Formula of an Ionic Compound with a Multivalent
- Slides: 16

Formula of an Ionic Compound with a Multivalent Metal • Some transitional metals are multivalent, meaning they have more than one ion form. w On the periodic table, the most common form of the ion is listed on top. See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

Formula of an Ionic Compound with a Multivalent Metal • Some transitional metals are multivalent, meaning they have more than one ion form. w On the periodic table, the most common form of the ion is listed on top. w In the name of the compound, Roman numerals are used following the positive ion to indicate which ion was used. See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

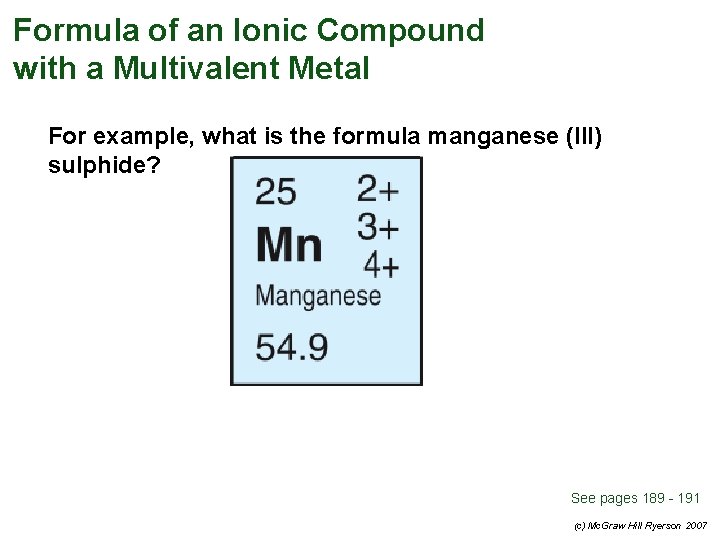
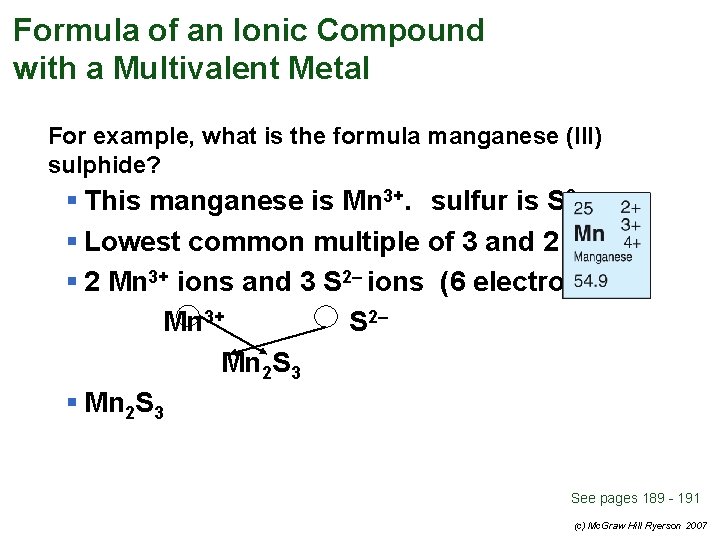
Formula of an Ionic Compound with a Multivalent Metal For example, what is the formula manganese (III) sulphide? See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

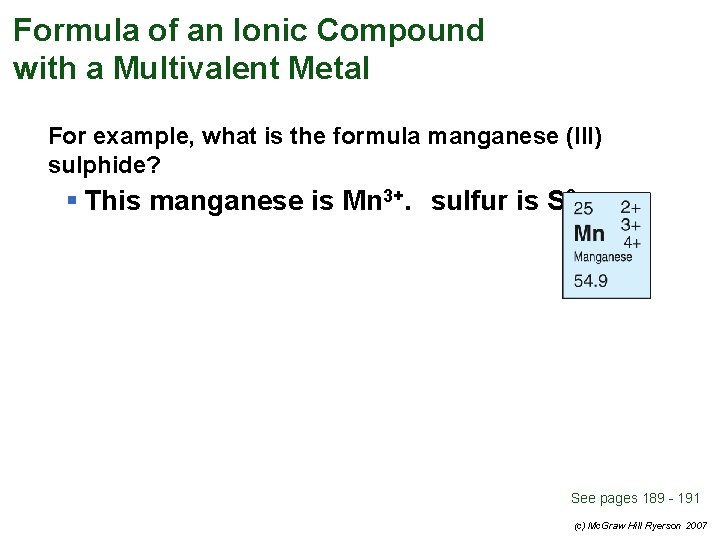
Formula of an Ionic Compound with a Multivalent Metal For example, what is the formula manganese (III) sulphide? § This manganese is Mn 3+. sulfur is S 2– See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

