Formula Weight Molecular Weight The FORMULA WEIGHT of







- Slides: 21

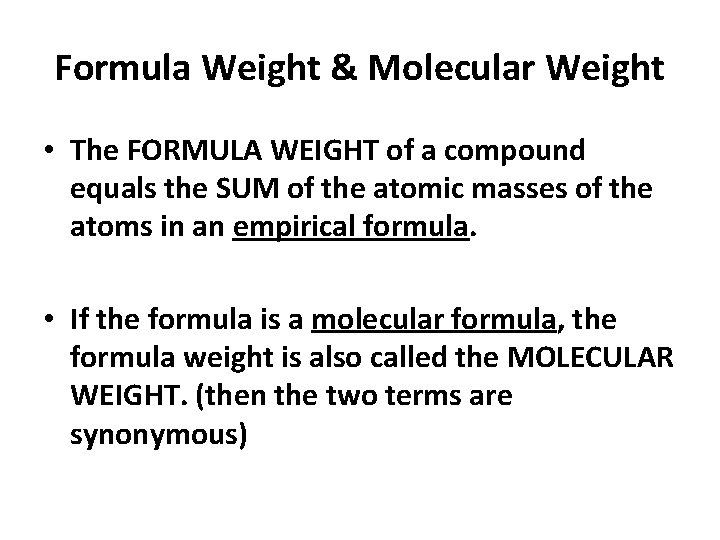
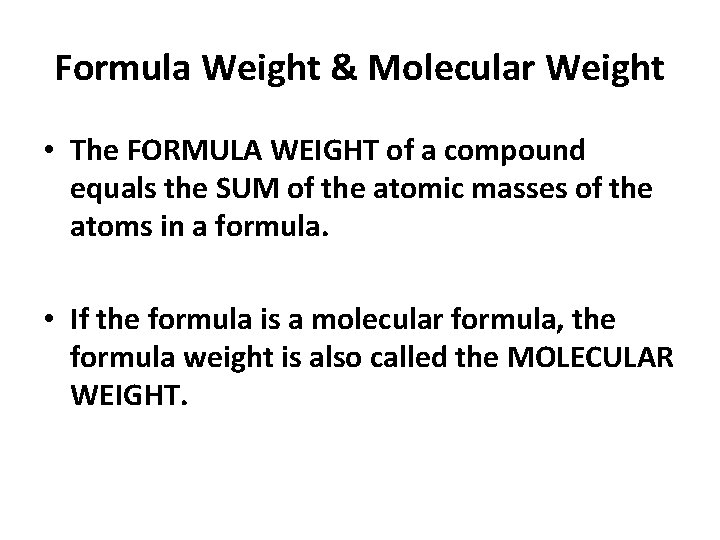
Formula Weight & Molecular Weight • The FORMULA WEIGHT of a compound equals the SUM of the atomic masses of the atoms in an empirical formula. • If the formula is a molecular formula, the formula weight is also called the MOLECULAR WEIGHT. (then the two terms are synonymous)

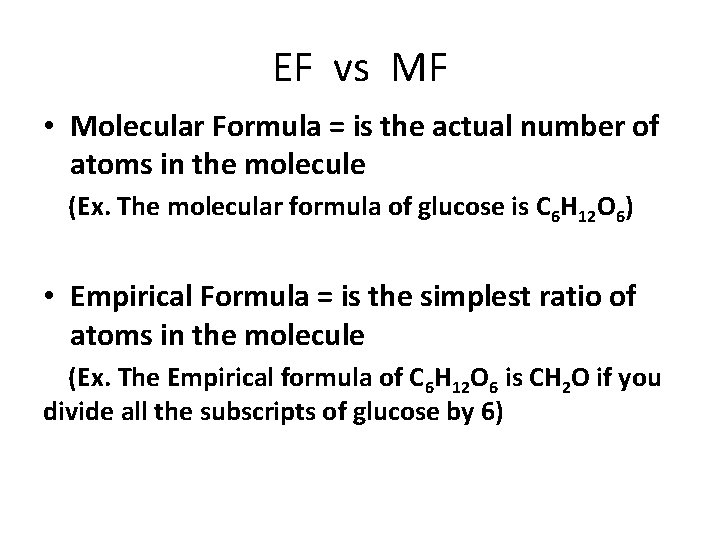
EF vs MF • Molecular Formula = is the actual number of atoms in the molecule (Ex. The molecular formula of glucose is C 6 H 12 O 6) • Empirical Formula = is the simplest ratio of atoms in the molecule (Ex. The Empirical formula of C 6 H 12 O 6 is CH 2 O if you divide all the subscripts of glucose by 6)

The MOLE • Amount of substance that contains an Avogadro’s number (6. 02 x 10 23)of formula units.

