FORMULATION AND PACKAGING STAGES OF THE DRUG DISCOVERY









































- Slides: 42

FORMULATION AND PACKAGING

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS • The development of a new medicinal product from a novel synthesized chemical compound, a chemical extracted from a natural source or a compound produced by biotechnological processes, is a long and complex process and involves many different disciplines working together. • The drug discovery and development process for a typical research-based pharmaceutical company can be broken down into five distinct stages. • At each stage, there will be several activities running in parallel, with the overall objective of discovering a candidate drug and developing it to market as efficiently as possible.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 1. Strategic Research • Feasibility studies are conducted to demonstrate whether interfering in a particular biological mechanism has an effect that might be of therapeutic value. • The strategic research of a particular company is usually guided by factors such as its inherent research competence and expertise, therapeutic areas of unmet medical need and market potential/commercial viability. • Companies often wish to develop a portfolio of products within a specific therapeutic area to capture a segment of the market. • By focusing on a particular therapeutic area, a company can build on its existing expertise and competence in all of its functions with the aim of becoming a leading company in that field.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 2. Exploratory Research • Investigation of the biological mechanism and identification of a “chemical lead” that interferes with it. • During the exploratory research stage, diverse compounds are screened for the desired biological activity. The aim is to find a chemical or molecular entity that interferes with the process and to provide a valuable probe of the underlying therapeutic problem. • Traditionally, this has been achieved by the organic chemist synthesizing compounds one at a time for the biologist to test in a linear fashion. Over the last decade, there has been a rapid development in the technologies for creating very large and diverse quantities of synthetic and biosynthetic molecules and for testing large numbers for activity in less time. • These technologies have been labeled “combinatorial chemistry” and automated “highthroughput screening” (HTS), respectively. • The key impact has been to accelerate the synthesis of new compounds from, say, 50 compounds per chemist year to many tens of thousands and to be able to test these against many biological targets (e. g. , biological receptors or biochemical pathways) very quickly

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 2. Exploratory Research • The rate of technology development specifically associated with HTS for pharmaceutical drug discovery has increased markedly over recent years with automated techniques involving miniaturization, to allow assays on very small samples (e. g. , 1 L volume), and the ability to analyze thousands of samples a day using well microplates. • In addition to the use of HTS for pharmacological activity, HTS tests have been developed for assessing metabolism and pharmacokinetic and toxicity factors to speed up the drug discovery process. • In simple terms, a biologically active compound can be considered to consist of a supportive framework with biofunctional groups attached that bind to a target to induce a biological response. • Each compound is, in effect, a unique combination of numerous possible groups. Combinatorial techniques have replaced traditional synthetic approaches to generate many possible combinations rapidly for biological testing.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 2. Exploratory Research • Approaches to lead generation during exploratory research often depend on how much is already known about therapeutic target under consideration. For example, if the threedimensional structure of the target (such as an enzyme-inhibitor complex) is known, chemical leads could be found and optimized through combinatorial chemistry and HTS. • Alternatively, in some cases, the only available biochemical knowledge might be the structure of a ligand for the enzyme. If there were no information at all, then the only approach might be limited to HTS of batches of compounds from combinatorial libraries. • Even with combinatorial chemistry and HTS, lead generation can be extremely laborious because of the vast number of different molecules possible (framework and biofunctional group combinations). To ease this burden, some rational drug design and quantitative structure activity relationships (QSARs) are often introduced to direct the programme and utilize a company’s finite screening resource as efficiently as possible.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 2. Exploratory Research • “Representative” libraries of compounds, where each member is selected to give information about a larger cluster of compounds, are designed and used to reduce the amount of compounds that have to be made and tested. • Together with combinatorial chemistry and rational drug design, genomics is rapidly emerging as a useful technique to enable companies to significantly increase the number of drug targets and improve on candidate selection success. • A number of companies have seen the potential in defining patient groups based on their genotypes and are now investing lots of money to gain a clearer understanding of the genes that are important to drug action.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 3. Candidate Drug Selection • The chemical lead is used to generate specific chemical compounds with the optimal desired characteristics, for example, potency, specificity, duration, safety and pharmaceutical aspects. • One or more candidate drugs are nominated for development. • During the candidate drug selection stage, the chemical lead is optimized by testing a range of selected compounds in in vitro and in vivo (animal) studies. The objective is to select one or more candidate drugs for development with the most desired characteristics. • Pharmacological characteristics might include acceptable absorption, potency, duration of action and selectivity for the receptor or enzyme. Safety characteristics will normally include noncarcinogenicity, non-teratogenicity, non-mutagenicity, and general non-toxicity. • The potential for these characteristics can be predicted from relatively short-term preclinical toxipharmacological animal studies and in vitro tests.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 3. Candidate Drug Selection • Higher priority in the selection process will, in most cases, be given to a compound’s optimal pharmacological and safety characteristics. However, in the event of having a choice from a range of compounds all possessing similar pharmacological and safety properties, there may be a significant advantage formulation development in selecting a compound with the most preferred pharmaceutical properties. It is useful to conduct preformulation studies and biopharmaceutics studies at the candidate drug selection stage to determine the most relevant physicochemical and biopharmaceutical properties of potential candidate drugs to aid candidate selection. • Biopharmaceutics is the study of how the physicochemical properties of the candidate drugs, the formulation/delivery system and the route of administration affect the rate and extent of drug absorption. Appropriate biopharmaceutical information generated at this stage can also be very important in directing the candidate selection process and for future dosage form design during development

