Fouriertransform microwave and millimeterwave spectroscopy of CH 2

- Slides: 21

Fourier–transform microwave and millimeter–wave spectroscopy of CH 2 IBr (v = 0) Stéphane Bailleux stephane. bailleux@univ-lille 1. fr University of Lille June 17, 2014 – 69 th ISMS Meeting

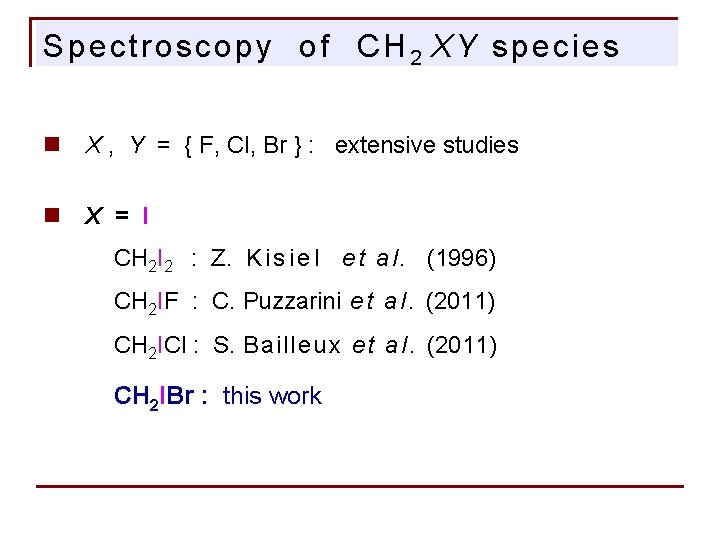
Spectroscopy of CH 2 XY species n X , Y = { F, Cl, Br } : extensive studies n X = I CH 2 I 2 : Z. K i s i e l e t a l. (1996) CH 2 IF : C. Puzzarini e t a l. (2011) CH 2 ICl : S. Bailleux e t a l. (2011) CH 2 IBr : this work

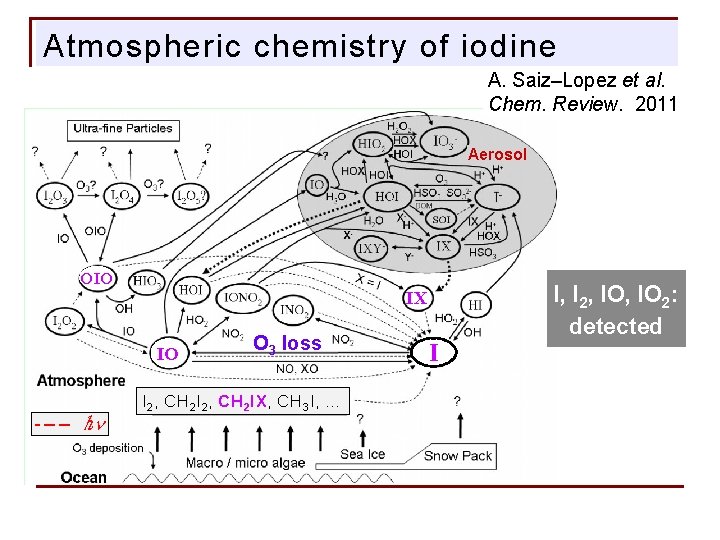
Atmospheric chemistry of iodine A. Saiz–Lopez et al. Chem. Review. 2011 Aerosol OIO IO - – – ℎn I, I 2, IO 2: detected IX O 3 loss I 2, CH 2 IX, CH 3 I, … I