The MOLE • The mass of 1 mole of atoms, molecules or ions = the formula weight of that element or compound in grams. Ex. Mass of 1 mole of water is 18 grams so molar mass of water is 18 grams/mole.

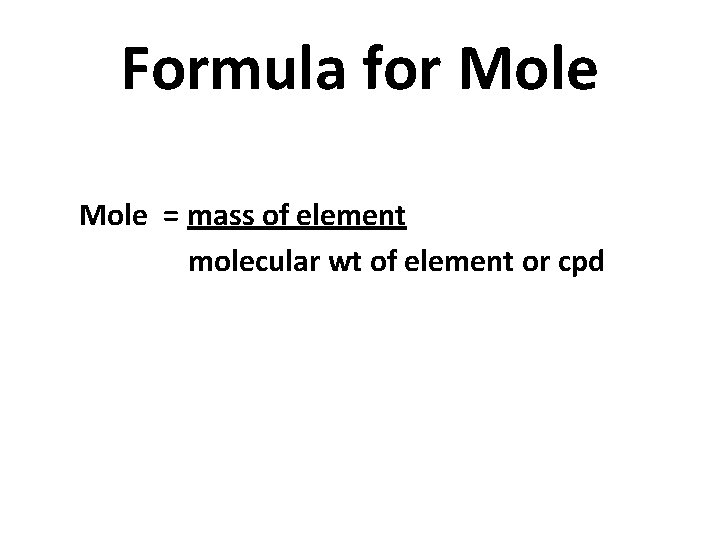
Formula for Mole = mass of element molecular wt of element or cpd

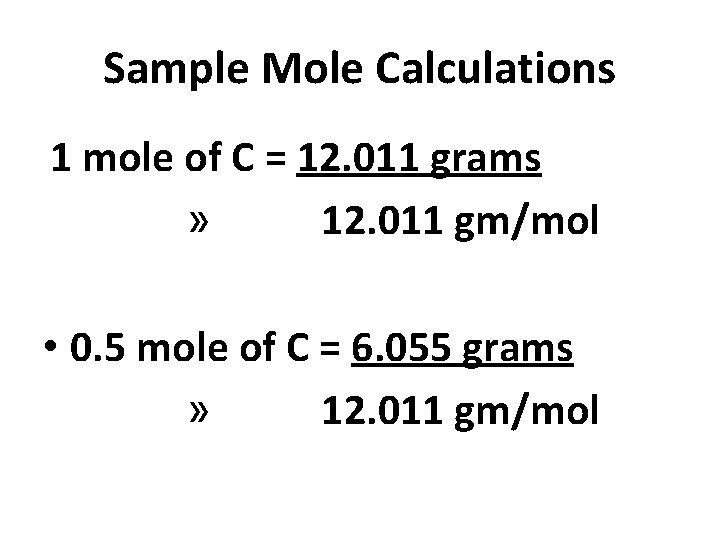
Sample Mole Calculations 1 mole of C = 12. 011 grams » 12. 011 gm/mol • 0. 5 mole of C = 6. 055 grams » 12. 011 gm/mol

Avogadro’s Number • Way of counting atoms • Avogadro’s number 23 = 6. 022 x 10

Point to Remember One mole of anything is: 6. 022 x 1023 units of that substance.

And……. . • 1 mole of C has the same number of atoms as one mole of any element

Formula Weight & Molecular Weight • The FORMULA WEIGHT of a compound equals the SUM of the atomic masses of the atoms in a formula. • If the formula is a molecular formula, the formula weight is also called the MOLECULAR WEIGHT.