Formula of an Ionic Compound with a Multivalent Metal For example, what is the formula manganese (III) sulphide? § This manganese is Mn 3+. sulfur is S 2– § Lowest common multiple of 3 and 2 is 6 § 2 Mn 3+ ions and 3 S 2– ions (6 electrons) Mn 3+ S 2– Mn 2 S 3 § Mn 2 S 3 See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

Formula of an Ionic Compound with a Multivalent Metal w What would the name be for Ti. F 4 See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

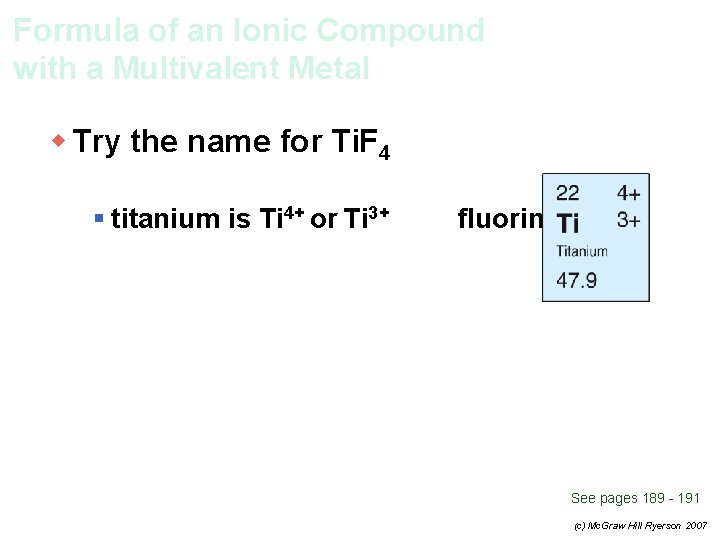
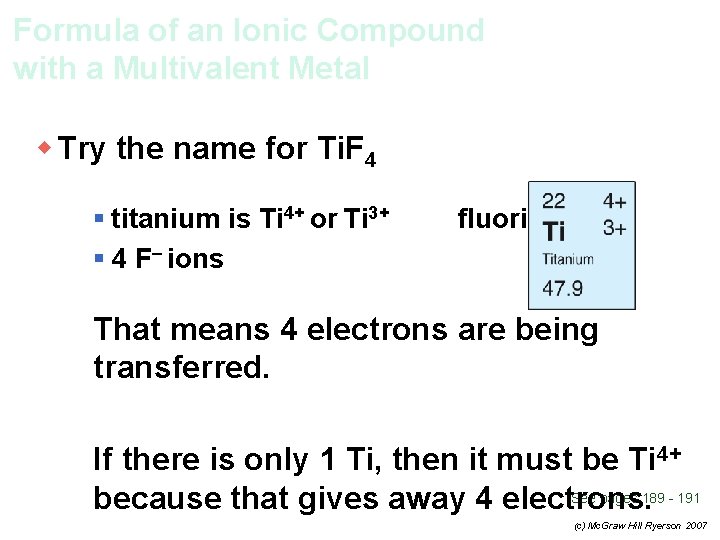
Formula of an Ionic Compound with a Multivalent Metal w Try the name for Ti. F 4 § titanium is Ti 4+ or Ti 3+ fluorine is F– See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