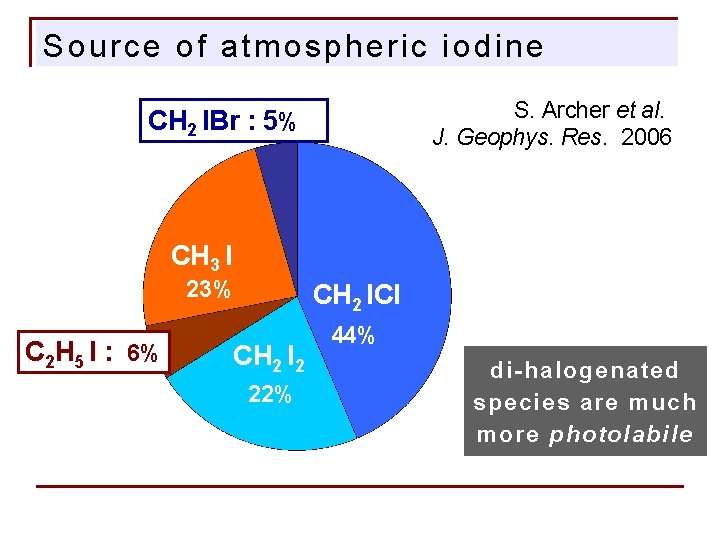
Source of atmospheric iodine S. Archer et al. J. Geophys. Res. 2006 CH 2 IBr : 5% CH 3 I 23% C 2 H 5 I : 6% CH 2 ICl CH 2 I 2 22% 44% di-halogenated species are much more photolabile

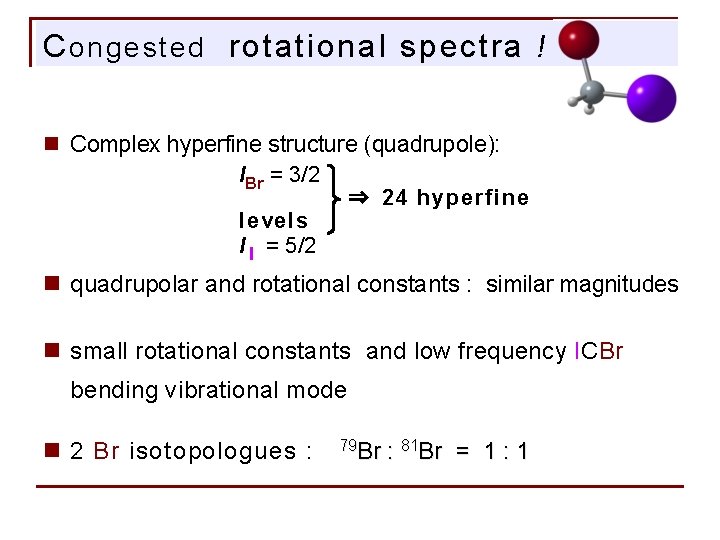
C ongested rotational spectra ! n Complex hyperfine structure (quadrupole): IBr = 3/2 ⇒ 24 hyperfine levels I I = 5/2 n quadrupolar and rotational constants : similar magnitudes n small rotational constants and low frequency ICBr bending vibrational mode n 2 Br isotopologues : 79 Br : 81 Br = 1 : 1

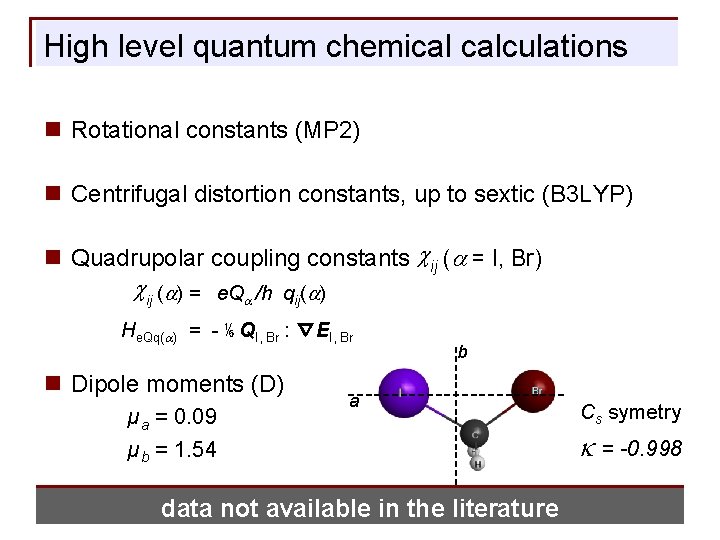
High level quantum chemical calculations n Rotational constants (MP 2) n Centrifugal distortion constants, up to sextic (B 3 LYP) n Quadrupolar coupling constants cij (a = I, Br) cij (a) = e. Qa /h qij(a) He. Qq(a) = - ⅙ QI, Br : ∇EI, Br n Dipole moments (D) µa = 0. 09 µb = 1. 54 b a data not available in the literature Cs symetry k = -0. 998

