Foundations of Chemistry Chapter 7 Understanding Matter Matter















- Slides: 28

Foundations of Chemistry Chapter 7

Understanding Matter • Matter is anything that has mass and takes up space. • An atom is a small particle that is a building block of matter. Atoms A nucleus is at the center of the atom. The nucleus is made of protons, which have a positive charge and neutrons which have no charge. Electrons have a negative charge and move quickly around the nucleus. Not all atoms have the same number of protons,

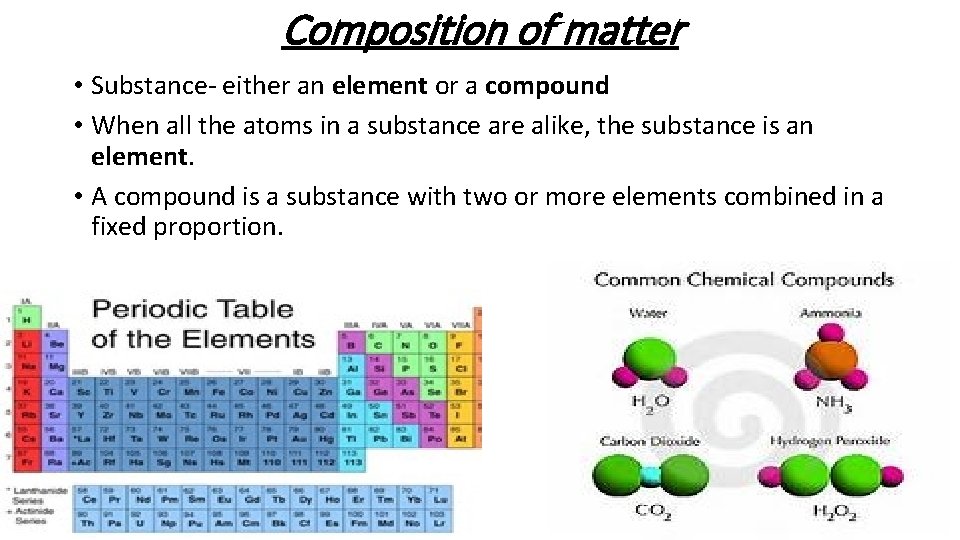
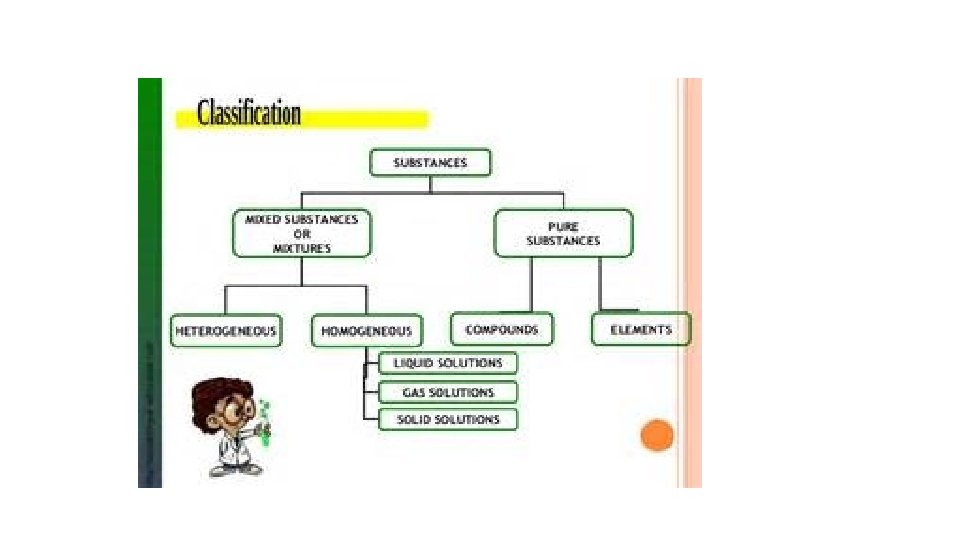
Composition of matter • Substance- either an element or a compound • When all the atoms in a substance are alike, the substance is an element. • A compound is a substance with two or more elements combined in a fixed proportion.

MIXTURES • A mixture is matter that can vary in composition. • The components of a mixture are physically blended together, so they can be separated by physical means. • The amounts of different components of a mixture can vary from one sample to another.

