Formulas of Molecular Compounds Many compoundsmade up of

- Slides: 9

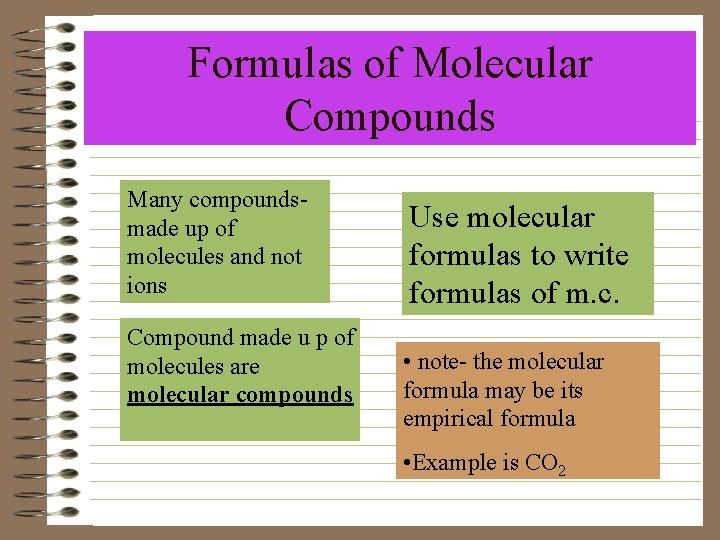
Formulas of Molecular Compounds Many compoundsmade up of molecules and not ions Compound made u p of molecules are molecular compounds Use molecular formulas to write formulas of m. c. • note- the molecular formula may be its empirical formula • Example is CO 2

UNLIKE IONIC COMPOUNDS, THERE IS NO SIMPLE WAY TO PREDICT AND WRITE FORMULAS FOR MOLECULAR COMPOUNDS!!! • The same elements can form a number of different compounds, which have different formulas Nitrogen and Oxygen can form 5 different compounds 1. N 2 O - dinitrogen monoxide 2. NO- nitrogen monoxide 3. N 2 O 3 - dinitrogen trioxide 4. NO 2 - nitrogen dioxide 5. N 2 O 5 - dinitrogen pentoxide

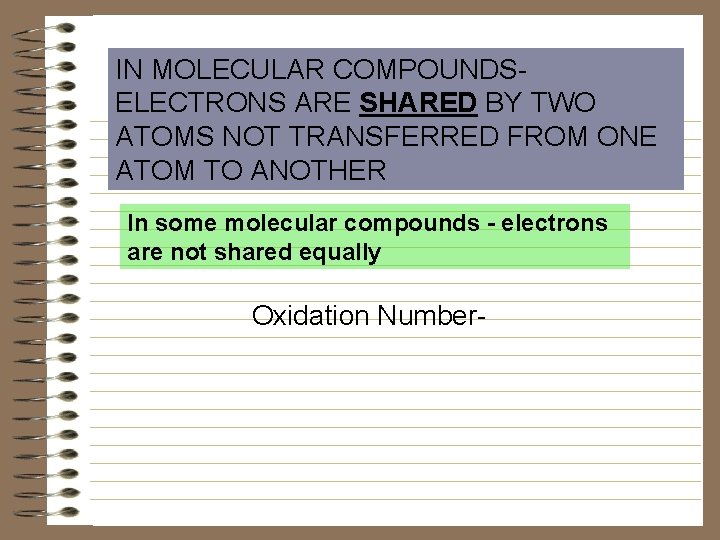
IN MOLECULAR COMPOUNDSELECTRONS ARE SHARED BY TWO ATOMS NOT TRANSFERRED FROM ONE ATOM TO ANOTHER In some molecular compounds - electrons are not shared equally Oxidation Number-

