Foundations for quantum mechanics Atomic Spectra A little








- Slides: 18

Foundations for quantum mechanics Atomic Spectra

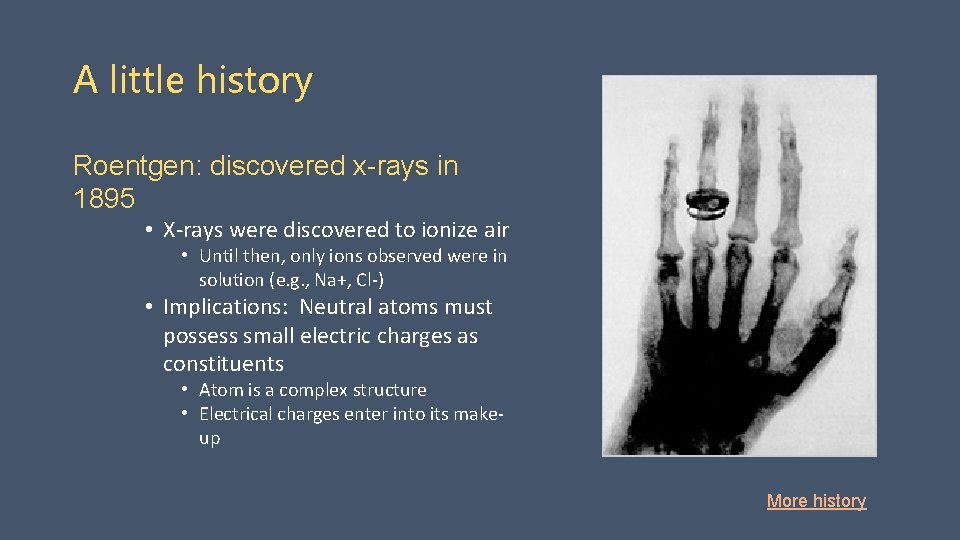
A little history Roentgen: discovered x-rays in 1895 • X-rays were discovered to ionize air • Until then, only ions observed were in solution (e. g. , Na+, Cl-) • Implications: Neutral atoms must possess small electric charges as constituents • Atom is a complex structure • Electrical charges enter into its makeup More history

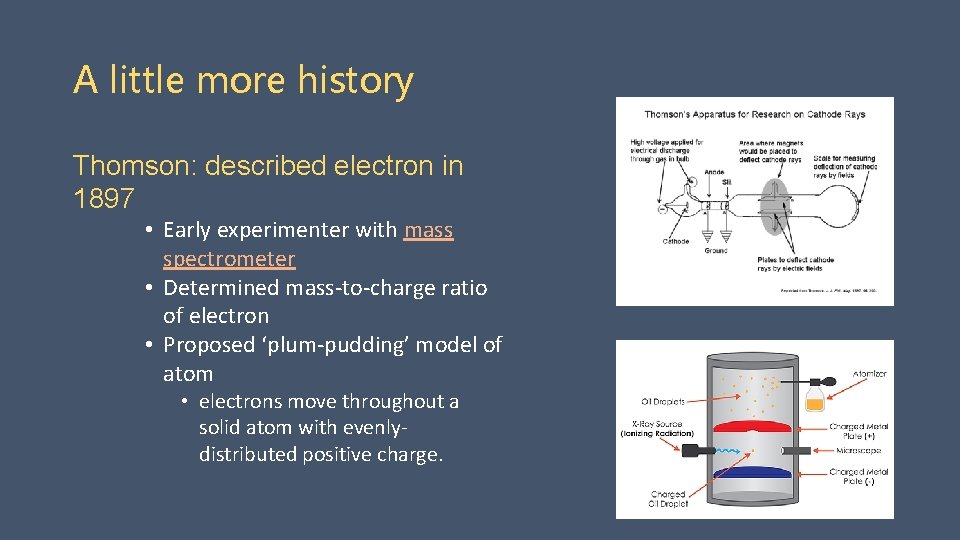
A little more history Thomson: described electron in 1897 • Early experimenter with mass spectrometer • Determined mass-to-charge ratio of electron • Proposed ‘plum-pudding’ model of atom • electrons move throughout a solid atom with evenlydistributed positive charge.