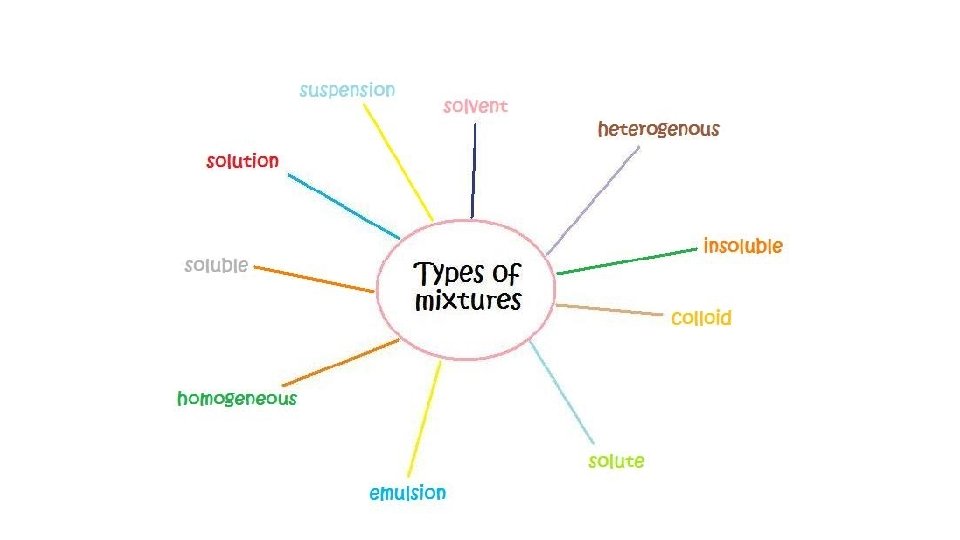
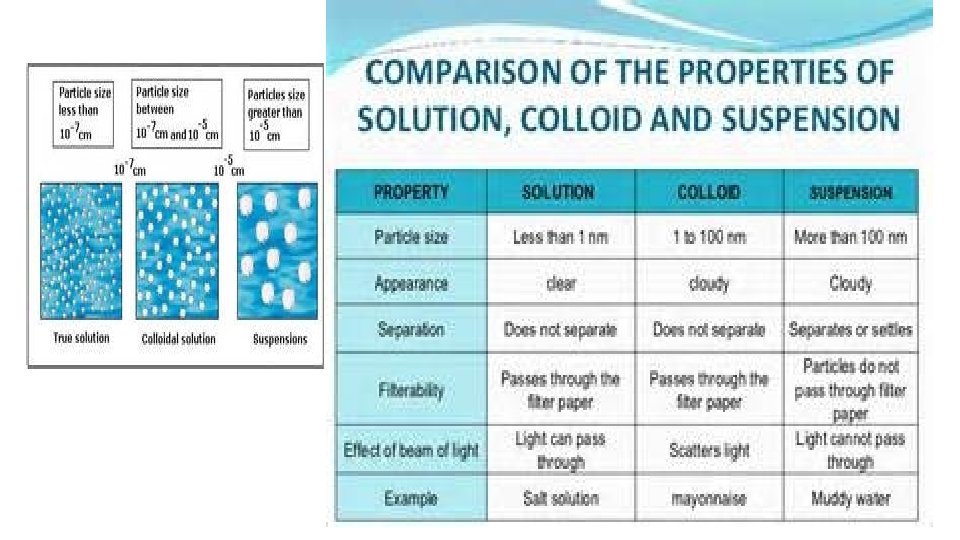
Two or more substances that can be easily separated by physical means form a mixture. 1. Heterogeneous mixture- mixture of different and easily distinguishable materials. 2. Homogeneous mixture- contains two or more gaseous, liquid, or solid substances blended evenly; also called a solution. 3. Colloid- heterogeneous mixture with larger particles that never settle; colloids scatter light in the Tyndall Effect. 4. A heterogeneous mixture containing a liquid in which visible particles settle is called a suspension.