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 3. Candidate Drug Selection • Generally, any pharmaceutical issues can be discovered earlier, before the candidate drug reaches development, and any implications for product design and development considered in advance. • Pharmaceutical development’s involvement in the selection process and “buy-in” to the nomination decision can often enhance the team’s working relationship with their research colleagues. • The objective is to achieve a seamless transition from research to development, as opposed to the traditional “over-the-wall” approach that many pharmaceutical companies experience to their costs. • Earlier involvement by the Pharmaceutical Development group at the preclinical stage should also result in better planning for full development.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 3. Candidate Drug Selection • In spite of all these potential advantages of early pharmaceutical involvement to candidate drug selection, there may be several barriers within a company which can hinder this way of working. Distance between the Research group and the Development group should not really be considered a barrier, although this can be the case for groups on different continents with different cultures and languages. • The important factor for success seems to be the development of a formal mechanism for interaction, supported by senior management in the company. • This often takes the form of a joint project team with regular meetings to review progress. However, there may still be a lack of appreciation of what input or expertise pharmaceutical development can offer at the candidate drug selection stage. Opportunities to demonstrate what can be done and to educate Research colleagues should be sought to try and overcome this attitude.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 3. Candidate Drug Selection • Another potential barrier is any overlapping expertise there may be in Research and Development groups. For example, overlap may occur between Preformulation in Pharmaceutical Development and Physical Chemistry in Research, or between Biopharmaceutics in Development and Drug Metabolism in Research. In these cases, it is important to clarify and agree which group does what activity. • A common perceived barrier to providing early preformulation and biopharmaceutics input can be the quantity of compound required for evaluation at this stage. The Research group may believe that significantly more compound is required; with modern instrumental techniques, however, this is often not the case.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 3. Candidate Drug Selection • Other potential barriers which can influence the success of the relationship with Research at the candidate drug selection stage are the Pharmaceutical Development response time not being fast enough to support Research and the lack of resources that Pharmaceutical Development can give to support the candidate drug selection programme. • Several compounds may have to be evaluated simultaneously to generate comparative data to aid the selection process. Preformulation and Biopharmaceutics have to keep pace with the pharmacologica and safety testing, otherwise there is no point in generating the data. • One way of achieving this is to allocate dedicated resources to these projects using people trained to rapidly respond to the preformulation and biopharmaceutics requirements. • Simple formulations can be used at this stage, and rank order information is often acceptable, rather than definitive quantitative information. • Analytical methods should not require rigorous validation at this stage to provide these data. Excessive documentation and rigid standard operating procedures that can slow down the work are not usually necessary and should be avoided.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 4. Exploratory Development • The aim of exploratory development is to gauge how the candidate drug is absorbed and metabolized in healthy human volunteers before studying its effect on those actually suffering from the disease for which it is intended. Occasionally, it is necessary to conduct further smallscale studies in patients in order to make a decision whether to progress the candidate drug into full development. • This stage is often referred to as Phase I clinical studies or Concept Testing (Proof of Concept). Usually a small number of healthy volunteers (who do not have the condition under investigation or any other illness) receives the drug candidate provided as a simple formulation, which can be different from the intended commercial formulation. • For example, a simple aqueous oral solution or suspension may be used, rather than a capsule or tablet, to minimize the formulation development work at this early stage • If the candidate drug does not produce the expected effects in human studies, or produces unexpected and unwanted effects, the development programme is likely to be stopped at this stage.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 5. Full Development • Completion of longer-term safety and clinical studies (Phase II and Phase III) in patients suffering from the disease are accomplished at this stage. Phase II studies are dose ranging studies in a reasonable patient population (several hundred) to evaluate the effectiveness of the drug and common side-effects. • During Phase II, the intended commercial formulation should be developed, and the product/process optimized and eventually scaled up to commercial production scale. • The candidate drug should ideally be in the intended commercial formulation for the Phase III trials. After the satisfactory completion of Phase II trials, large patient populations (several hundred to thousands) are involved to statistically confirm efficacy and safety.