A little more history Rutherford conducts gold foil experiment in 1911 • Directed high-energy, positively-charged alpha particles (helium ions from radon) at gold foil surrounded by cylindrical detector Expectation Thomson’s model = no massive concentration of positive charge = nothing to significantly deflect ions Results • Most ions passed through as if foil were empty space • Few deflected at very large angles Conclusion relatively small nucleus with positive charge

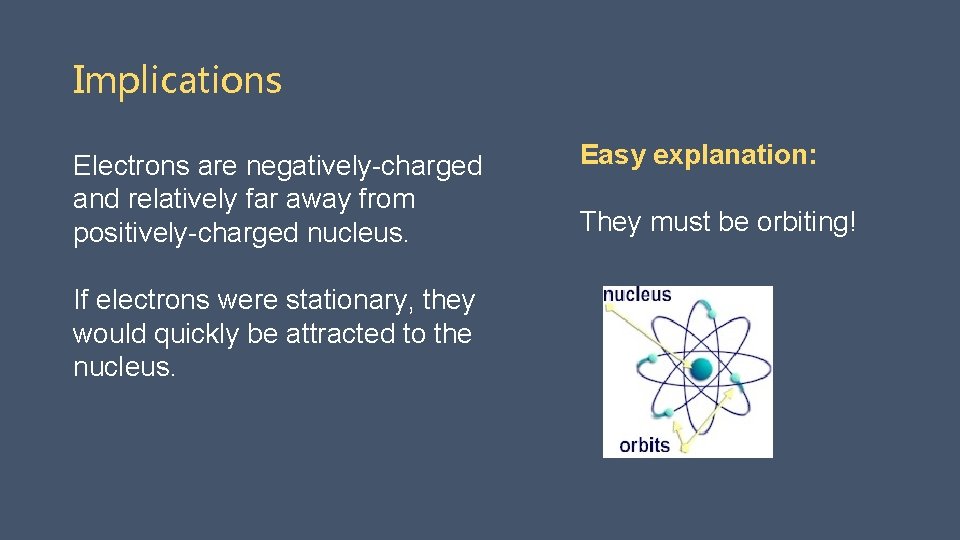
Implications Electrons are negatively-charged and relatively far away from positively-charged nucleus. If electrons were stationary, they would quickly be attracted to the nucleus. Easy explanation: They must be orbiting!

Implications 2 Since… • Electrons orbit stable nuclei. • Moving charges emit energy in the form of electromagnetic waves. • Energy is conserved. We expect that … • As electrons orbit the nucleus, their energy must decrease. • As their energy decreases, they spiral into the nucleus. • As they spirals inward, their speed increases and so would frequency of light emitted. • The process repeats. • The universe does not last

Oops.

If our assumptions are true, we would expect to see light emitted from atoms shift from red (low-energy) to orange to yellow to green to blue (high-energy) relatively quickly. (and also, we would expect not to exist as the electrical potential in atoms would have evened out long ago, making life as we know it impossible. )

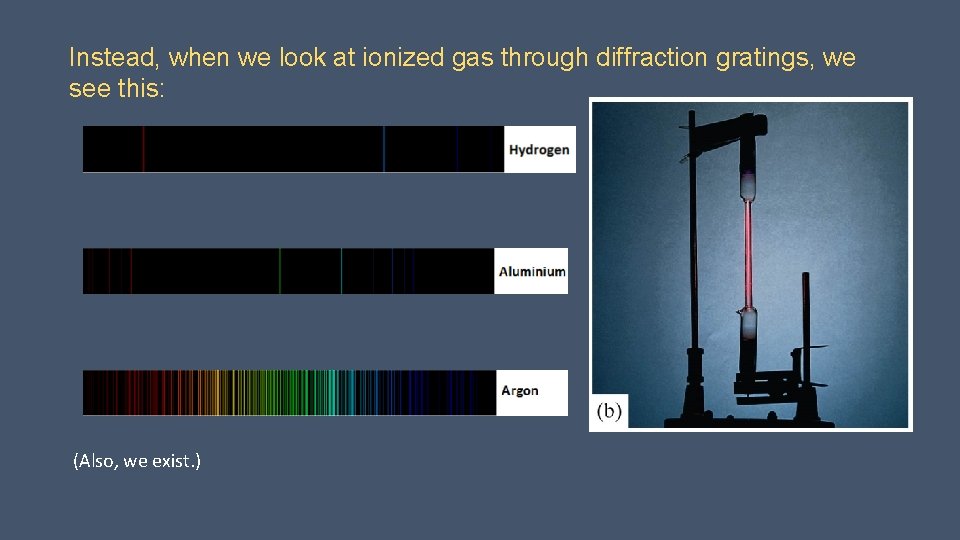
Instead, when we look at ionized gas through diffraction gratings, we see this: (Also, we exist. )