Mixtures HETEROGENEOUS-proportions of substance may vary -different materials can be identified - Particles settle in a suspension HOMOGENEOUS- Particles are too small to be seen with a microscope. - Particles never settle - They remain constantly mixed COLLOID-particles are larger than in solutions -Contains substances in varying proportions -particles will not settle Particles scatter light





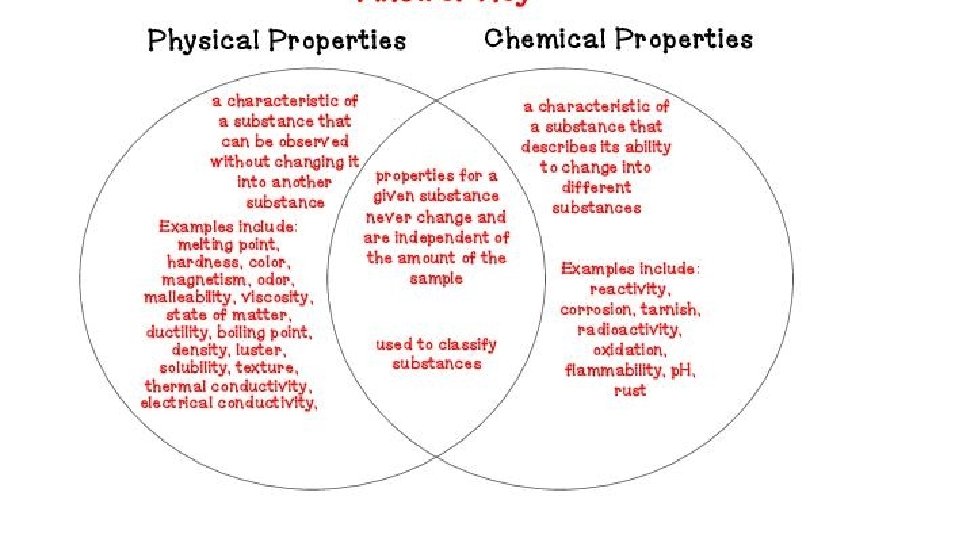
Properties of matter • Physical Property- characteristics of a material which can be observed without changing the identity of the substance in the material. ; examples include color, shape, size, melting point, and boiling point

Physical Properties: • Appearance- physical description of a substance • Behavior- how a substance acts; for example magnetism, viscosity, ductility • Physical properties such as size and magnetism can be used to separate mixtures.

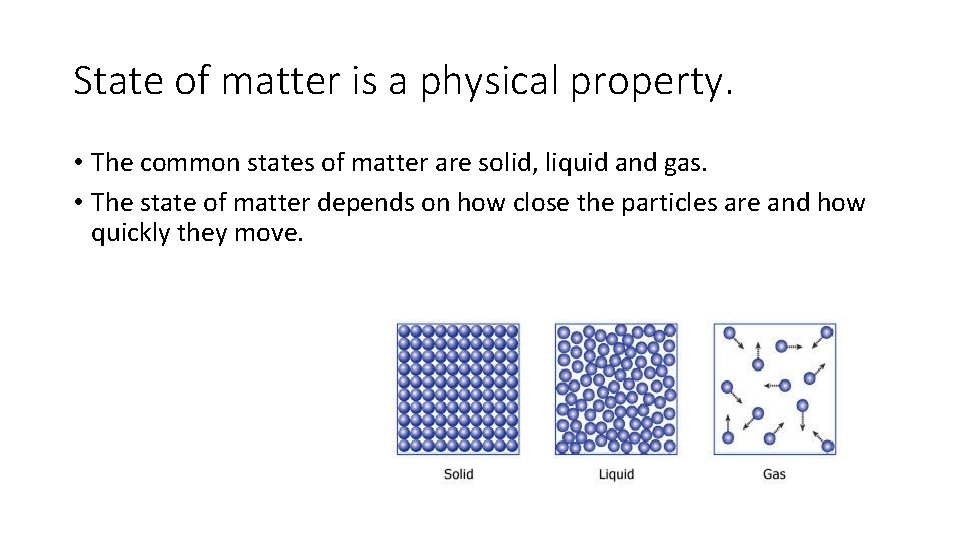
State of matter is a physical property. • The common states of matter are solid, liquid and gas. • The state of matter depends on how close the particles are and how quickly they move.

