FORMULA MATH THE MOLE The Mole Measuring Matter

















































- Slides: 58

FORMULA MATH & THE MOLE

The Mole Measuring Matter

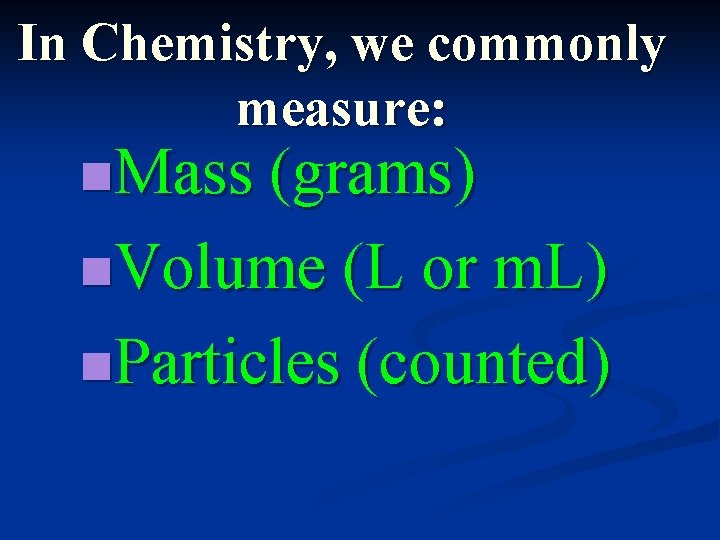
In Chemistry, we commonly measure: n. Mass (grams) n. Volume (L or m. L) n. Particles (counted)

n. We can relate each of these measurements to a single quantity called the “Mole”.

n. The Mole is the SI unit for the “amount of something”

Why use it? n. To estimate the number of particles that are too small or too numerous to actually count.

n 1. 0 mole = 22. 4 Liters of a gas, at standard temperature and pressure (STP) n Std. temp. = 0º C or 273 K n Std. pressure = 1 atmosphere (atm) or 760 mm Hg (Torr)

n 1 mole = 6. 022 x 23 10 particles of a pure substance. * Element = atom * Molecular = molecule (mlc) *Ionic = formula unit (fmu)

n 1. 0 mole = ____ grams of a pure substance. (“Molar Mass” of that substance)

Molar Mass – mass, in grams, of 1 mole of a pure substance.

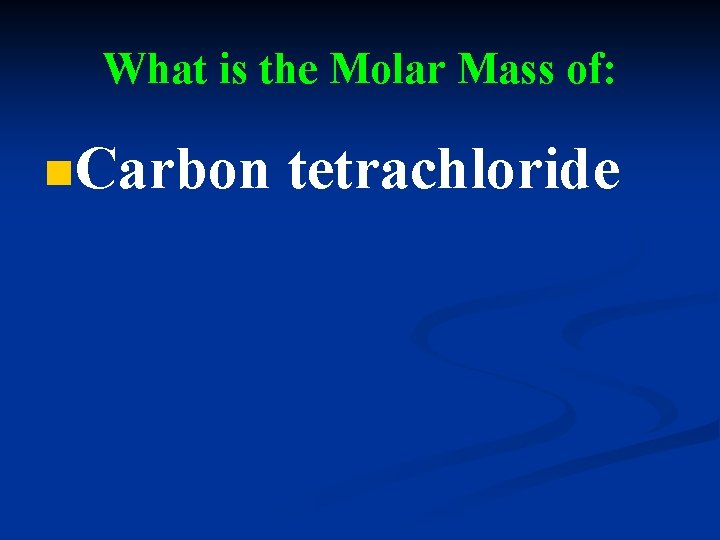
What is the Molar Mass of: n. Carbon tetrachloride

n. Oxygen

n. Aluminum carbonate

Calculating with Moles

Remember…. 1 mole = 23 6. 022 x 10 particles = 22. 4 liters of gas, at STP ______ grams (Molar Mass)

Steps to calculate: READ directions in Lecture Packet.

Example What is the mass of 45. 8 L of carbon dioxide gas, at STP?

Example: How many moles are equal to 3. 01 x 10 22 atoms of magnesium?

Example: How many molecules are in 38. 69 grams of chlorine gas?