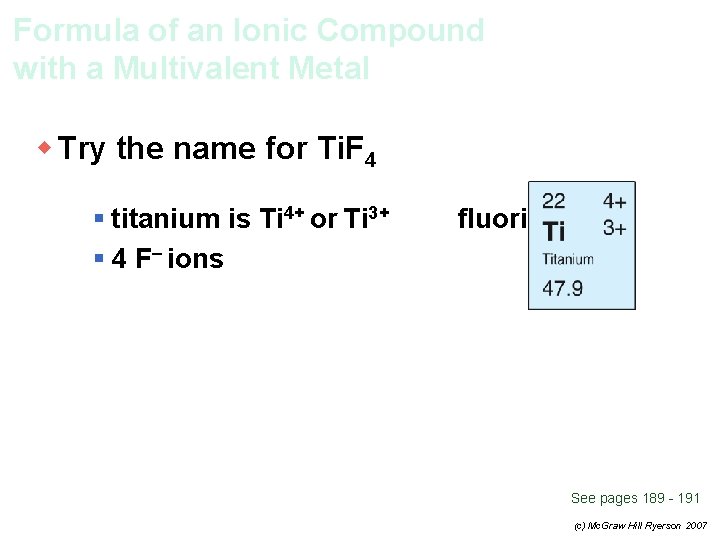
Formula of an Ionic Compound with a Multivalent Metal w Try the name for Ti. F 4 § titanium is Ti 4+ or Ti 3+ § 4 F– ions fluorine is F– See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

Formula of an Ionic Compound with a Multivalent Metal w Try the name for Ti. F 4 § titanium is Ti 4+ or Ti 3+ § 4 F– ions fluorine is F– That means 4 electrons are being transferred. If there is only 1 Ti, then it must be Ti 4+ See pages 189 - 191 because that gives away 4 electrons. (c) Mc. Graw Hill Ryerson 2007

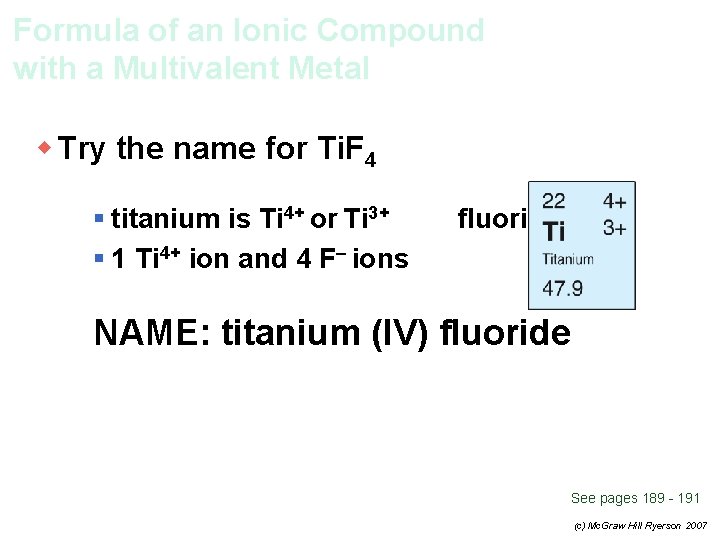
Formula of an Ionic Compound with a Multivalent Metal w Try the name for Ti. F 4 § titanium is Ti 4+ or Ti 3+ § 1 Ti 4+ ion and 4 F– ions fluorine is F– NAME: titanium (IV) fluoride See pages 189 - 191 (c) Mc. Graw Hill Ryerson 2007