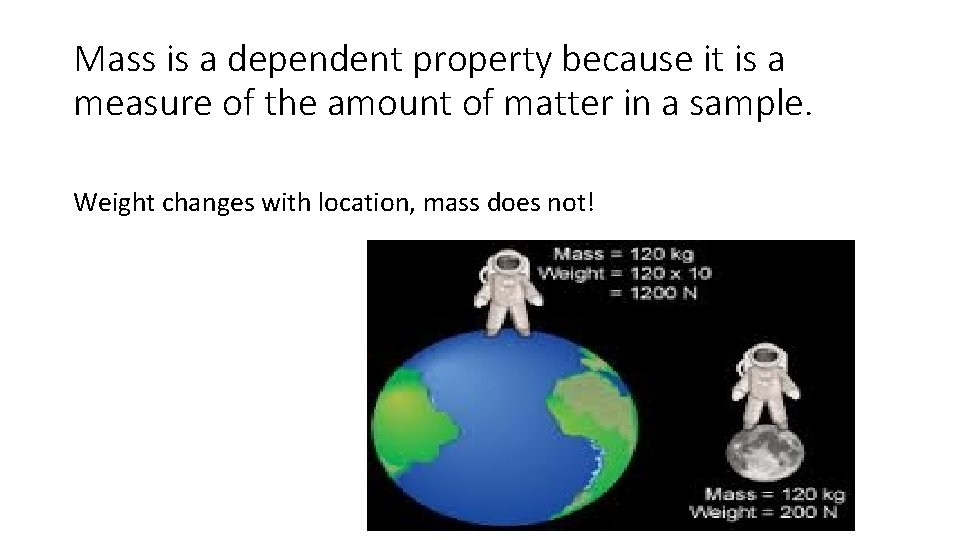
Mass is a dependent property because it is a measure of the amount of matter in a sample. Weight changes with location, mass does not!

The parts of a mixture can be separated using the physical properties of the components. • Salt and water can be separated because the boiling point is much lower than salt. • A solid can be separated from a liquid by filtering if the solid does not dissolve in the liquid. • Oil and water can be separated because they have different densities. • A magnet can be used to separate materials that contain iron from other materials.

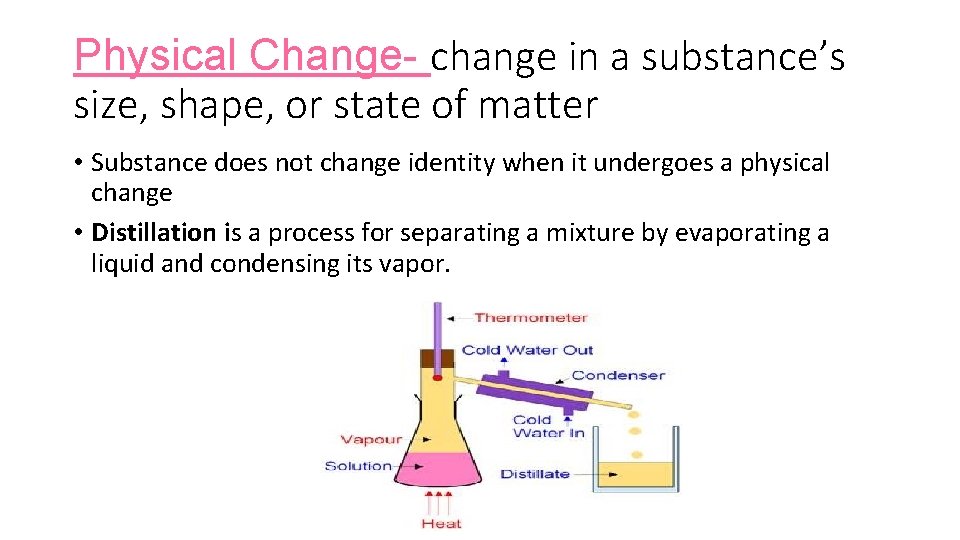
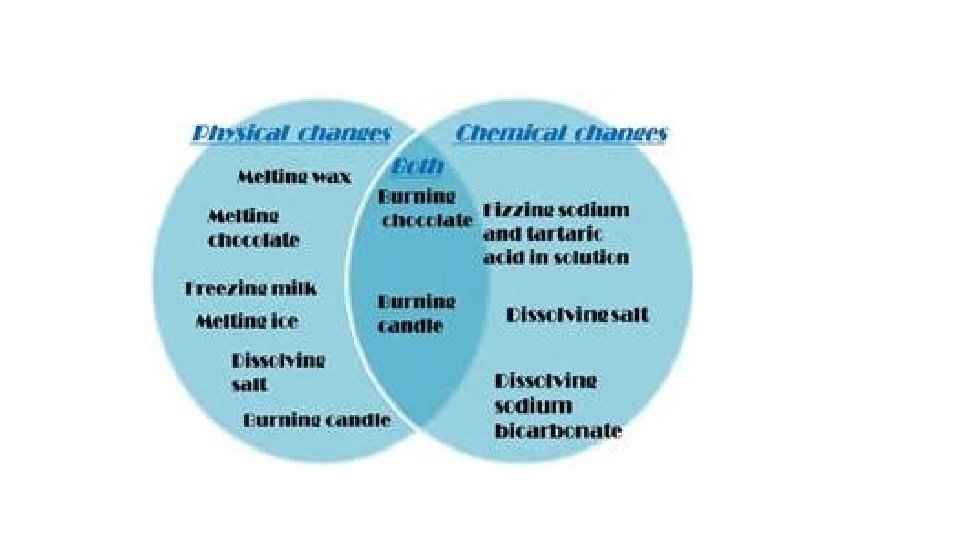
Physical Change- change in a substance’s size, shape, or state of matter • Substance does not change identity when it undergoes a physical change • Distillation is a process for separating a mixture by evaporating a liquid and condensing its vapor.

