Foundations of chemistry Chapter 1 Key concepts in















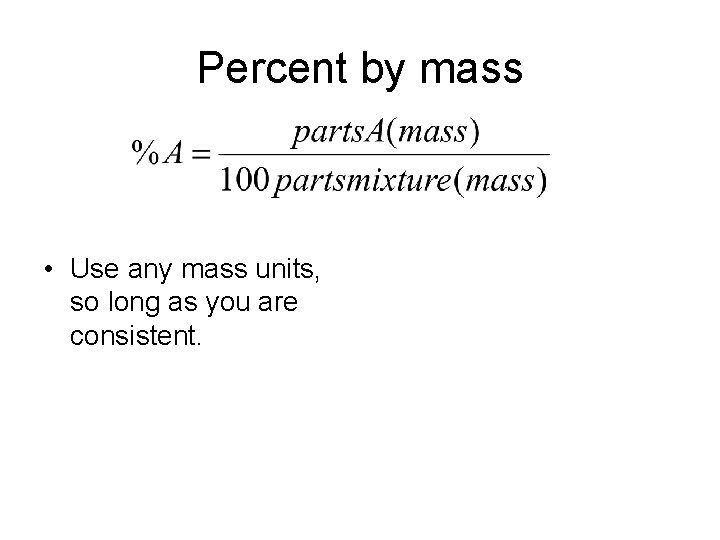

- Slides: 31

Foundations of chemistry Chapter 1

Key concepts in this unit • The scientific method • The definition of chemistry • Matter and energy – States of matter – Chemical and physical properties – Chemical and physical changes • Measurements in chemistry – – – Units and SI system Uncertainty in measurement: precision and accuracy Using dimensional analysis Mass percent Density and specific gravity Heat, temperature, specific heat

The Scientific Method • The generally accepted approach to solving problems in the sciences. • The pattern is used to generate rigorous, reliable, and repeatable research procedures in the discovery of new scientific concepts.

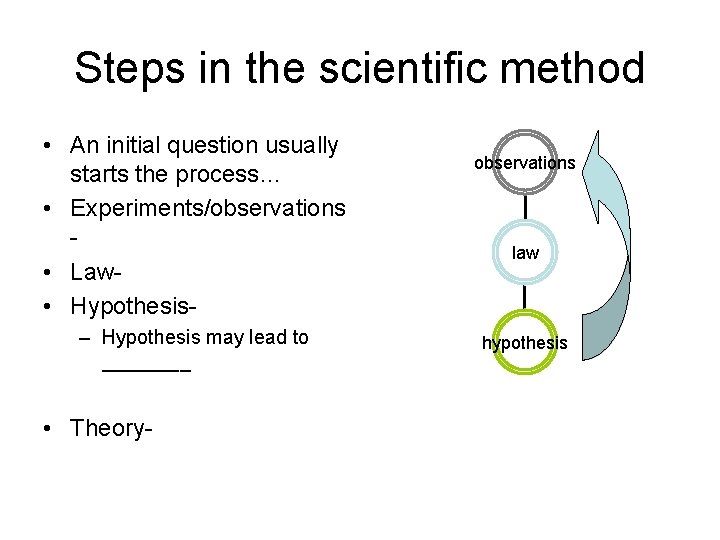
Steps in the scientific method • An initial question usually starts the process… • Experiments/observations • Law • Hypothesis– Hypothesis may lead to ____ • Theory- observations law hypothesis

Chemistry—A study of matter and energy • Matter— – Mass— – D & C 131: 7—spiritual things are also material – Since matter is everywhere, a basic understanding of chemistry is essential. • Energy— • Types of energy – kinetic – potential

Laws of conservation • Conservation of matter: • Conservation of energy: • Most chemical changes (reactions) observe these two laws. • Nuclear reactions—changing matter into energy E=mc 2 • Conservation of matter and energy: – D & C 131: 7