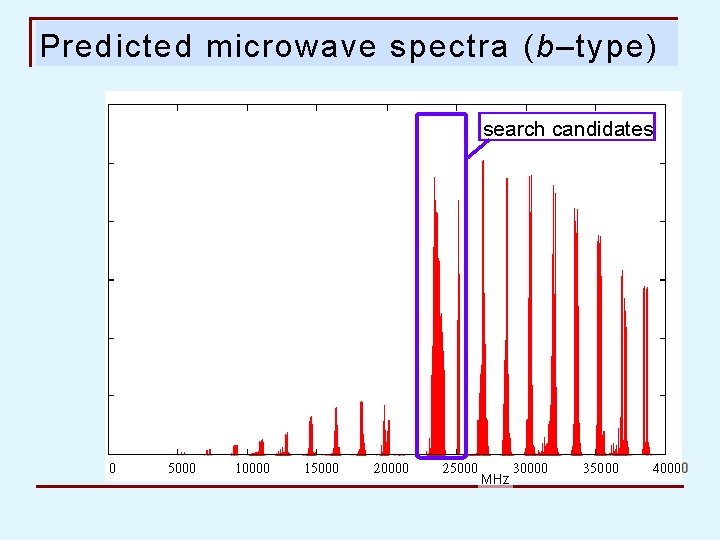
Predicted microwave spectra (b–type) search candidates MHz

Predicted microwave spectra 23000 23500 24000 24500 25000 25500 26000

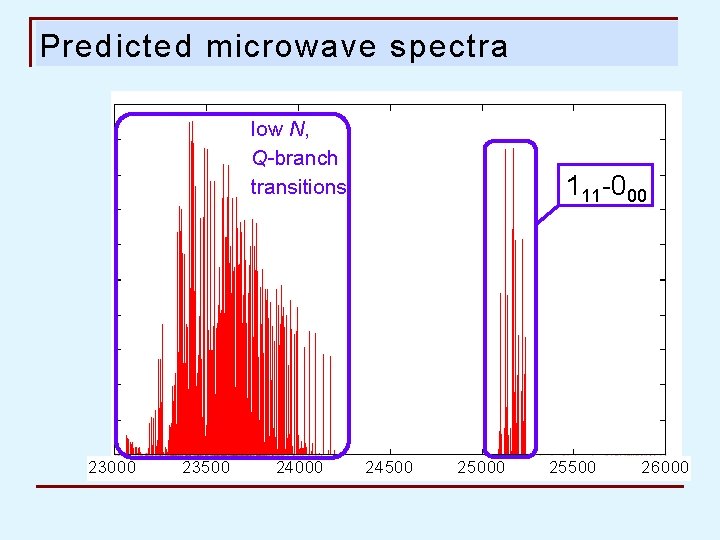
Predicted microwave spectra low N, Q-branch transitions 23000 23500 24000 111 -000 24500 25000 25500 26000