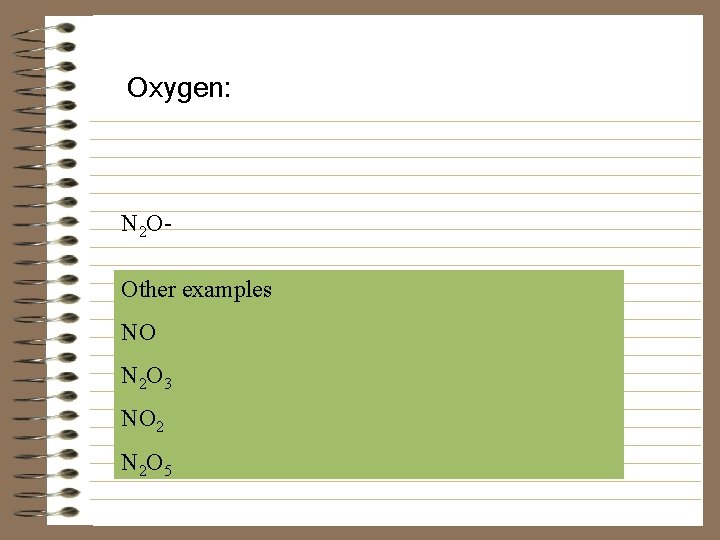
Oxygen: N 2 OOther examples NO N 2 O 3 NO 2 N 2 O 5

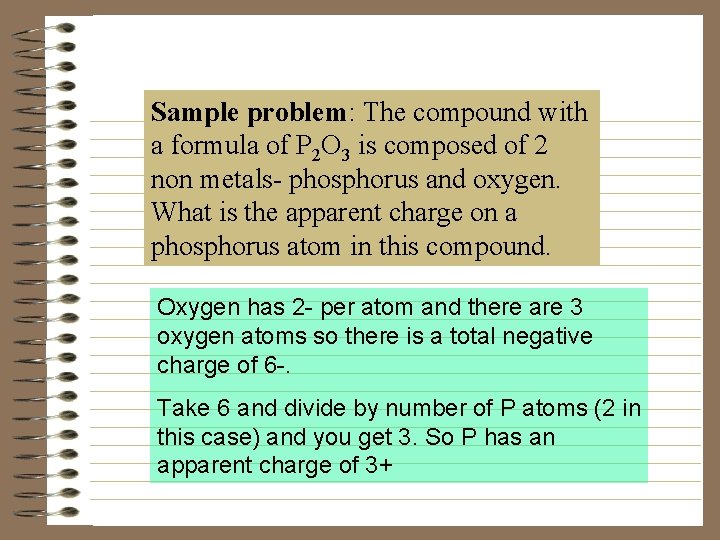
Sample problem: The compound with a formula of P 2 O 3 is composed of 2 non metals- phosphorus and oxygen. What is the apparent charge on a phosphorus atom in this compound. Oxygen has 2 - per atom and there are 3 oxygen atoms so there is a total negative charge of 6 -. Take 6 and divide by number of P atoms (2 in this case) and you get 3. So P has an apparent charge of 3+

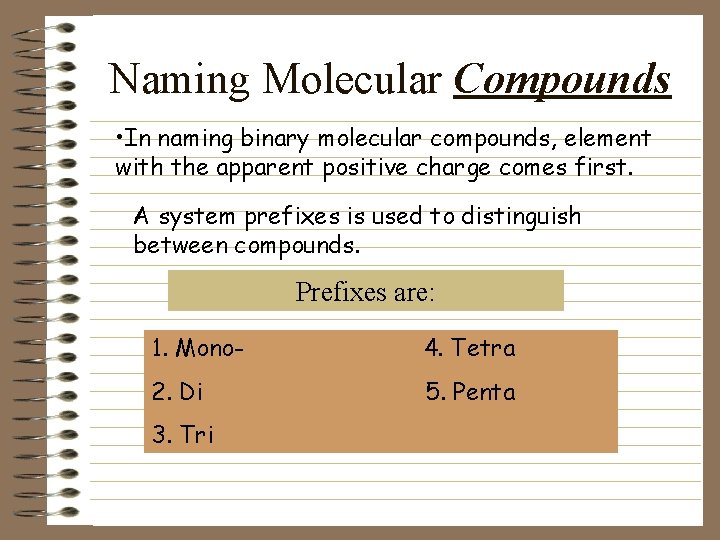
Naming Molecular Compounds • In naming binary molecular compounds, element with the apparent positive charge comes first. A system prefixes is used to distinguish between compounds. Prefixes are: 1. Mono- 4. Tetra 2. Di 5. Penta 3. Tri