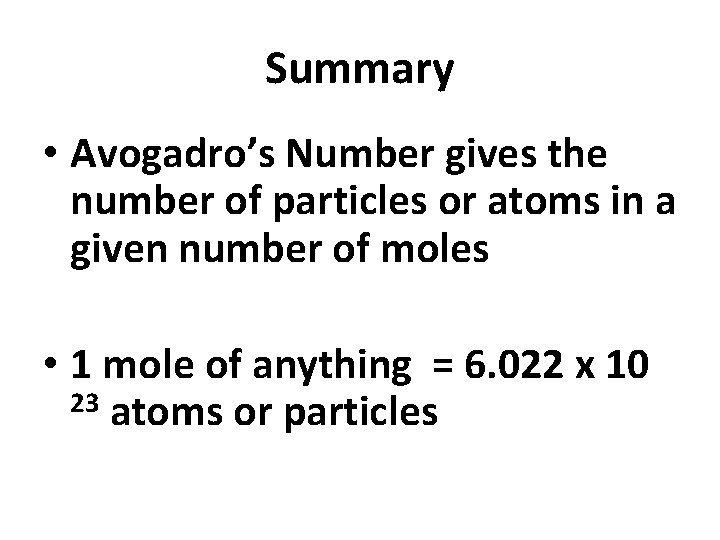
Summary • Avogadro’s Number gives the number of particles or atoms in a given number of moles • 1 mole of anything = 6. 022 x 10 23 atoms or particles

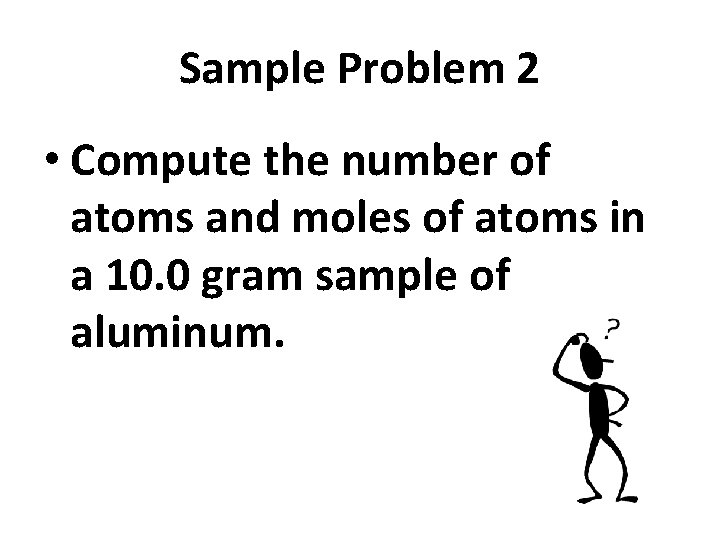
Sample Problem 2 • Compute the number of atoms and moles of atoms in a 10. 0 gram sample of aluminum.

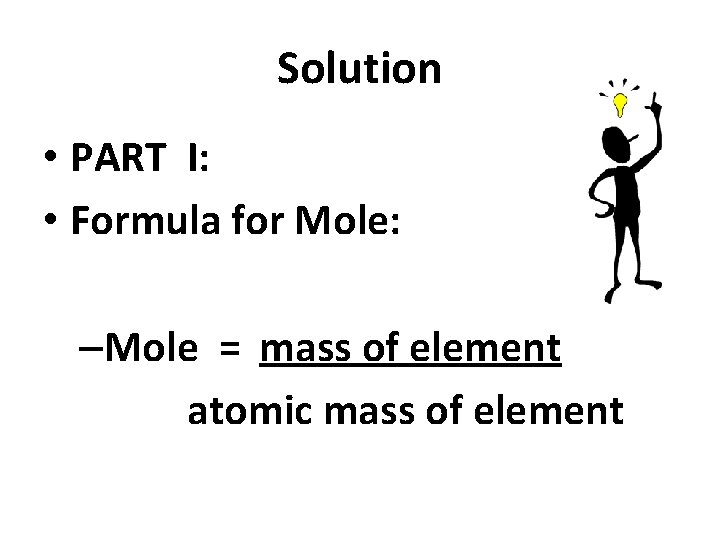
Solution • PART I: • Formula for Mole: –Mole = mass of element atomic mass of element

Solution (cont. ) • Part II: atoms To determine # of • # atoms = moles x Avogadro’s number

Problem # 2 • A diamond contains 5. 0 x 1021 atoms of carbon. How many moles of carbon and how many grams of carbon are in this diamond?

