Fractional Distillation of Hydrocarbons Carbon Chain Length and

Fractional Distillation of Hydrocarbons

Carbon Chain Length and Boiling Point As the length of the carbon chain in an alkane increases, the attraction between molecules becomes stronger because there are more attractive forces holding the molecule together. As a result, the boiling point of hydrocarbons increases as the size of the hydrocarbon increases.
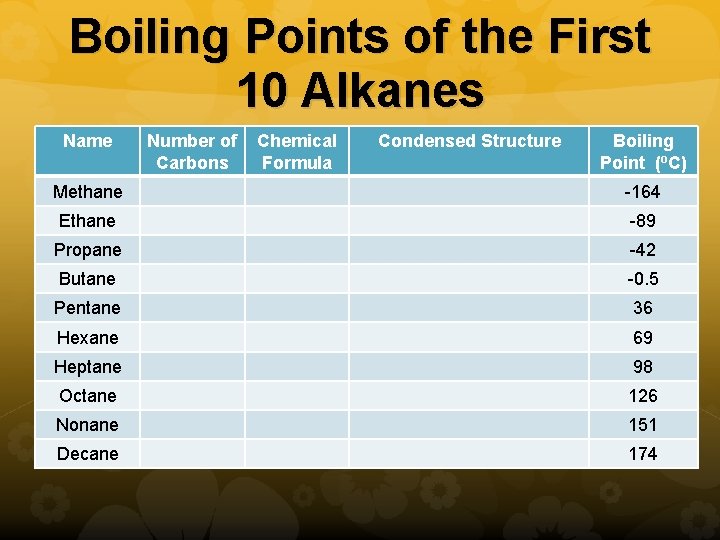
Boiling Points of the First 10 Alkanes Name Number of Carbons Chemical Formula Condensed Structure Boiling Point (o. C) Methane -164 Ethane -89 Propane -42 Butane -0. 5 Pentane 36 Hexane 69 Heptane 98 Octane 126 Nonane 151 Decane 174
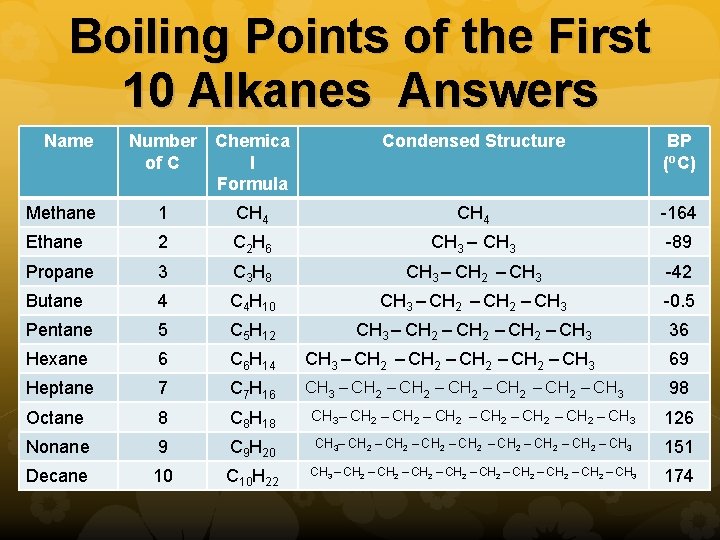
Boiling Points of the First 10 Alkanes Answers Name Number of C Chemica l Formula Condensed Structure BP (o. C) Methane 1 CH 4 -164 Ethane 2 C 2 H 6 CH 3 – CH 3 -89 Propane 3 C 3 H 8 CH 3 – CH 2 – CH 3 -42 Butane 4 C 4 H 10 CH 3 – CH 2 – CH 3 -0. 5 Pentane 5 C 5 H 12 CH 3 – CH 2 – CH 3 36 Hexane 6 C 6 H 14 CH 3 – CH 2 – CH 3 69 Heptane 7 C 7 H 16 CH 3 – CH 2 – CH 2 – CH 3 98 Octane 8 C 8 H 18 CH 3– CH 2 – CH 2 – CH 3 126 Nonane 9 C 9 H 20 CH 3– CH 2 – CH 2 – CH 3 151 Decane 10 C 10 H 22 CH 3 – CH 2 – CH 2 – CH 3 174

Boiling Point vs. Number of Carbon Atoms 200 150 100 Boiling Point (C) 50 0 0 1 2 3 4 5 6 -50 -100 -150 -200 Number of Carbon Atoms 7 8 9 10 11

The Structure of Hydrocarbons Determines their Uses The structure of hydrocarbons influences their physical properties. Their physical properties determine what they are used for.