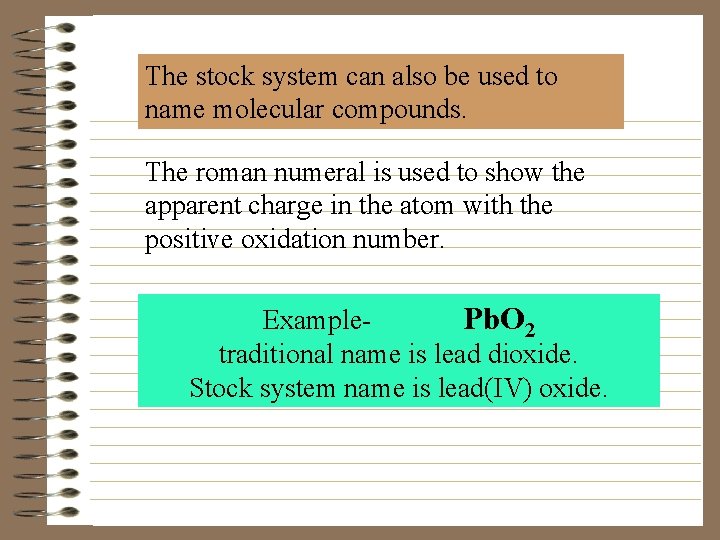
The stock system can also be used to name molecular compounds. The roman numeral is used to show the apparent charge in the atom with the positive oxidation number. Example. Pb. O 2 traditional name is lead dioxide. Stock system name is lead(IV) oxide.

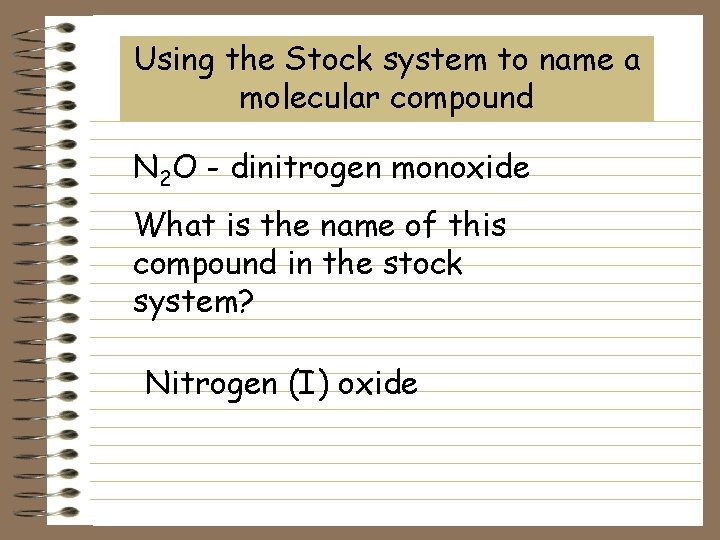
Using the Stock system to name a molecular compound N 2 O - dinitrogen monoxide What is the name of this compound in the stock system? Nitrogen (I) oxide

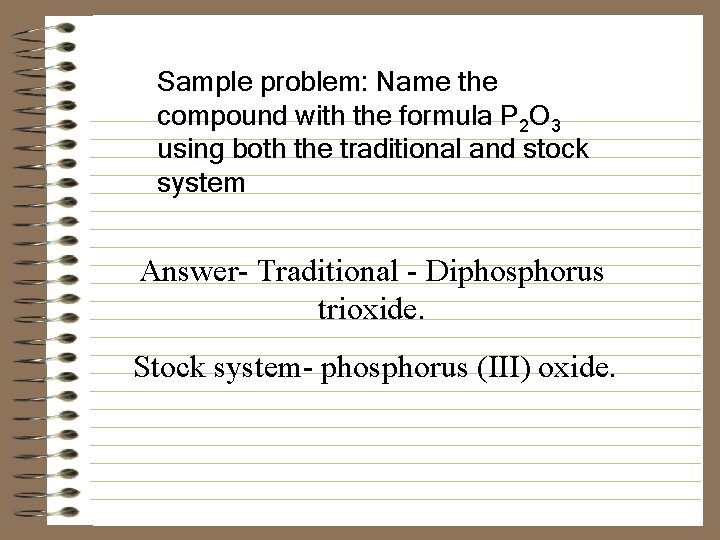
Sample problem: Name the compound with the formula P 2 O 3 using both the traditional and stock system Answer- Traditional - Diphosphorus trioxide. Stock system- phosphorus (III) oxide.