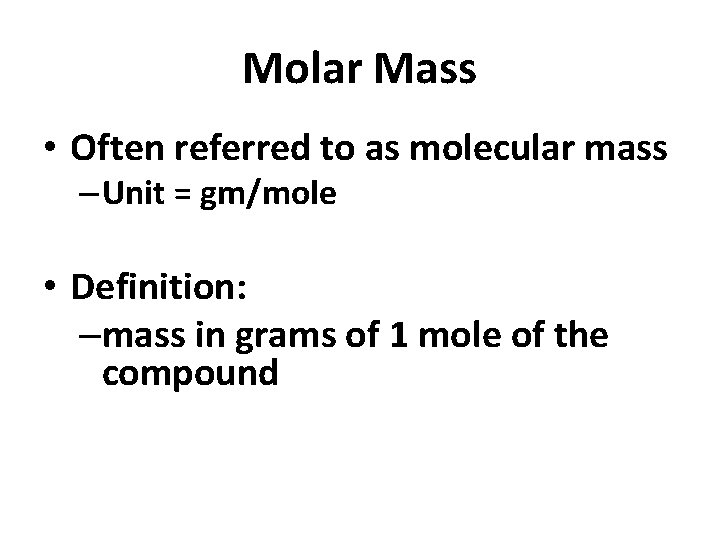
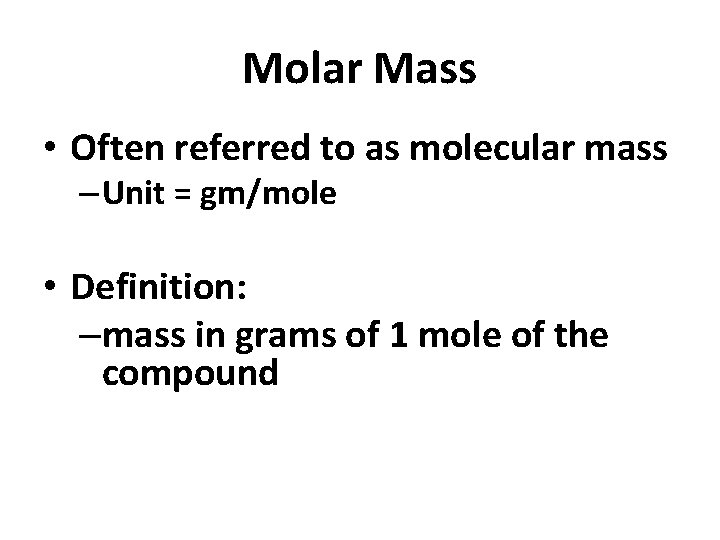
Molar Mass • Often referred to as molecular mass – Unit = gm/mole • Definition: –mass in grams of 1 mole of the compound

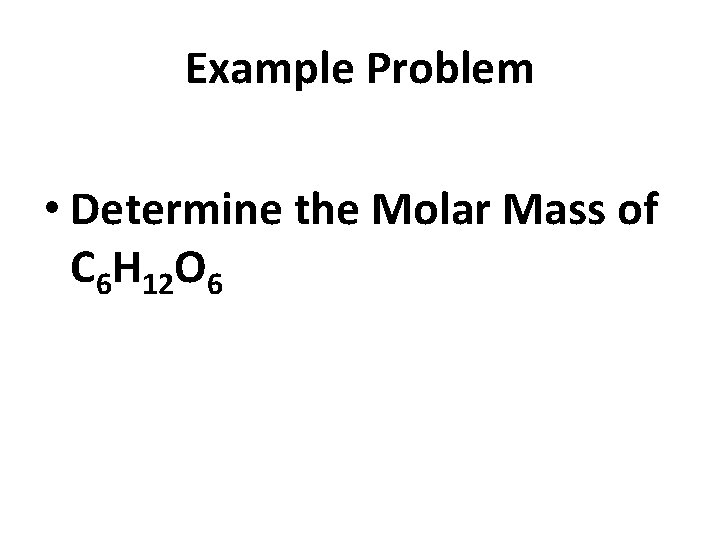
Example Problem • Determine the Molar Mass of C 6 H 12 O 6