STAGES OF THE DRUG DISCOVERY AND DEVELOPMENT PROCESS 5. Full Development • Some patients will be given the drug, some a placebo product (required to be identical in appearance) and some may be given a known market leader (with all products appearing identical). • The doctors and patients in the study will not know whether the patients are getting the test drug, placebo or market leader; by switching the medication in a controlled way (doubleblind trials), objectivity and statistical assessment of the treatment under investigation are assured. • Most regulatory authorities, including the U. S. Food and Drugs Administration (FDA), the Medicines Control Agency (MCA) in the United Kingdom and the European Agency for the Evaluation of Medicinal Products (EMEA), require three phases of clinical trials and sufficient data to demonstrate that the new product can be licensed as safe, effective and of acceptable quality. • Once these clinical studies are complete, the company can decide whether it wishes to submit a marketing authorization application to a regulatory authority for a medicinal drug product. Approval is usually followed by product launch to market.

PACKAGING OF PHARMACEUTICALS • Packaging means a collection of different packaging materials which encase the pharmaceutical product from the time of manufacturing to the end of the user. encasing of drugs is important for life-saving drugs, medical devices, medical treatments, and new products like medical nutritionals. • Poultice, liquid, solid, powder, suspension it should be transparent to the user about its whole information on the drug different types of pharmaceutical packaging materials are present but it depends upon its function and type of the material used. • Finally packaging materials are evaluated for sterilization, storage and certain stability studies. Packaging protects the harmful drugs from children direct contact because different types of packaging are produced in to market, they can’t easily open the packaging. Packaging is an multiple user means provide presentation, protection, identification information, about a product during storage, carriage, display and until the product is consumed.

PACKAGING OF PHARMACEUTICALS The quality of the packaging of pharmaceutical products plays a very important role in the quality of such products. It must: � protect against all adverse external influences that can alter the properties of the product, e. g. moisture, light, oxygen and temperature variations; �protect against biological contamination; �protect against physical damage; � Carry the correct information and identification of the product.

PACKAGING OF PHARMACEUTICALS • A distinction must be made between primary and secondary packaging components. The primary packaging components (e. g. bottles, vials, closures, blisters) are in direct physical contact with the product, whereas the secondary components are not (e. g. aluminium caps, cardboard boxes). • The choice of primary and/or secondary packaging materials will depend on the degree of protection required, compatibility with the contents, the filling method and cost, but also the presentation for over-the-counter (OTC) drugs and the convenience of the packaging for the user (e. g. size, weight, method of opening/reclosing (if appropriate), legibility of printing). • Containers may be referred to as primary or secondary, depending on whether they are for immediate use after production of the finished product or not. Both single-dose and multi-dose containers exist. • Containers may be well-closed, tightly closed, hermetically closed or light-resistant, airtight. [

PACKAGING OF PHARMACEUTICALS Functions of packaging: Containment: • The containment of the product is the most fundamental function of packaging for medicinal products. The design of high-quality packaging must take into account both the needs of the product and of the manufacturing and distribution system. This requires the packaging: • Not to leak, nor allow diffusion and permeation of the product; • To be strong enough to hold the contents when subjected to normal handling. • Not to be altered by the ingredients of the formulation in its final dosage form. • Protection

PACKAGING OF PHARMACEUTICALS Functions of packaging: Containment: • The packaging must protect the product against all adverse external influences that may affect its quality or potency, such as: • �light • �moisture • �oxygen • �biological contamination • �Mechanical damage

PACKAGING OF PHARMACEUTICALS Functions of packaging: Stability: • Information on stability is given in the guidelines for stability testing of pharmaceutical products containing well established drug substances in conventional dosage forms. • For primary packaging, it is necessary to know the possible interactions between the container and the contents. • Normally, product/component stability and compatibility are confirmed during the primary research and development stage.