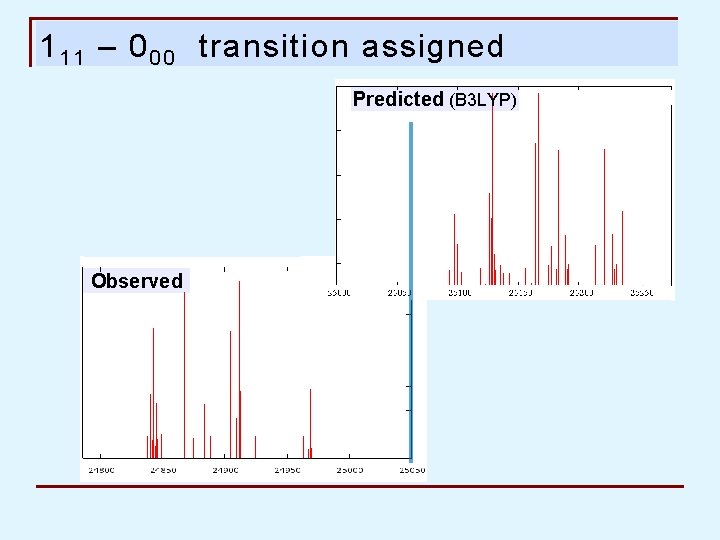
1 1 1 – 0 0 0 transition assigned Predicted (B 3 LYP) Observed

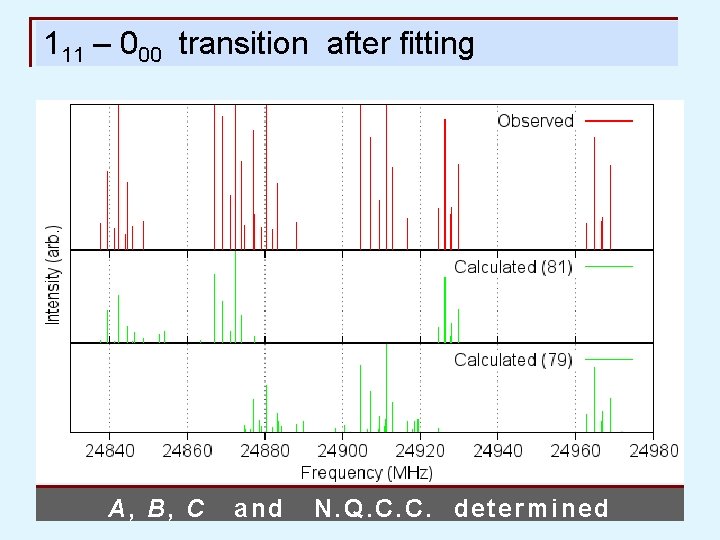
111 – 000 transition after fitting A, B, C and N. Q. C. C. determined

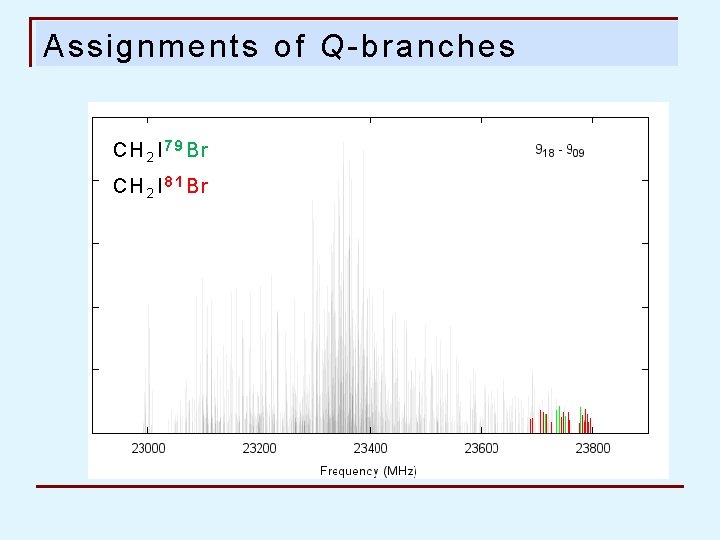
Assignments of Q-branches CH 2 I 79 Br CH 2 I 81 Br