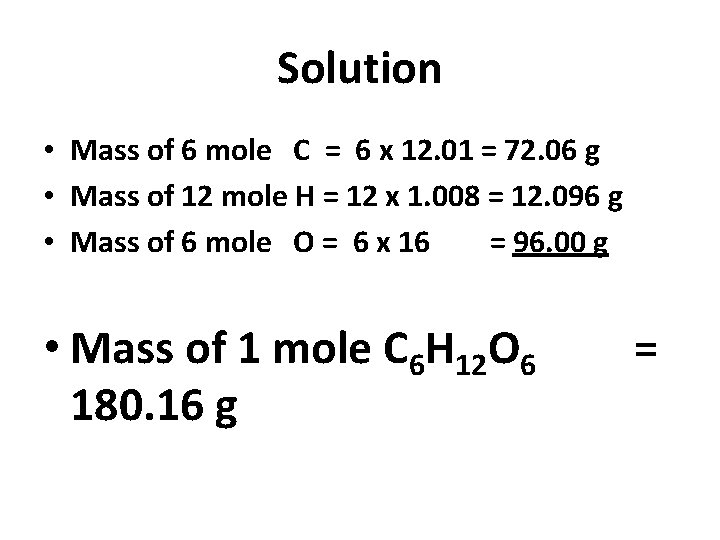
Solution • Mass of 6 mole C = 6 x 12. 01 = 72. 06 g • Mass of 12 mole H = 12 x 1. 008 = 12. 096 g • Mass of 6 mole O = 6 x 16 = 96. 00 g • Mass of 1 mole C 6 H 12 O 6 180. 16 g =

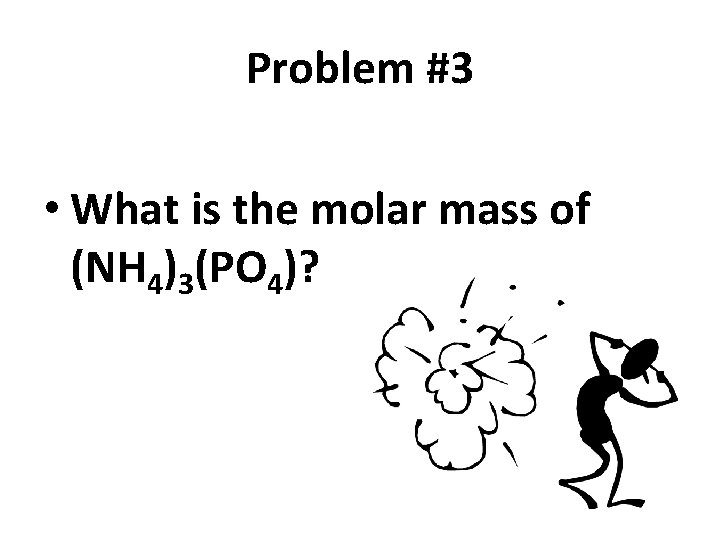
Problem #3 • What is the molar mass of (NH 4)3(PO 4)?

Molar Mass • Often referred to as molecular mass – Unit = gm/mole • Definition: –mass in grams of 1 mole of the compound

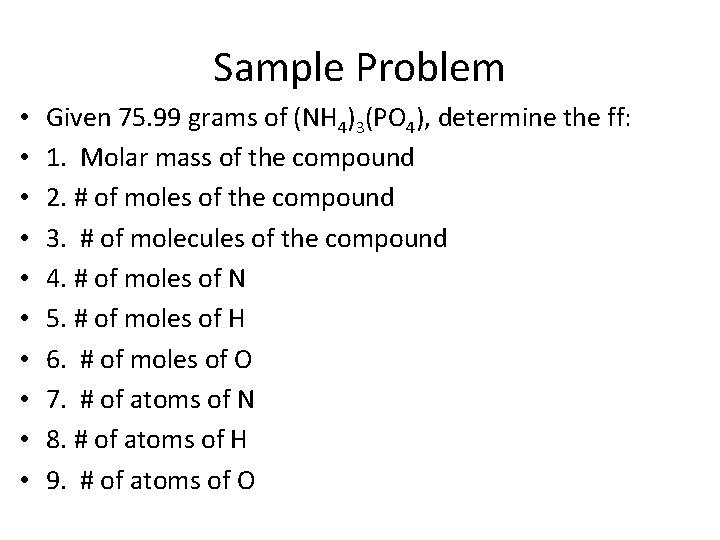
Sample Problem • • • Given 75. 99 grams of (NH 4)3(PO 4), determine the ff: 1. Molar mass of the compound 2. # of moles of the compound 3. # of molecules of the compound 4. # of moles of N 5. # of moles of H 6. # of moles of O 7. # of atoms of N 8. # of atoms of H 9. # of atoms of O