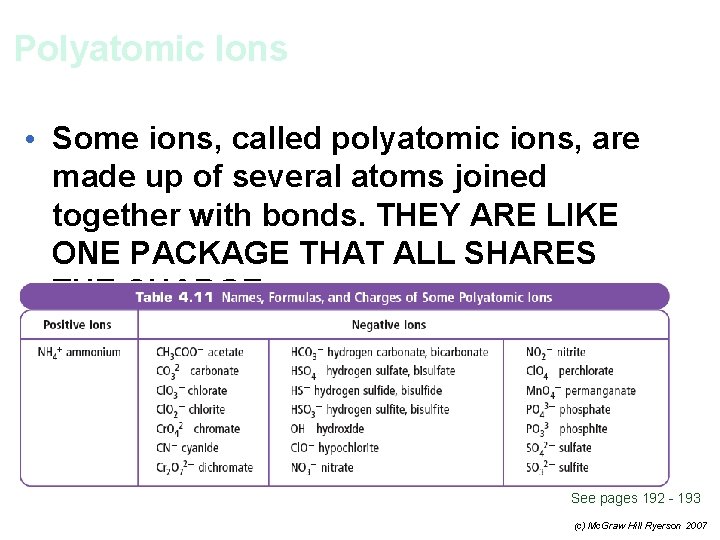
Polyatomic Ions • Some ions, called polyatomic ions, are made up of several atoms joined together with bonds. THEY ARE LIKE ONE PACKAGE THAT ALL SHARES THE CHARGE. See pages 192 - 193 (c) Mc. Graw Hill Ryerson 2007

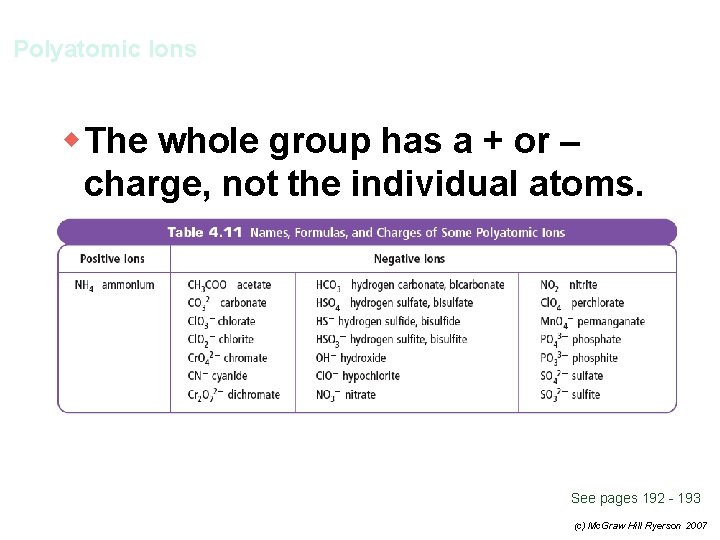
Polyatomic Ions w The whole group has a + or – charge, not the individual atoms. See pages 192 - 193 (c) Mc. Graw Hill Ryerson 2007

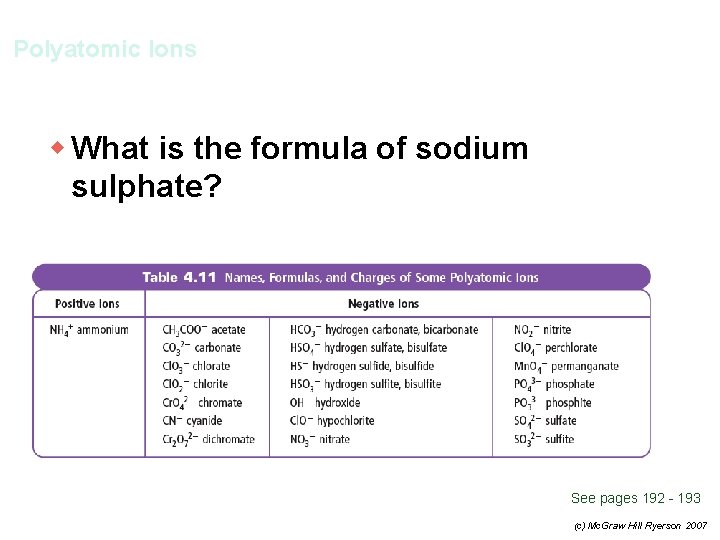
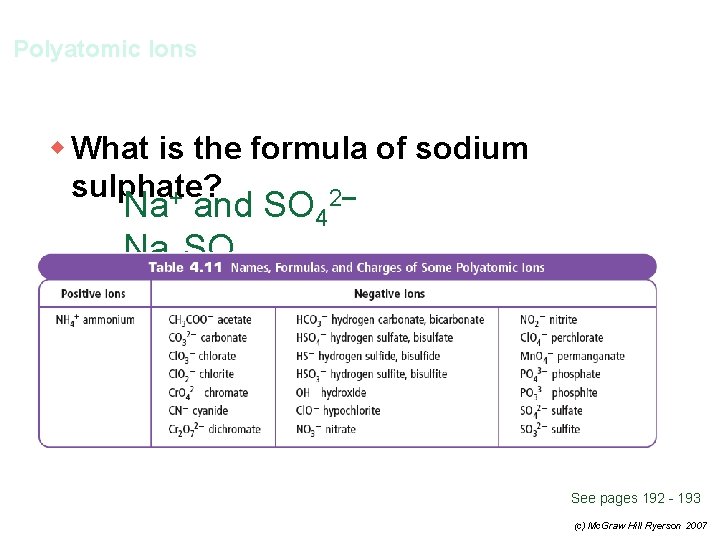
Polyatomic Ions w What is the formula of sodium sulphate? See pages 192 - 193 (c) Mc. Graw Hill Ryerson 2007