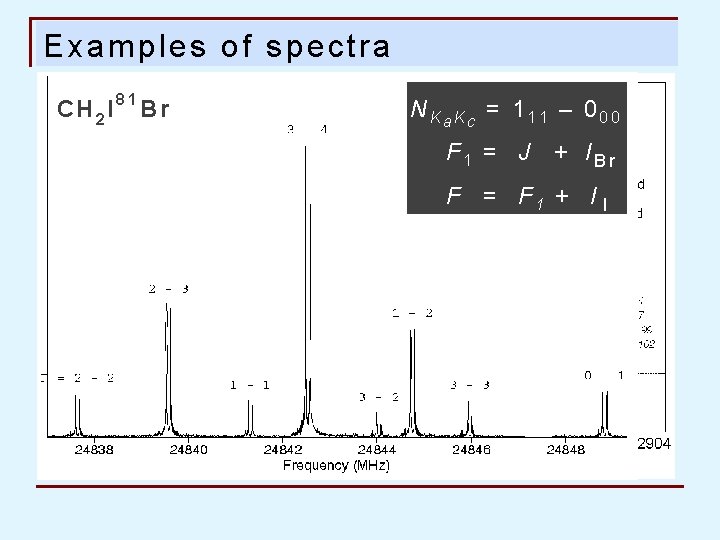
Examples of spectra CH 2 I 81 Br NKa. Kc = 111 – 000 F 1 = J + IBr F 1 = F 1 + I I

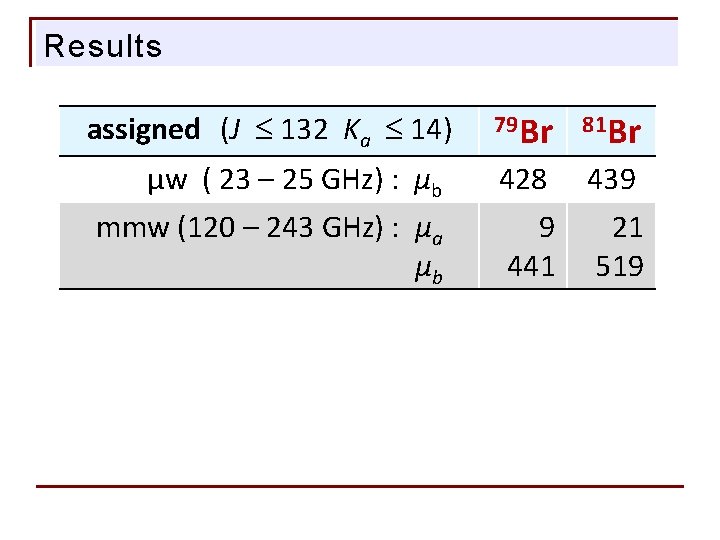
Results assigned (J 132 Ka 14) µw ( 23 – 25 GHz) : µb mmw (120 – 243 GHz) : µa mmw (200 – 243 GHz): µb 79 Br 81 Br 428 439 0021 0441 0519

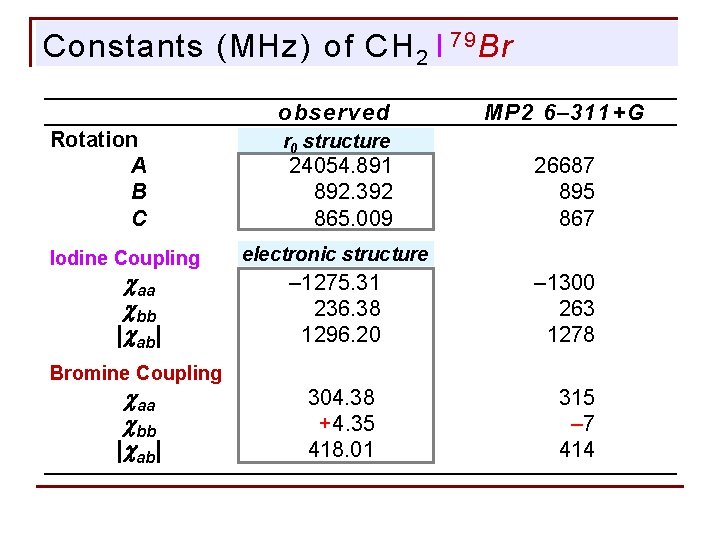
Constants (MHz) of CH 2 I 79 Br observed Rotation A B C Iodine Coupling caa cbb |cab| r 0 structure 0024054. 8910 0000892. 3920 0000865. 0090 MP 2 6– 311+G 26687 00895 00867 electronic structure – 1275. 31 00236. 38 01296. 20 – 1300 00263 01278 0304. 38 00+4. 35 0418. 01 00315 000– 7 00414 Bromine Coupling caa cbb |cab|