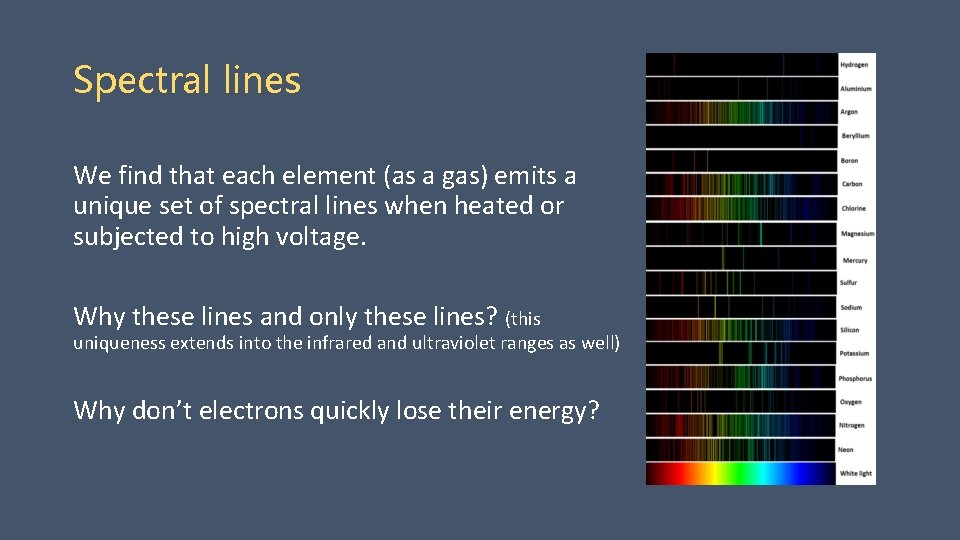
Spectral lines We find that each element (as a gas) emits a unique set of spectral lines when heated or subjected to high voltage. Why these lines and only these lines? (this uniqueness extends into the infrared and ultraviolet ranges as well) Why don’t electrons quickly lose their energy?

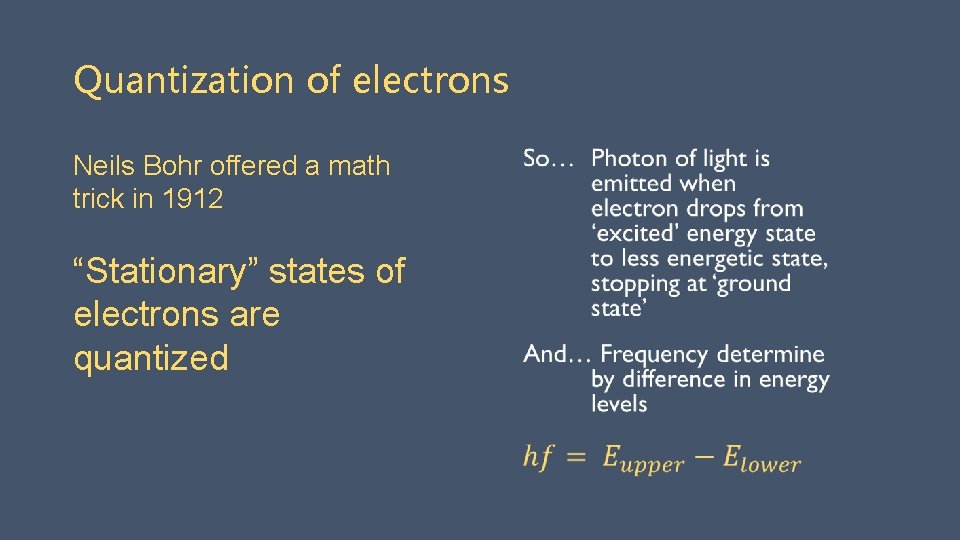
Quantization of electrons Neils Bohr offered a math trick in 1912 “Stationary” states of electrons are quantized

Implications Reasons we trust the model Does a nice job explaining spectral lines for hydrogen Reasons we question the model: Math trick without a very good explanation: • Stationary state? • Ground state? • Moving charged particles don’t emit light? To explain the rest of the periodic table, we need to quantize angular momentum, “magnetic quantum number”, “spin quantum number”, and allow only one electron per quantum state.