PACKAGING OF PHARMACEUTICALS Functions of packaging: Stability: • There are numerous possibilities of interactions between (primary) packaging materials and pharmaceutical products, such as: - The release of chemicals from components of the packaging materials; - The release of visible and/or sub visible particles; - The absorption or adsorption of pharmaceutical components by the packaging materials; - Chemical reactions between the pharmaceutical product and the packaging materials; - The degradation of packaging components in contact with the pharmaceutical products; - The influence of the manufacturing process (e. g. sterilization) on the container.

PACKAGING OF PHARMACEUTICALS Functions of packaging: Labels: Throughout manufacturing, a succession of specific outer labels is applied to the container of the medicinal product. The level of processing is indicated by the following words: • �Quarantine • �Storage • �Distribution. • Specifications for labels for finished drug products are defined in the WHO guidelines on GMP for pharmaceutical products. - Written labels on the packaging: • � Permit the identification of each active ingredient by means of its INN, and also give the dosage form and the trade name/trademark. All information concerning the medicinal product, as required by national legislation, must be stated on the packaging. • �Preserve the stability of the medicinal product by giving advice on its storage

PACKAGING OF PHARMACEUTICALS Functions of packaging: Labels: After the stability of the product has been evaluated, one of the following recommendations as to storage conditions can be prominently indicated on the label: • Store under normal storage conditions; • Store between 2 and 8 °C (under refrigeration, no • freezing); • Store below 8 °C (under refrigeration); • Store between -5 and -20 °C (in a freezer); • Store below -18 °C (in a deep freezer).

PACKAGING OF PHARMACEUTICALS Functions of packaging: Repacking, relabeling and dispensing: In some countries, it is common practice not to dispense drugs in the original packaging, but rather in a personalized manner to each patient. This applies especially to solid oral dosage forms, and involves the “repacking” and “relabeling” of drugs in small quantities. Different drugs may even be included in “customized” medication packages, also referred to as “patient med packs”. The quantities of drugs supplied in this way are usually enough only for a short period of time, i. e. to provide drugs for immediate use. It should be remembered, however, that data obtained in stability studies undertaken by the manufacturer are no longer valid for drugs removed from the original package.

PACKAGING OF PHARMACEUTICALS Functions of packaging: Package inserts for patients (patient information leaflets): • Product information must help patients and other users to understand the medication. The patient package insert, together with the label, provides the patient with key information concerning the proper use of the product, potential adverse drug reactions and interactions, storage conditions and the expiry date. • In OTC medicinal products, the package insert, together with the label, may constitute the only pharmaceutical advice that the patient receives.

PACKAGING OF PHARMACEUTICALS Functions of packaging: • Compliance: • Packaging and labeling may help to reinforce the instructions given by the physician or the pharmacist, and improve compliance with drug therapy. In this respect, packaging becomes a compliance aid. • The design of pharmaceutical packaging should be such that the product can easily be administered in a safe manner to the patient. If the patient feels at ease with the packaging and route of administration, the design of the packaging may become a key factor in increasing compliance. This is also an important factor in clinical trials.

PACKAGING OF PHARMACEUTICALS Functions of packaging: Protection of patients: • Packaging must not only increase compliance through its design, but must also protect the patient and indicate the integrity of the product. • Packaging equipped with a tamperevident device protects against incidental and accidental poisoning. To protect children, several child-resistant closures have been developed

PACKAGING OF PHARMACEUTICALS Packaging materials and closures Glass: • For a large number of pharmaceuticals, including medicinal products for oral and local administration, glass containers are usually the first choice (e. g. bottles for tablets, injection syringes for unit- or multi dose administration. • Classifications of types of glass are given in the European and United States pharmacopoeias, whereas no such classification exists in the Japanese pharmacopoeia. • Glass can be tested for light transmission and hydrolytic resistance. In the Japanese pharmacopoeia, such tests are described only for glass containers for injection, whereas in the European and United States pharmacopoeias they are given for all types of glass containers.

PACKAGING OF PHARMACEUTICALS Packaging materials and closures Plastics: • Some containers are now being made of plastics; • the main use is for bags for parenteral solutions. • Plastic containers have several advantages compared with glass containers unbreakable, collapsible, light resistant. Metal: • Metal containers are used solely for medicinal products for non parenteral administration. They include tubes, packs made from foil or blisters, cans, and aerosol and gas cylinders. • Aluminum and stainless steel are the metals of choice for both primary and secondary packaging for medicinal products. They have certain advantages and provide excellent tamper-evident containers. Metal is strong, impermeable to gases and shatter proof, it is the ideal packaging material for pressurized containers.