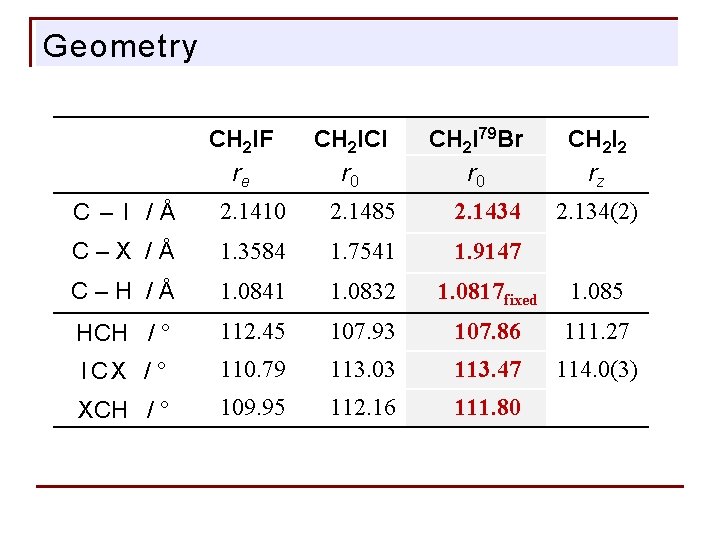
Geometry CH 2 IF re CH 2 ICl r 0 CH 2 I 79 Br r 0 CH 2 I 2 rz C – I /Å 2. 1410 2. 1485 2. 1434 C–X /Å 1. 3584 1. 7541 1. 9147 C–H /Å 1. 0841 1. 0832 1. 0817 fixed 1. 085 HCH / ° 112. 45 107. 93 107. 86 111. 27 ICX / ° 110. 79 113. 03 113. 47 114. 0(3) XCH / ° 109. 95 112. 16 111. 80 2. 134(2)

Concluding remarks n Organic iodine in the atmosphere: q ozone depletion q impact on the radiative balance n our data (hopefully) … q will prompt vibrational spectroscopic studies q give the potential for atmospheric monitoring

Contributors Measurement & analysis n Toho University (MMW) K. Taniguchi Computations n Kean University W. Bailey S. Sakai H. Ozeki n Shizuoka University (FTMW) T. Okabayashi n acknowledgements French National Research Agency n Lille University D. Duflot

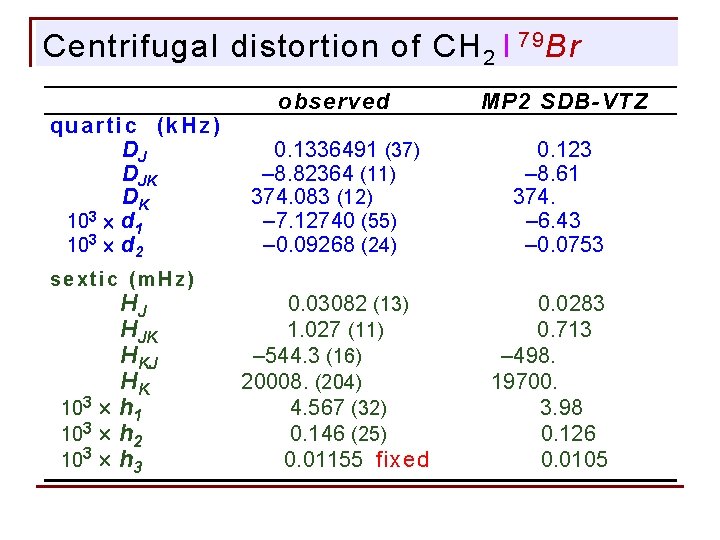
Centrifugal distortion of CH 2 I 79 Br quartic (k. Hz) DJK DKJ 103 d 1 20000000 103 d 2 20000000 sextic (m. Hz) observed MP 2 SDB-VTZ 000. 1336491 (37) – 8. 82364 (11)0 374. 083 (12) 0001 – 7. 12740 (55) 0 – 0. 09268 (24) 0 0. 123 – 8. 6100 374. 0001 – 6. 4300 0 – 0. 0753 HJK 000. 03082 (13) HJK 001. 027 (11)00 HKJ 0– 544. 3 (16) 00000 HKJ 20008. (204) 00000 103 h 1 20000000 04. 567 (32) 103 h 2 20000000 00. 146 (25) 103 h 3 20000000. 01155 fixed 00. 0283 000. 71300 0– 498. 0000000 19700. 000000 3. 9800 00. 126 000. 0105 0