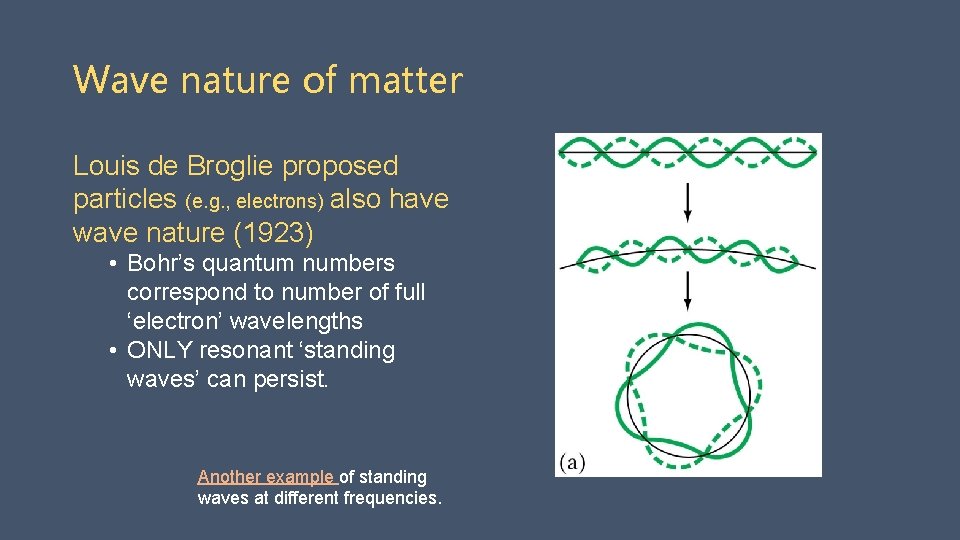
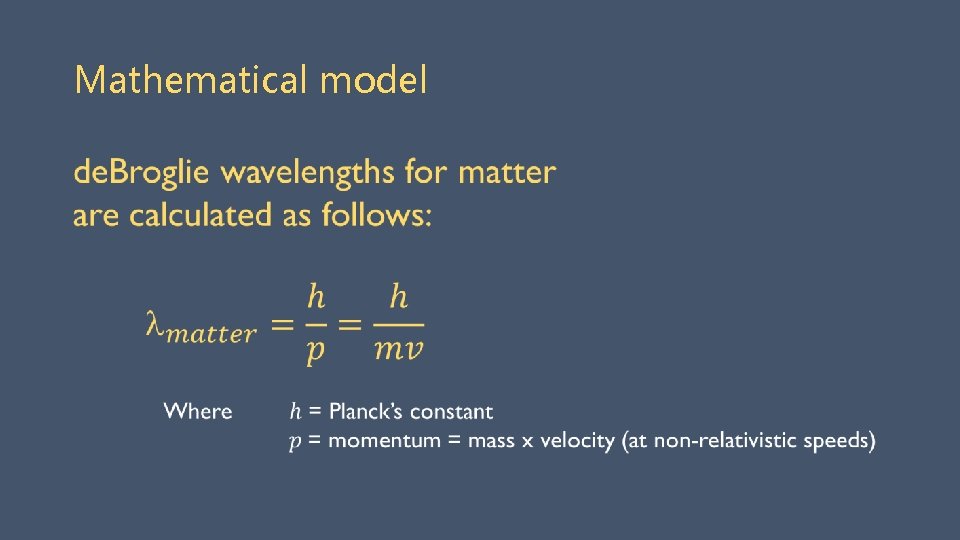
Wave nature of matter Louis de Broglie proposed particles (e. g. , electrons) also have wave nature (1923) • Bohr’s quantum numbers correspond to number of full ‘electron’ wavelengths • ONLY resonant ‘standing waves’ can persist. Another example of standing waves at different frequencies.

Mathematical model

Example Calculate the de. Broglie wavelength of a 0. 20 -kg ball moving at a speed of 15 m/s. NOTE: Far too small to diffract around anything. Matter waves of macroscopic objects are undetectable. What quantities are given? What is the relationship between wavelength and momentum? Substitute Solve.

Example Calculate the de. Broglie wavelength of an electron moving at a speed of 6. 0 x 106 m/s NOTE: Very small, but could diffract around atomsized objects. This physics is the basis for the electron microscope. What quantities are known or given? What is the relationship between wavelength and momentum? Substitute Solve.

Just a math trick? No. Not just a trick. • Electrons diffract? • Demonstrated 1927 • Davison – Germer • Electrons interfere with each other in double-slit experiment? • Yes: Demonstrated in 1961

Implications Light is not a wave or a particle; rather, it has properties of both. Microscopic matter is not a wave or a particle; rather, it has properties of both. All models are wrong. Some models are useful.