Example: What is the volume, in L, at STP, occupied by 0. 600 moles of sulfur dioxide gas?

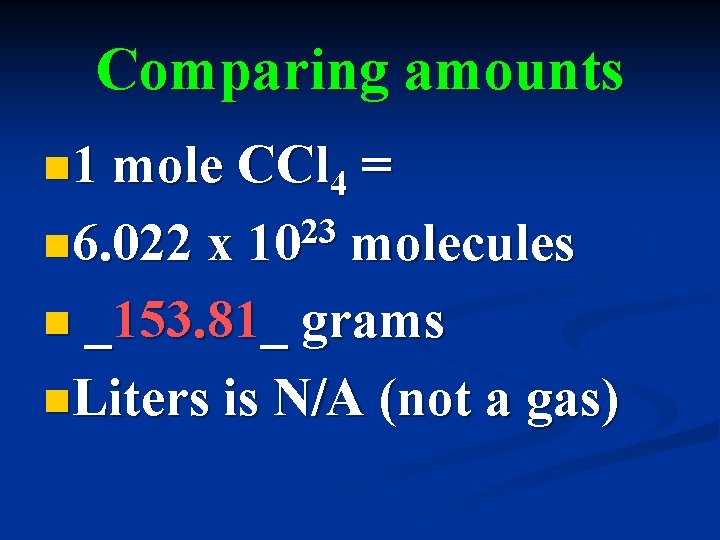
Comparing amounts n 1 mole CCl 4 = 23 n 6. 022 x 10 molecules n _153. 81_ grams n. Liters is N/A (not a gas)

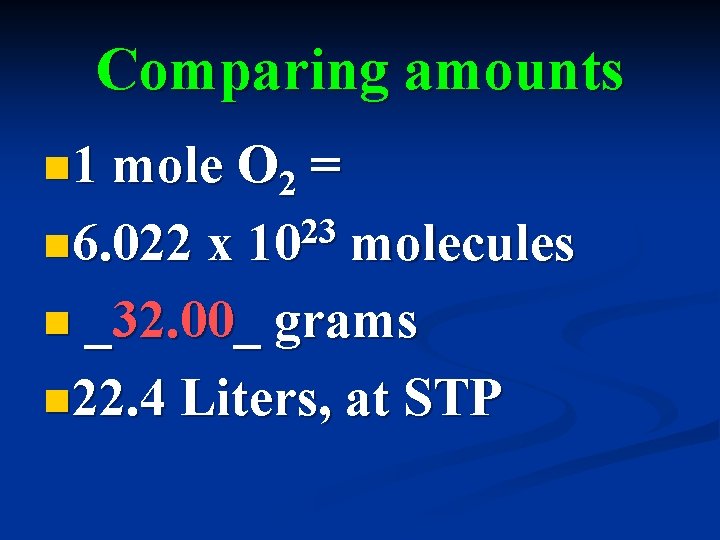
Comparing amounts n 1 mole O 2 n 6. 022 x = 23 10 molecules n _32. 00_ grams n 22. 4 Liters, at STP

Comparing amounts n 1 mole Al 2(CO 3)3 = 23 n 6. 022 x 10 formula units n _233. 99_ grams n. Liters is N/A (not a gas)

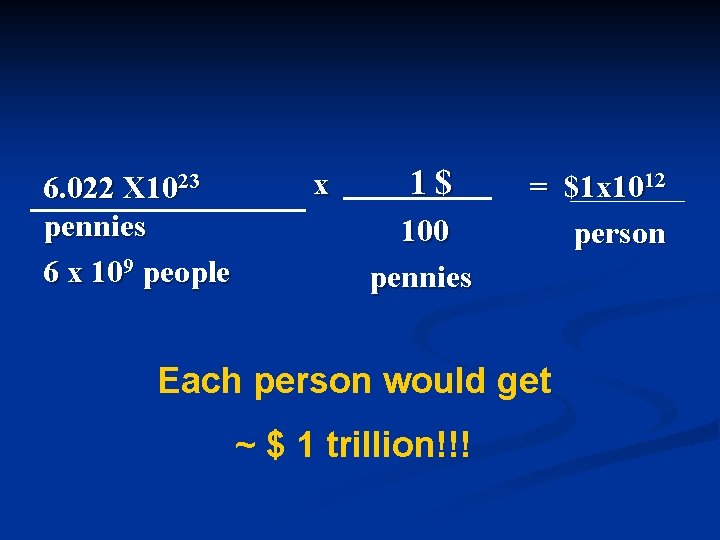
n. How big is a Mole? n. If you have a mole of pennies and divide them equally among the 6 billion people on Earth, how many dollars would each person get?