PACKAGING OF PHARMACEUTICALS Packaging materials and closures • Closures used for the purpose of covering drug containers after the filling process should be as inert as possible. They should not give rise to undesired interactions between the contents and the outside environment, and should provide a complete seal. • Besides their protective function, closures must also allow the easy and safe administration of the drug. • Depending on the application, closures may have to be pierced with a needle for intravenous sets. Such closures are made from elastomeric materials (rubbers), while those that cannot be pierced are generally made from plastics such as polyethylene or polypropylene. • Depending on the type of container, closures may have different shapes and sizes, e. g. stoppers for infusion or injection bottles or plungers for prefilled syringes. A special design of stopper may also be required for some pharmaceutical production processes such as lyophilization. • Closures, as primary packaging components, are of critical importance and must be carefully selected.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Solid dosage forms: Tamper resistant packaging: • The requirement for tamper resistant packaging is now one of the major consideration in the development of packaging for pharmaceutical products. Tamper evident containers are closed containers fitted with a device that irreversibly indicates if the container has been opened. • The following package configuration have been identified by the FDA as examples of packaging systems that are capable of meeting the requirements of tamper resistant packaging as defined by FDA regulation • �Film wrappers • �Blister package • �Strip package • �Bubble pack • �Shrink seal and bands • �Foil paper or plastic pouches • �Bottle seals • �Tape seals • �Breakable caps • �Sealed tubes

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Solid dosage forms: Strip packages: • A strip package is a form of unit dose packaging that is commonly used for the packaging of tablets and capsules. A strip package is formed by feeding two webs of a heatsealable flexible film through either a heated crimping roller or a heated reciprocating plate. The product is dropped into the pocket formed prior to forming the final set of seals. A continuous strip of packets is formed, generally several packets wide depending on the packaging machine's limitations. The strip of packets is cut to the desired number of packets in length. • The strips formed are usually collated and packaged into a folding carton. The product sealed between the two sheets of film usually has a seal around each tablet, with perforations usually separating adjacent packets. The seals can be in a simple rectangular or "picture-frame" format or can be contoured to the shape of the product. Different packaging materials are used for strip packaging based on their properties for high-barrier applications a paper/polyethylene/foil/polyethylene lamination is commonly used.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Solid dosage forms: Blister packages • When one thinks of unit dose in pharmaceutical packaging, the package that invariably comes to mind is the blister package. This packaging mode has been used extensively for pharmaceutical packaging for several good reasons. It is a packaging configuration capable of providing excellent environmental protection, coupled with an esthetically pleasing and efficacious appearance. It also provides user functionality in terms of convenience, child resistance, and no, tamperresistance. • The blister package is formed by heat-softening a sheet of thermoplastic resin and vacuum-drawing the softened sheet of plastic into a contoured mold. After cooling, the sheet is released from the mold and proceeds to the filling station of the packaging machine.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Containers for liquids Parentrals Injectable formulations are packaged in to containers made of plastic or glass. Container system includes ampoules, syringes, vials, bottles, cartridges, bags ampoules are all glass, and plastic are all bags. Rubber materials for rubber stoppers for vials and bottles, rubber plungers and rubber seals for syringes, cartridges. Irrigation solutions are packaged in glass bottles with aluminum screwcaps. A single-dose container is one that holds a quantity of drug intended as a single dose and when opened cannot be resealed with assurance that sterility has been maintained. These containers include fusion-sealed ampoules and prefilled syringes and cartridges