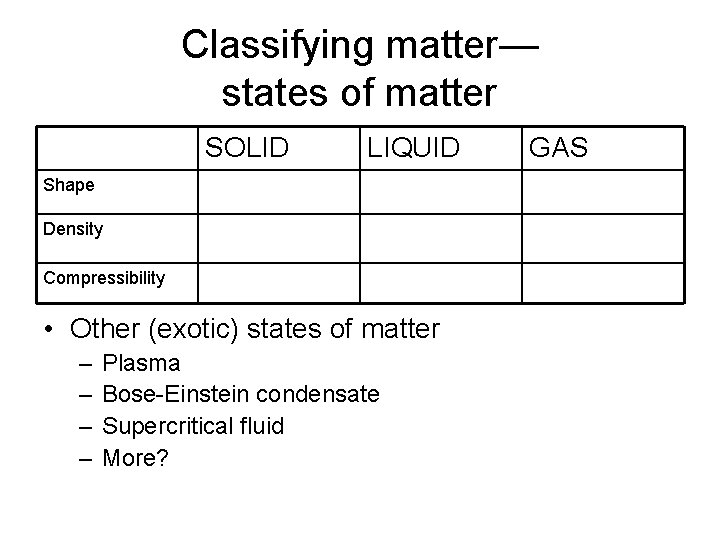
Classifying matter— states of matter SOLID LIQUID Shape Density Compressibility • Other (exotic) states of matter – – Plasma Bose-Einstein condensate Supercritical fluid More? GAS

Properties of matter • Physical properties— • Chemical properties— • Which are physical? Chemical? Melting point density flammability conductivity Matter states

Properties of matter • Intensive properties— • Extensive properties— • Which are intensive? Extensive? mass volume density melting point boiling point

Changes in matter • Physical changes— • Chemical changes also called chemical reactions • Which changes are physical? Chemical? – – Combustion Evaporation Dissolution Fission

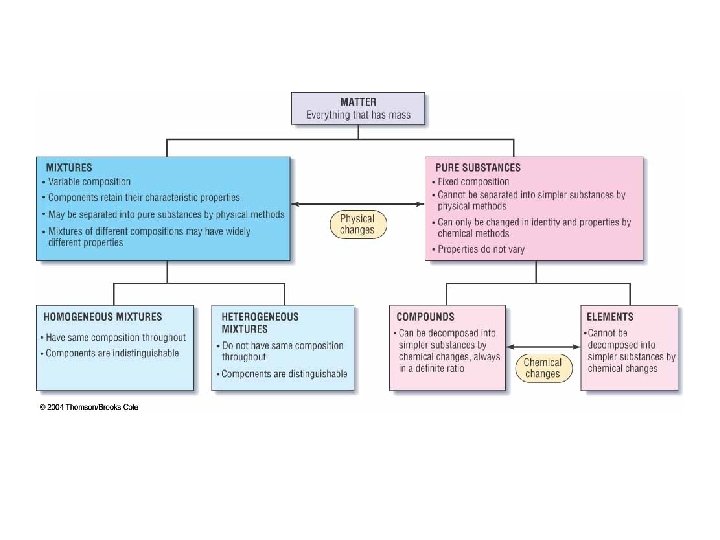
Mixtures • Two types – Heterogeneous – Homogeneous. A homogeneous mixture also called a ______ • Water and salt vs water and sand. – A homogeneous mixture may have some physical properties differing from its components (such as melting point or boiling point) – NO change in chemical properties.

• Mixtures may be separated by physical means. Examples: • Distillation— • Filtration— • Chromatography—

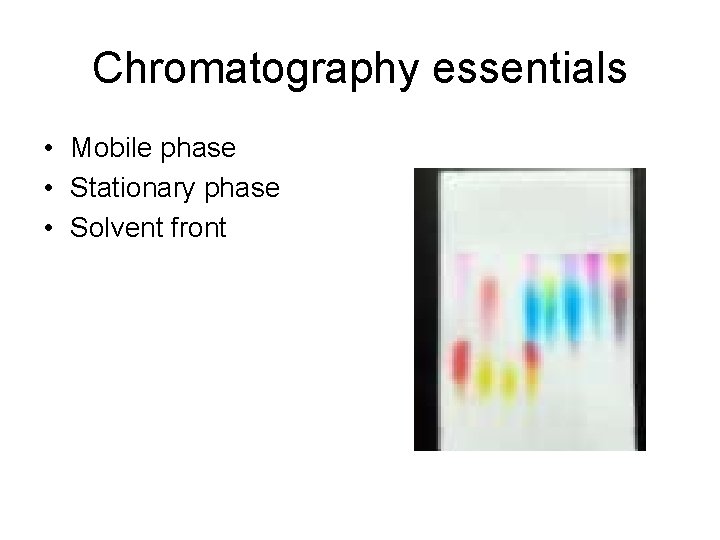
Chromatography essentials • Mobile phase • Stationary phase • Solvent front