6. 022 X 1023 pennies 6 x 109 people x 1$ 100 pennies = $1 x 1012 person Each person would get ~ $ 1 trillion!!!

Percent Composition

Think about it. . . What is the percent composition of the number of boys to girls in this classroom?

Now, think about this. . . What is the percent composition of the weight of boys to girls in this classroom?

In Chemistry, it’s calculated the same way, except we consider the make-up of compounds.

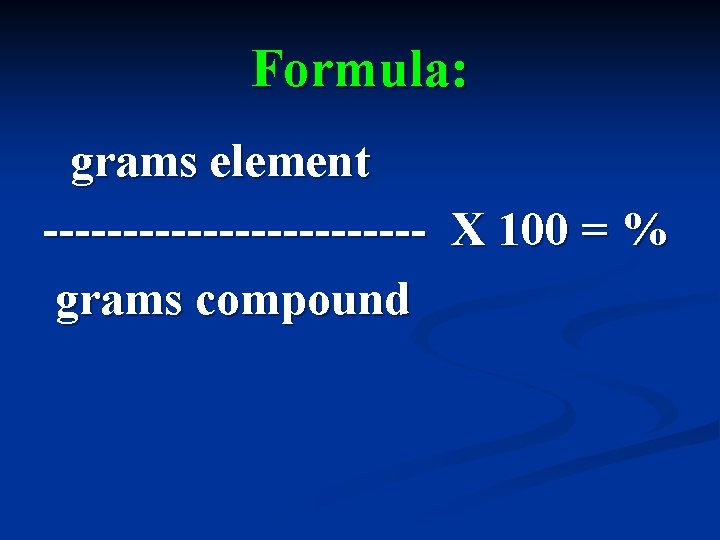
What information does % Composition give us? The percent, by mass, of each element in a compound.

Formula: grams element ------------ X 100 = % grams compound

2 Ways To Calculate: 1) Chemical Formula is given 2) Masses are given (no formula given)

If formula is given: 1) 2) 3) 4) 5) Write chemical formula. Count atoms of each element in compound. Multiply by Mass Number from P. T. (round all mass # to the 10 th). Add answers. Plug numbers into formula and solve.

Example #1 What is the percent composition of water?

Example#2 What is the % composition of aluminum chromate?

If masses are given: g element + g element g compound *Remember the Law Of Conservation Of Mass 1)

Example#3 What is the percent composition of a compound if 27. 07 grams of calcium completely reacts with 47. 93 grams of chlorine?

More Formula Math!!!

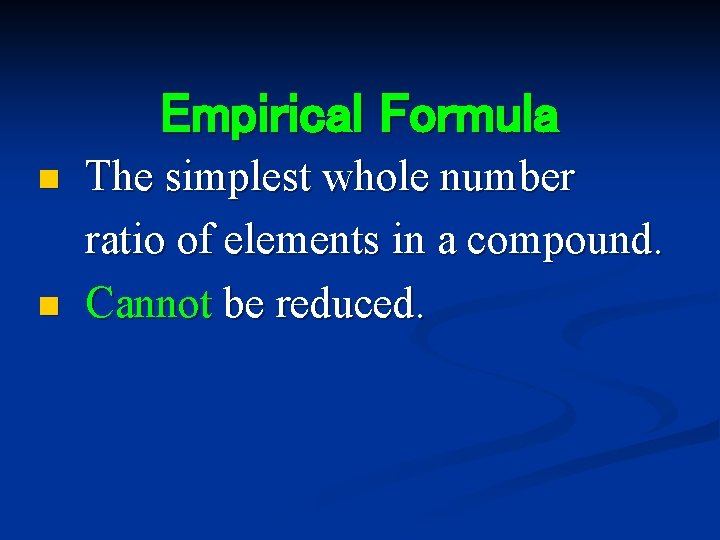
Empirical Formula n n The simplest whole number ratio of elements in a compound. Cannot be reduced.