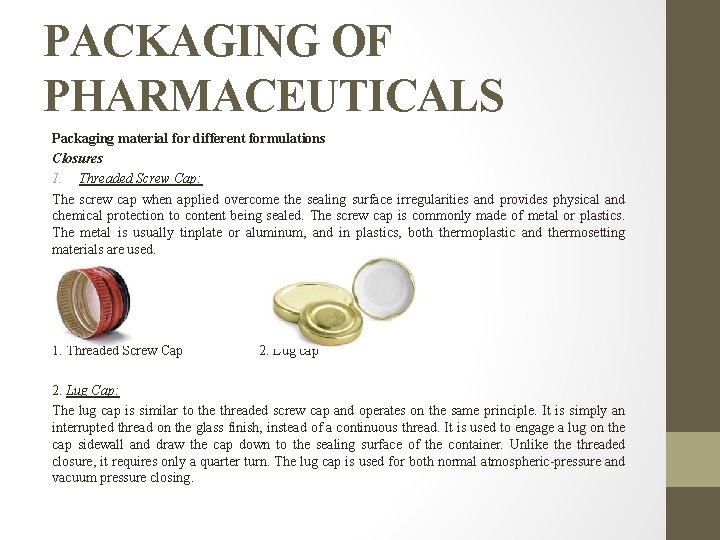
PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Closures The closure is normally the most vulnerable and critical component of a container in so far as stability and compatibility with the product are concerned. An effective closure must prevent the contents from escaping and allow no substance to enter the container. Function of a closure: �Provide a totally hermetic seal. �Provide an effective seal which is acceptable to the products. �Provide an effective microbiological seal. Types of closures Closures are available in five basic designs �Threaded screw cap �Lug cap �Crimp-on (crowns) �pilfer proof closure �Roll-on Many variations of these basic types exist, including vacuum, tamperproof, safety, child resistant, and liner less types, and dispenser applicators.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Closures 1. Threaded Screw Cap: The screw cap when applied overcome the sealing surface irregularities and provides physical and chemical protection to content being sealed. The screw cap is commonly made of metal or plastics. The metal is usually tinplate or aluminum, and in plastics, both thermoplastic and thermosetting materials are used. 1. Threaded Screw Cap 2. Lug cap 2. Lug Cap: The lug cap is similar to the threaded screw cap and operates on the same principle. It is simply an interrupted thread on the glass finish, instead of a continuous thread. It is used to engage a lug on the cap sidewall and draw the cap down to the sealing surface of the container. Unlike threaded closure, it requires only a quarter turn. The lug cap is used for both normal atmospheric-pressure and vacuum pressure closing.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Closures 3. Crown Caps: This style of cap is commonly used as a crimped closure for beverage bottles and has remained essentially unchanged for more than 50 years. 4. Roll-On Closures: The aluminum roll-on cap can be sealed securely, opened easily, and resealed effectively. It finds wide application in the packaging of food, beverages, chemicals, and pharmaceuticals. The roll-on closure requires a material that is easy to form, such as aluminum or other light-gauge metal. Re sealable, non re sealable, and pilfer proof types of the roll-on closure available for use on glass or plastic bottles and jars.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Closures 5. Pilfer proof Closures The pilfer proof closure is similar to the standard roll-on closure except that it has a greater skirt length. This additional length extends below the threaded portion to form a bank, which is fastened to the basic cap by a series of narrow metal "bridges. " When the pilfer proof closure is removed, the bridges break, and the bank remains in place on the neck of the container. The closure can be re sealed easily and the detached band indicates that the package has been opened. The torque is necessary to remove the cap.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Packaging for liposomes 1. Hermetically sealed borosilicate glass ampoules Provide a secure environment for lipids sensitive to oxidation. The ampules are shipped in cardboard liners for protection and storage convenience. Ampules are pre-scored for easier opening. Once the seal is broken, sample may be transferred to a Screw Cap Storage Vial. 2. Narrow-mouth borosilicate glass bottles Convenient for shipping and storage of larger volumes of lipids. The closure system is composed of a closed-top screw cap with a teflon liner fused to a silicone rubber backing. The liner is sonically welded to the cap, therefore no glue can come in contact with the organic solution.

PACKAGING OF PHARMACEUTICALS Packaging material for different formulations Packaging for liposomes 3. Wide-mouth borosilicate glass bottles Convenient for shipping and storage of dry powder lipids. Larger openings provide easier access to lipid samples. The closure system is composed of a closed-top screw cap with a teflon liner. 4. The Screw Cap Storage Vial Designed as a storage option for materials shipped in glass ampules or bottles. The closure system contains an open-top screw cap with a teflon liner fused to a silicone rubber septum.
 Brc standard 8
Brc standard 8 Packaging and packaging waste directive
Packaging and packaging waste directive Why problem formulation follow goal formulation
Why problem formulation follow goal formulation Nature reviews drug discovery
Nature reviews drug discovery Qsar notes
Qsar notes Chapter 37 discipline chart
Chapter 37 discipline chart Define adulteration of crude drugs
Define adulteration of crude drugs Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan