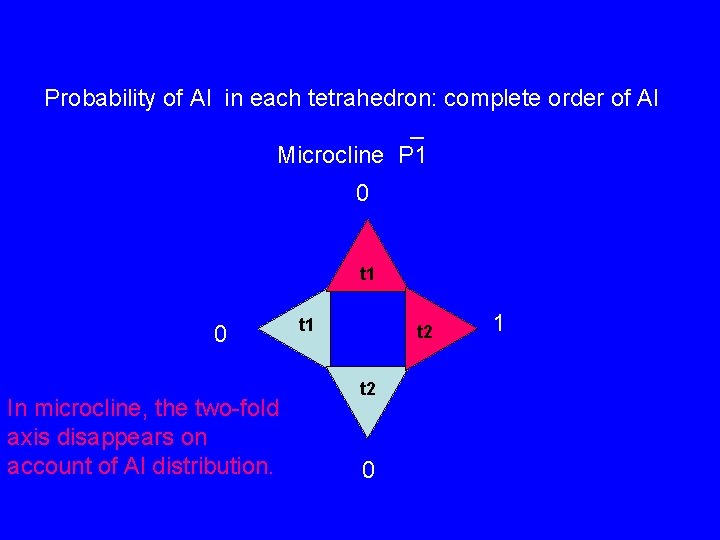
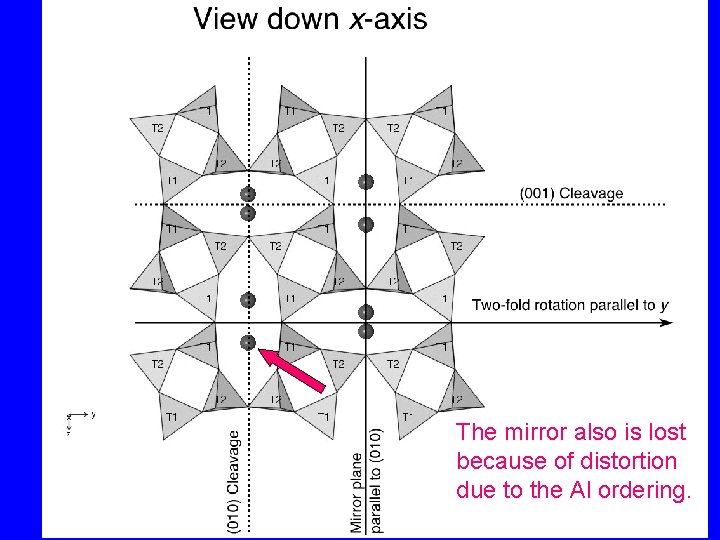
Probability of Al in each tetrahedron: complete order of Al _ Microcline P 1 0 t 1 0 In microcline, the two-fold axis disappears on account of Al distribution. t 1 t 2 0 1

The mirror also is lost because of distortion due to the Al ordering.

Al Alka kal l i feld lds spa par rs s Na - Feldspars

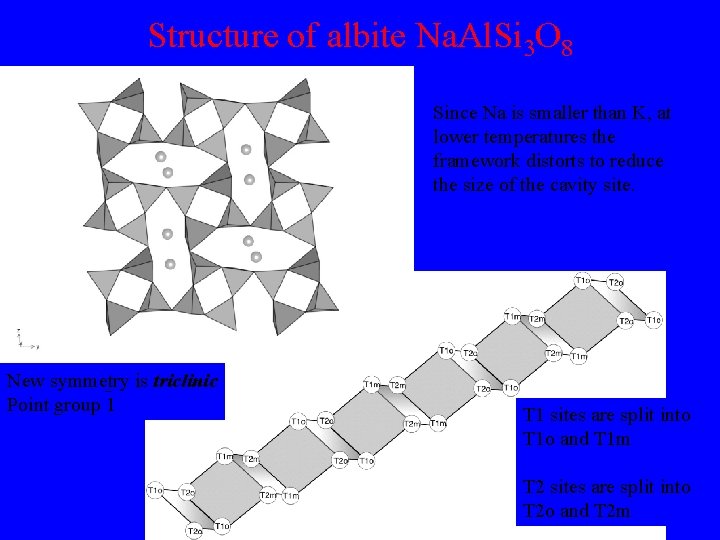
Phase transitions in Na-feldspar, Na. Al. Si 3 O 8 1. At very high temperature the structure is monoclinic with Al, Si disordered. This is called monalbite. But on cooling below about 1000 o. C monalbite undergoes a displacive transition to triclinic symmetry because the Na is too small to stop the structure from distorting. This triclinic albite is called high albite. In most rocks albite grows as high albite because the temperature is below that where albite is monoclinic. 2. As the temperature decreases Al, Si begin to order. There is no twinning associated with this because high albite is already triclinic and cannot reduce its symmetry further. Albite with ordered Al, Si is called low albite. It has no transformation twinning.

Structure of albite Na. Al. Si 3 O 8 Since Na is smaller than K, at lower temperatures the framework distorts to reduce the size of the cavity site. New symmetry is triclinic Point group 1 T 1 sites are split into T 1 o and T 1 m T 2 sites are split into T 2 o and T 2 m

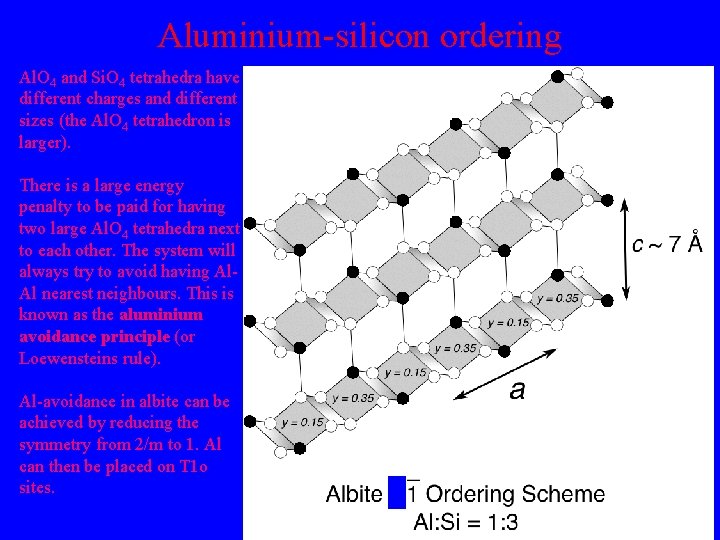
Aluminium-silicon ordering Al. O 4 and Si. O 4 tetrahedra have different charges and different sizes (the Al. O 4 tetrahedron is larger). There is a large energy penalty to be paid for having two large Al. O 4 tetrahedra next to each other. The system will always try to avoid having Al. Al nearest neighbours. This is known as the aluminium avoidance principle (or Loewensteins rule). Al-avoidance in albite can be achieved by reducing the symmetry from 2/m to 1. Al can then be placed on T 1 o sites.

i fe lds Al kal Anorthoclase par s Alkali - Feldspars

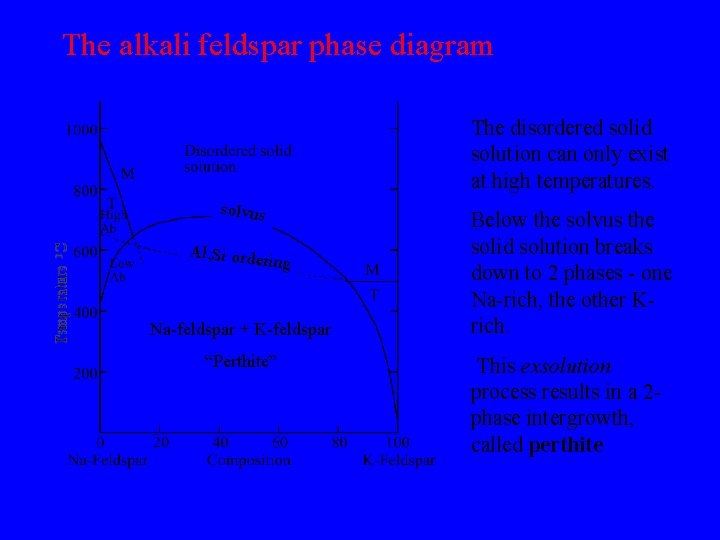
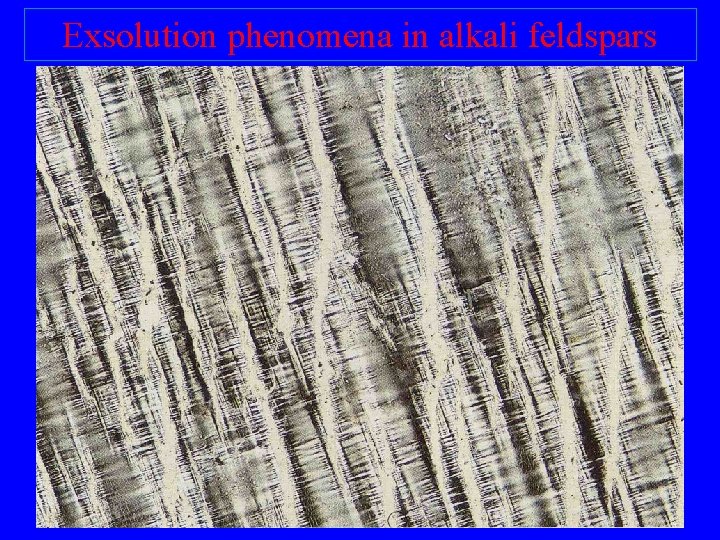
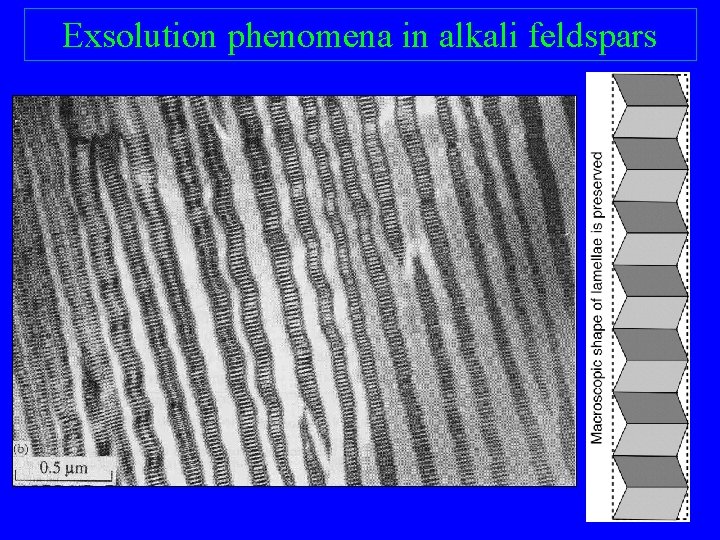
The alkali feldspar phase diagram The disordered solid solution can only exist at high temperatures. solvus Al, Si orde ring Na-feldspar + K-feldspar “Perthite” Below the solvus the solid solution breaks down to 2 phases - one Na-rich, the other Krich. This exsolution process results in a 2 phase intergrowth, called perthite

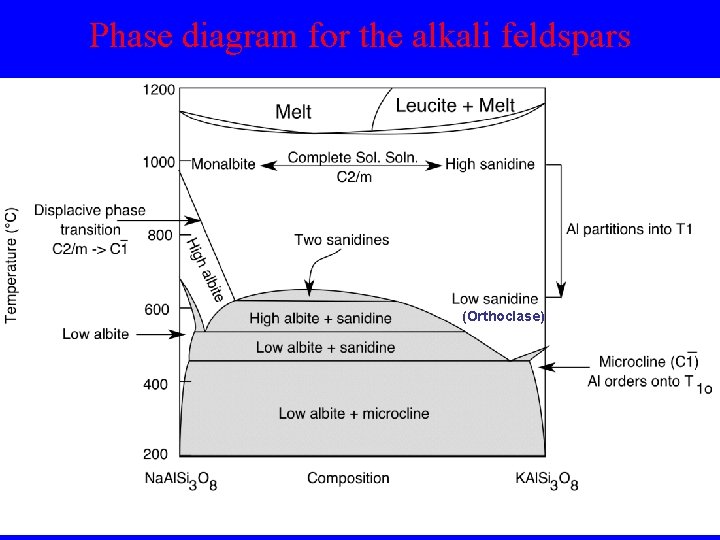
Phase diagram for the alkali feldspars (Orthoclase)

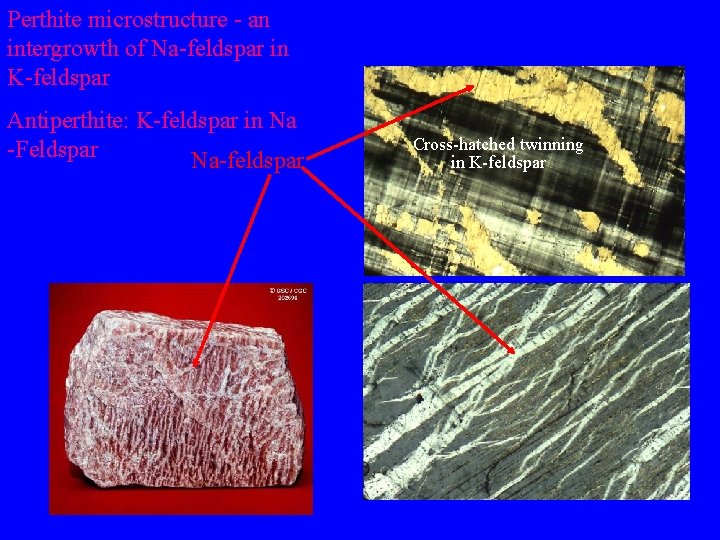
Perthite microstructure - an intergrowth of Na-feldspar in K-feldspar whi te Antiperthite: K-feldspar in Na -Feldspar Na-feldspar Cross-hatched twinning in K-feldspar

Exsolution phenomena in alkali feldspars

Exsolution phenomena in alkali feldspars

rs fel dsp a kal i Al Oligioclase Andesine Labradorite Bytowinite