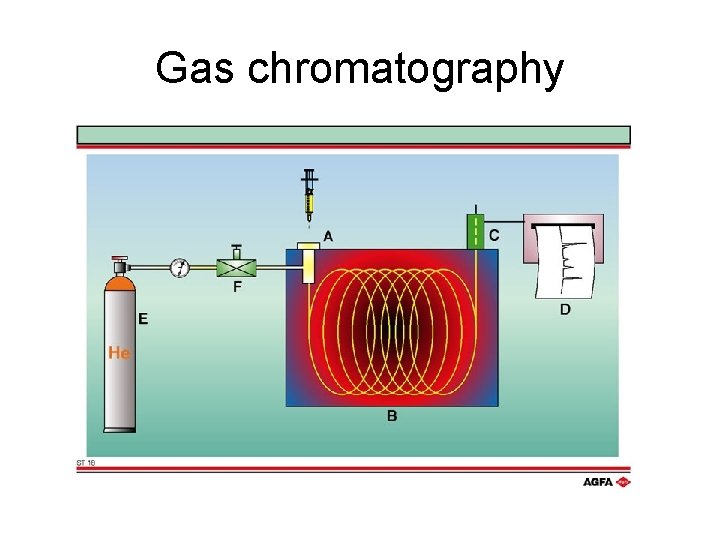
Gas chromatography

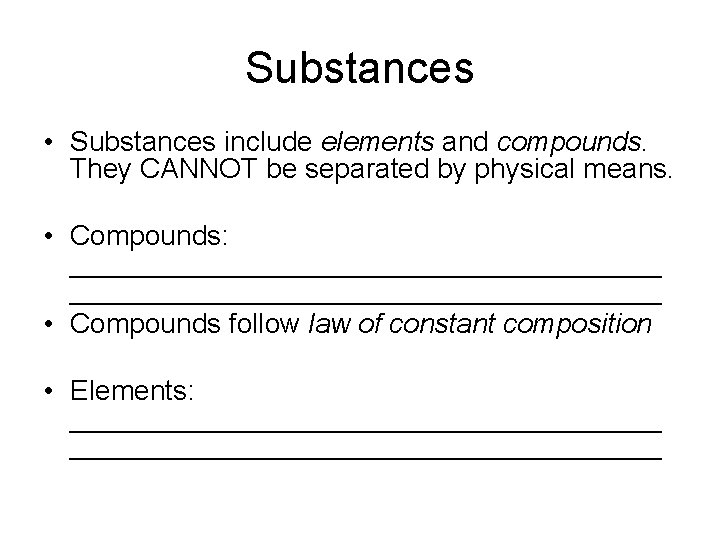
Substances • Substances include elements and compounds. They CANNOT be separated by physical means. • Compounds: ______________________________________ • Compounds follow law of constant composition • Elements: ______________________________________


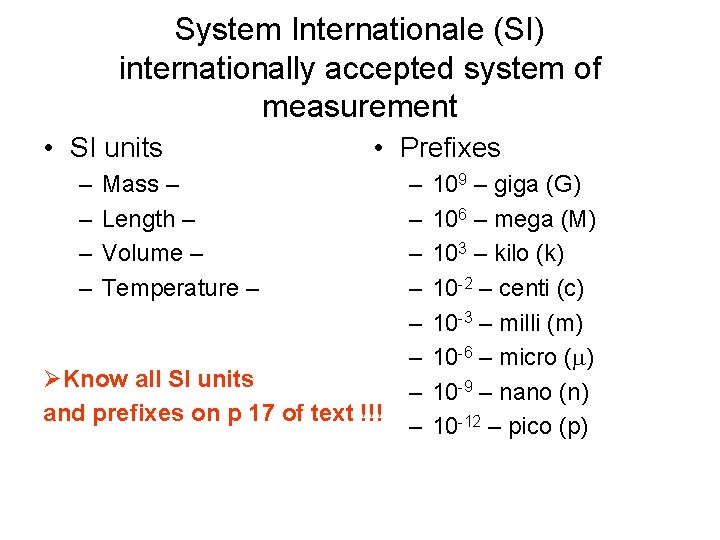
System Internationale (SI) internationally accepted system of measurement • SI units – – • Prefixes Mass – Length – Volume – Temperature – ØKnow all SI units and prefixes on p 17 of text !!! – – – – 109 – giga (G) 106 – mega (M) 103 – kilo (k) 10 -2 – centi (c) 10 -3 – milli (m) 10 -6 – micro ( ) 10 -9 – nano (n) 10 -12 – pico (p)