• When food is chewed, matter undergoes a physical change in its size and shape. • Matter undergoes a physical change when it changes from one state to another • A change in state involves a change in the energy of particles. • The energy of particles changes when thermal energy is added or removed. When thermal energy is added to particles, they move faster and temperature increases. When thermal energy is removed from particles, they move slower and temperature decreases.

• Melting and freezing are reverse processes, as are condensation and boiling, and sublimation and deposition. • Dissolving, during which one substance evenly mixes with another substance, is also a physical change. • Some types of physical changes are reversible, such as when a solid changes to a liquid and then the liquid changes back in to a solid. • Dissolving is also a physical change.

Law of Conservation of mass- mass of all substances present before a chemical change equals the mass of all substances after the change.


CHEMICAL PROPERTY- characteristics of a substance indicating that it can change chemically • For example- flammability or light sensitivity of a substance




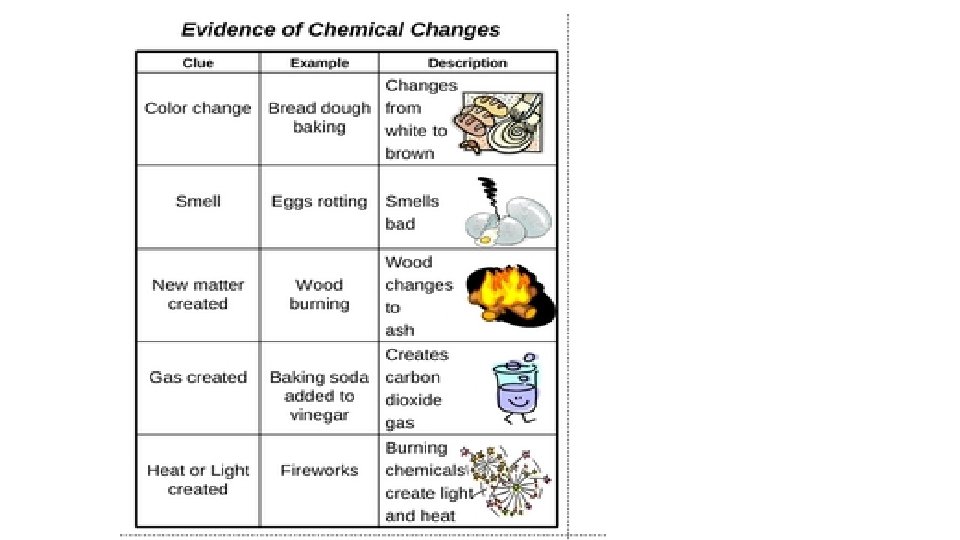
When one substance changes to another substance, a chemical change has occurred. • Some chemical changes are indicated by temperature change, smell, or bubble formation. • Other chemical changes occur very slowly such as the formation of rust. • Chemical changes can be used to separate substances such as metals from their ores. • THESE PROPERTIES of a substance NEVER CHANGE: density, specific heat, boiling point, melting point



WEATHERING of Earth’s surface involves both physical and chemical change. • Physical- big rocks split into smaller ones; streams carry rock particles from one location to another. • Chemical- Chemical change can occur in rocks when calcium carbonate in limestone changes to calcium hydrogen carbonate due to acid rain.