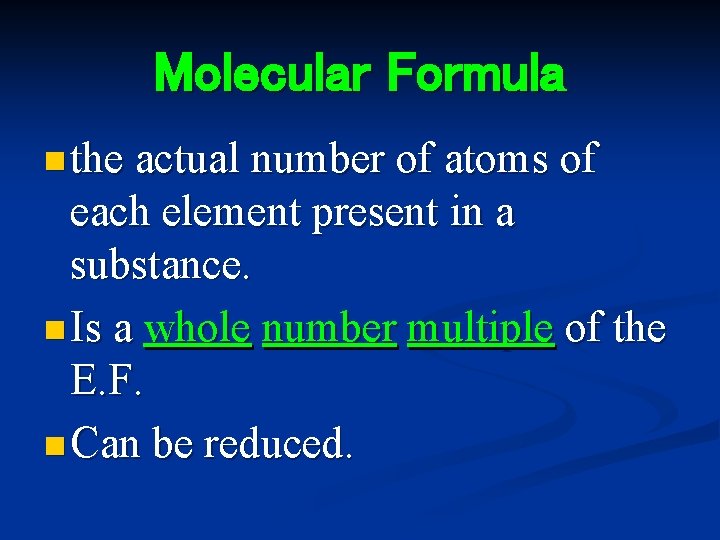
Molecular Formula n the actual number of atoms of each element present in a substance. n Is a whole number multiple of the E. F. n Can be reduced.

From the “mole” perspective… Ba 3 N 2 Al 2(SO 4)3

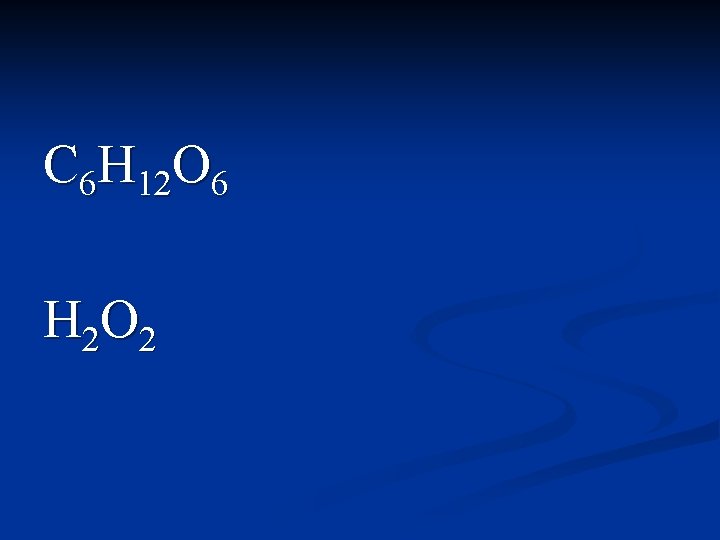
C 6 H 12 O 6 H 2 O 2

To calculate Empirical Formulas: READ directions in your LECTURE PACKET!

example… What is the E. F. of a compound composed of 32. 00% Carbon, 42. 66% Oxygen, 18. 67% Nitrogen, and 6. 67% Hydrogen?


example… What is the E. F. of a compound composed of 25. 9% Nitrogen and 74. 1% Oxygen?


example… What is the E. F. of a compound composed of 36. 6 grams of Carbon and 9. 2 grams of Hydrogen?


To calculate Molecular Formulas: READ direction in LECTURE PACKET!!!

example…. What is the M. F. of a compound with a mass of 92 grams and an E. F. of NO 2?


example…. What is the M. F. of a compound with a mass of 150 grams and an E. F. of CH 2 O?


example…. What is the M. F. of a compound with a mass of 132 grams that is composed of 54. 6% Carbon, 13. 6% Hydrogen, and 31. 8% Nitrogen?


Hydrate - crystals that absorb a specific amount of water 1. Determine the grams of dry crystal and the grams of water. 2. Calculate mols of each. 3. Determine the simplest whole number ratio.

example… A student had a 12. 151 g sample of hydrated barium iodide. After heating the sample, the dry sample had a mass of 9. 520 g. What is the formula of the hydrate?