Uncertainty in measurement • Two types of numbers: – Exact numbers (counting or defined) – Measured numbers

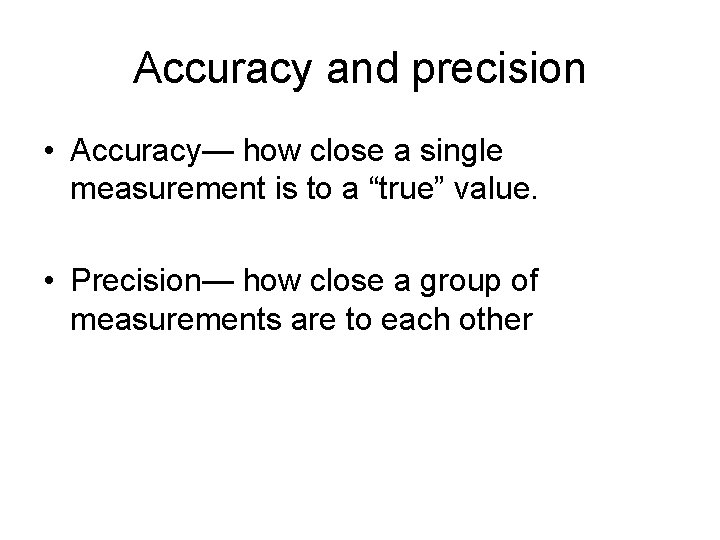
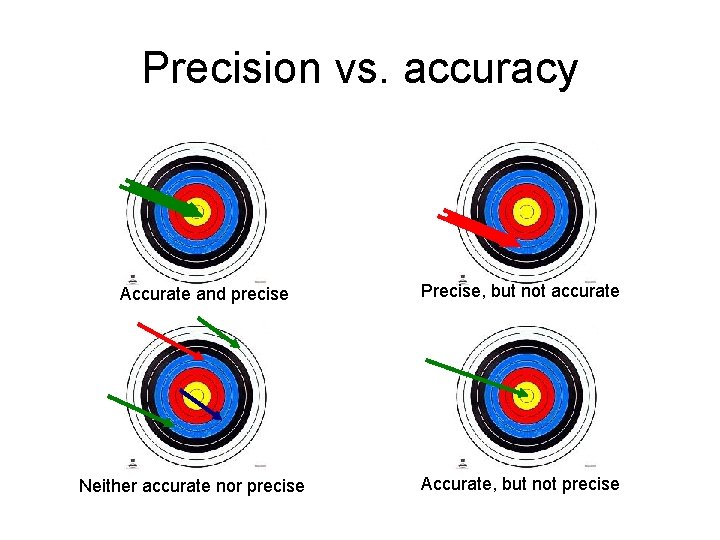
Accuracy and precision • Accuracy— how close a single measurement is to a “true” value. • Precision— how close a group of measurements are to each other

Precision vs. accuracy Accurate and precise Neither accurate nor precise Precise, but not accurate Accurate, but not precise

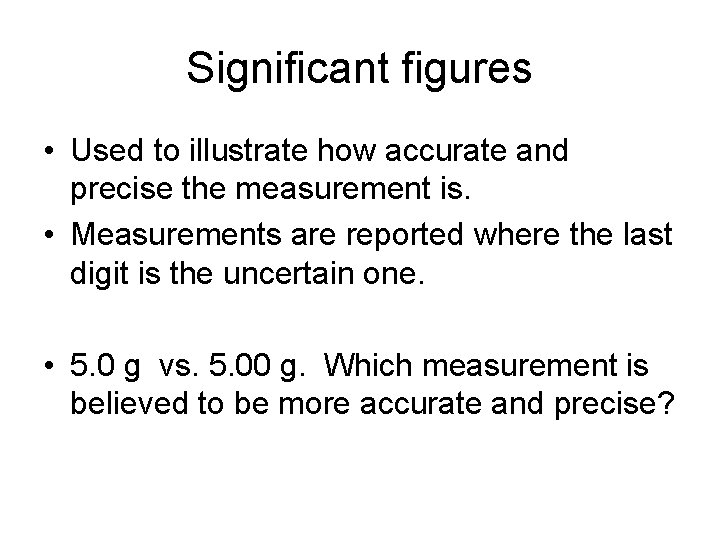
Significant figures • Used to illustrate how accurate and precise the measurement is. • Measurements are reported where the last digit is the uncertain one. • 5. 0 g vs. 5. 00 g. Which measurement is believed to be more accurate and precise?

Significant figure rules 1. Exact numbers have unlimited number of significant digits. 2. Nonzero digits are always significant. 3. Zeros between nonzero digits are always significant 4. Zeros at the beginning of a number (placeholders) are never significant 5. Zeros at the end of a number after the decimal point are always significant 6. Zeros at the end of a number without a decimal point are not very clear….

Scientific notation • Two purposes for SN – Removing ambiguity of zeros – Handling very large or very small quantities. number = N 10 x • N is a number between 1 and 10. • “x” is an exponent • Using a calculator, SN exponents entered with the EXP or EE button. – 4. 5 × 1014 entered as 4. 5 EE 14

Significant figures in calculations • Addition and subtraction— • Multiplication and division— • When carrying on several steps, retain extra digits from calculator in intermediate answers to avoid rounding errors. Round answer according to sig. fig. rules.

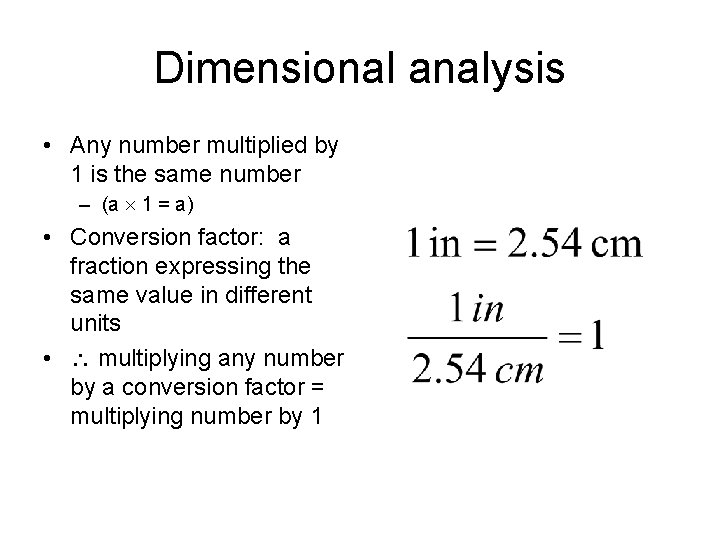
Dimensional analysis • Any number multiplied by 1 is the same number – (a 1 = a) • Conversion factor: a fraction expressing the same value in different units • multiplying any number by a conversion factor = multiplying number by 1

Process for dimensional analysis • ALWAYS CARRY UNITS. They are essential in determining if a calculation was done correctly. • Let’s try some examples…
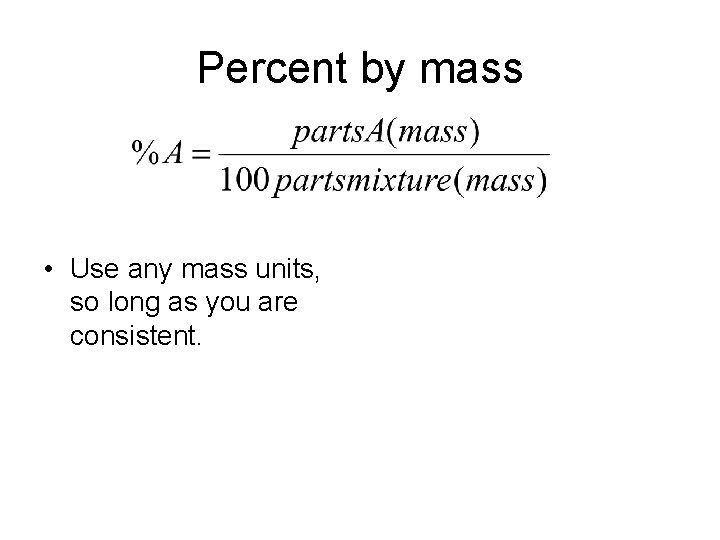
Percent by mass • Use any mass units, so long as you are consistent.