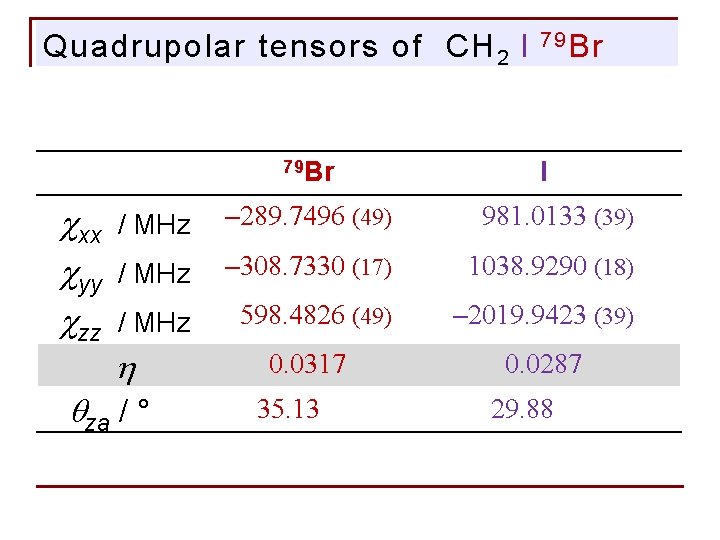
Quadrupolar tensors of CH 2 I 79 Br cxx cyy czz 7 9 Br I / MHz – 289. 7496 (49) 981. 0133 (39) / MHz – 308. 7330 (17) 1038. 9290 (18) / MHz 598. 4826 (49) – 2019. 9423 (39) h 0. 03170 0. 0287 qza / °Hz 35. 13000 29. 88000

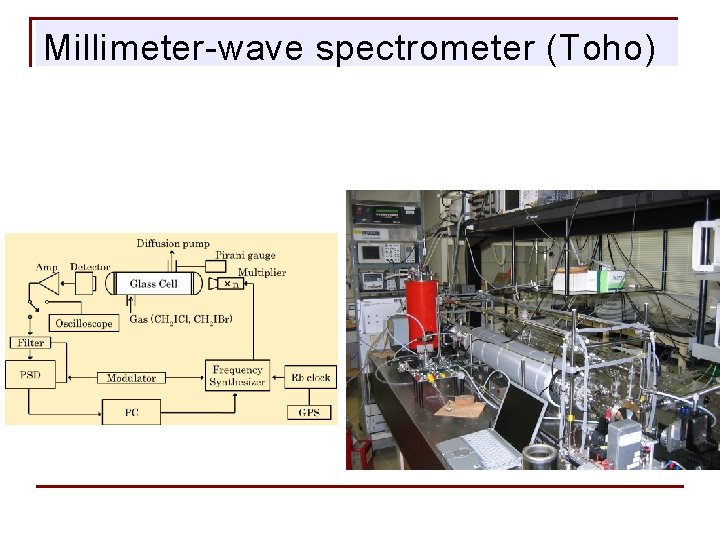
Millimeter-wave spectrometer (Toho)