Alkanes that contain between one and four carbons are gases at room temperature. They are used as fuels for cooking and heating.

Alkanes that contain between 5 and 16 carbons are liquids at room temperature. They are used as fuels to run devices with engines such as cars, planes, chainsaws, and generators.

Alkanes that contain between 16 and 22 carbons are heavy liquids. They are used as lubricating oils or in oilburning furnaces.

Alkanes that contain more than 18 carbons are semi-solids. They are used to make candles, waxed paper, and cosmetics.

Alkanes that contain more than 26 carbons are solid residues. They are used as asphalt and tars for roofing and paving.

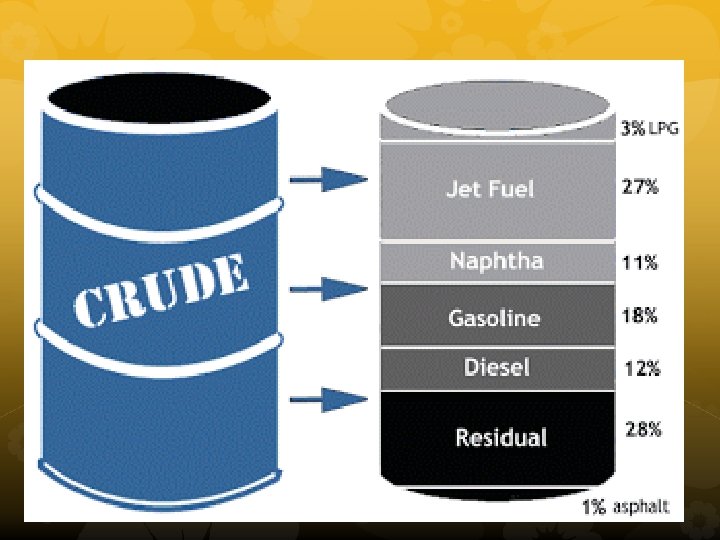
Petroleum Refineries An important source of hydrocarbons is petroleum (crude oil). Petroleum products include gasoline, jet fuel, diesel fuel, lubricating oils, and asphalt. Crude oil contains hundreds of different hydrocarbon compounds.

The hydrocarbons are separated depending on their boiling points in distillation towers by a process call fractional distillation. Each component of petroleum that is separated out is called a fraction, and each fraction has its own range of boiling points.

In distillation towers, petroleum is heated, vapourized, cooled, and condensed to separate the different fractions. The small chains have lower boiling points than longer chains. The temperature is higher near the bottom and cooler near the top of the tower.
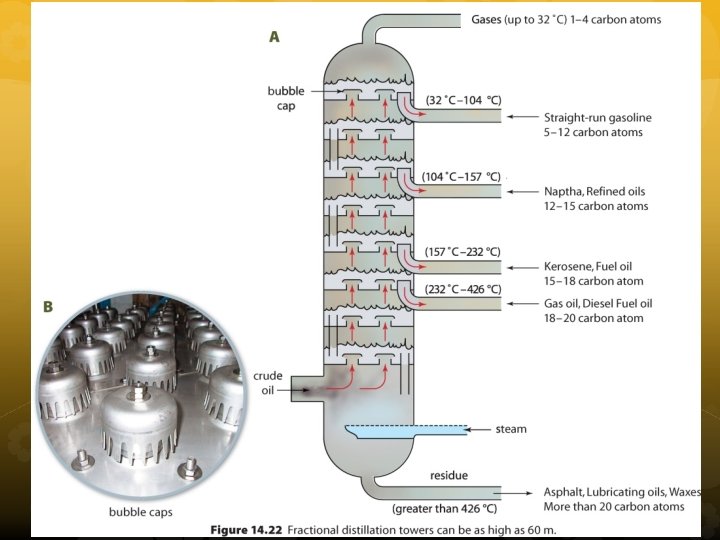


Once the fractions are collected they may be further processed to make them more marketable. The fractions may undergo a process called cracking or reforming to increase the yield of useful products from each barrel of crude oil.

Cracking involves breaking long hydrocarbon chains into smaller more useful hydrocarbon chains. There are 4 main types of cracking Thermal Cracking Catalytic Cracking Hydrocracking Steam Cracking

Each type of cracking involves large amounts of heat, pressure and some times the addition of a catalyst to make the desired products. Each type also produces different products.

Reforming This is the opposite of cracking and involves the breaking of straight chain alkenes to form branched alkanes to make more useful products. Smaller hydrocarbon chains are combined together with heat, pressure and a catalyst to produce a larger hydrocarbon. Naptha is typically used in the reforming process to make high octane gasoline such as aircraft fuel.

Practice Text Book Page 196 # 1 -6
- Slides: 22