Polyatomic Ions w What is the formula of sodium sulphate? + 2– Na and SO 4 Na 2 SO 4 See pages 192 - 193 (c) Mc. Graw Hill Ryerson 2007

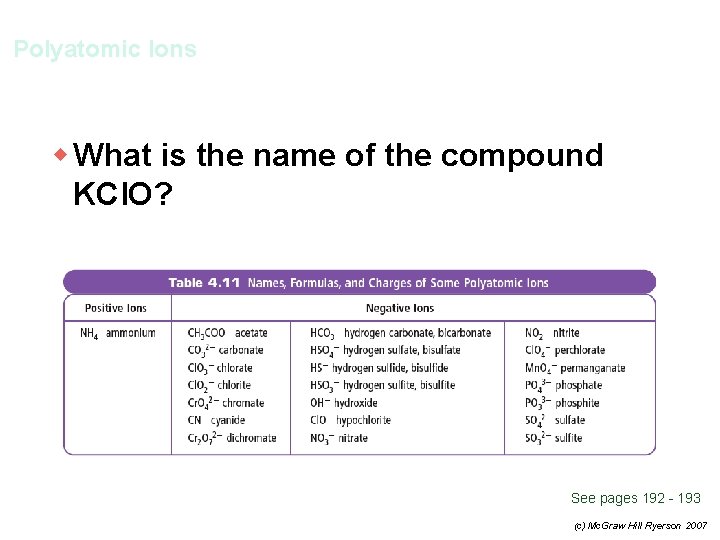
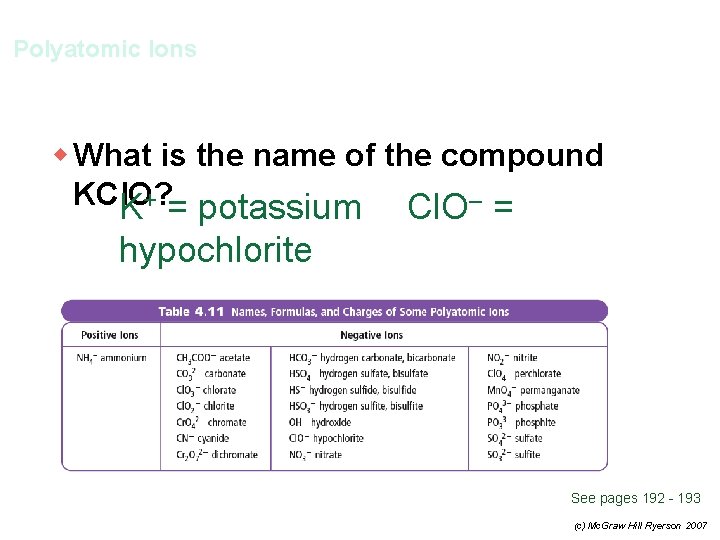
Polyatomic Ions w What is the name of the compound KCl. O? See pages 192 - 193 (c) Mc. Graw Hill Ryerson 2007

Polyatomic Ions w What is the name of the compound KCl. O? K+ = potassium Cl. O– = hypochlorite potassium hypochlorite See pages 192 - 193 (c) Mc. Graw Hill Ryerson 2007