Density • Ratio of mass per unit volume • D = m/V • Density is an intensive property. • Specific gravity is ratio of density of substance to density of water (1. 00 g/m. L @ room temp) • Sp. Gr. = Dsub/Dwater • Sp. Gr. is numerically equal to D, but it is unitless • Examples…

Heat and temperature • Temperature indicates _________________ • Heat flows in which direction? • • Temperature units Celsius Kelvin Fahrenheit • Conversions (just one or two)

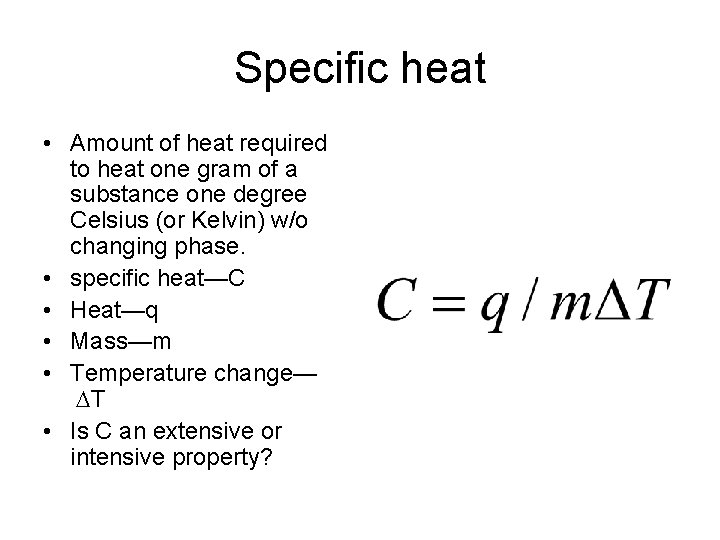
Specific heat • Amount of heat required to heat one gram of a substance one degree Celsius (or Kelvin) w/o changing phase. • specific heat—C • Heat—q • Mass—m • Temperature change— T • Is C an extensive or intensive property?

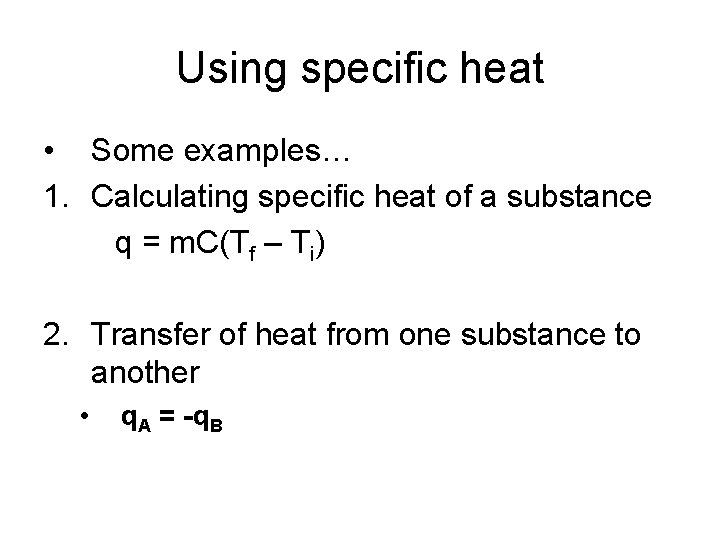
Using specific heat • Some examples… 1. Calculating specific heat of a substance q = m. C(Tf – Ti) 2. Transfer of heat from one substance to another • q. A = -q. B