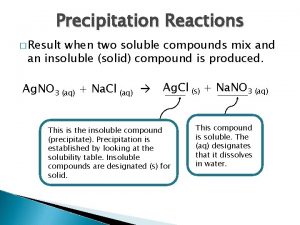
Formula Equation Stoichiometry What is stoichiometry n Deals






























- Slides: 48

Formula & Equation Stoichiometry

What is stoichiometry? n Deals with the specifics of QUANTITY in chemical formula or chemical reaction.

Review: Counting Atoms n n Remember subscripts tell you how many atoms… … Coefficients tell you how many TOTAL molecules compounds

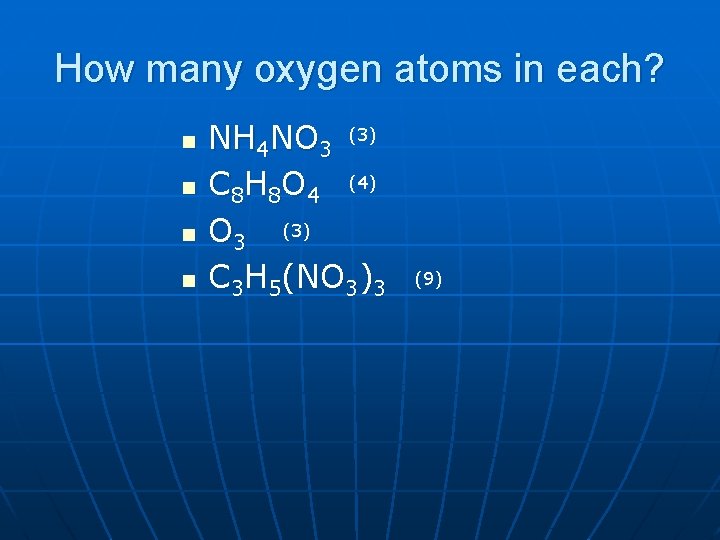
How many oxygen atoms in each? n n NH 4 NO 3 (3) C 8 H 8 O 4 (4) O 3 (3) C 3 H 5(NO 3)3 (9)

Counting Atoms Practice Ditto Example #1: (NH 4)2 CO 3 * First list the types of atoms and then count each. N: 2 H: 8 C: 1 O: 3

Counting Atoms Practice Ditto cont. Example #2: Ca. Cr. O 4 * First list the types of atoms and then count each. Ca: 1 Cr: 1 O: 4

Counting Atoms Practice Ditto cont. Example #3: Ca 3(PO 4)2 * First list the types of atoms and then count each. Ca: 3 P: 2 O: 8 **Do the rest on your own!**

Atomic Mass n n Mass of an atom of one element based on percent abundances and masses of all isotopes (on PT) Units: amu

Molecular Mass n n Mass of a molecular compound (ex: H 2 O, CO 2) Units: amu

Formula Mass n n Mass of an ionic compound (ex: Na. Cl, Mg. I 2) Units: amu

Gram Formula Mass n Mass expressed in grams

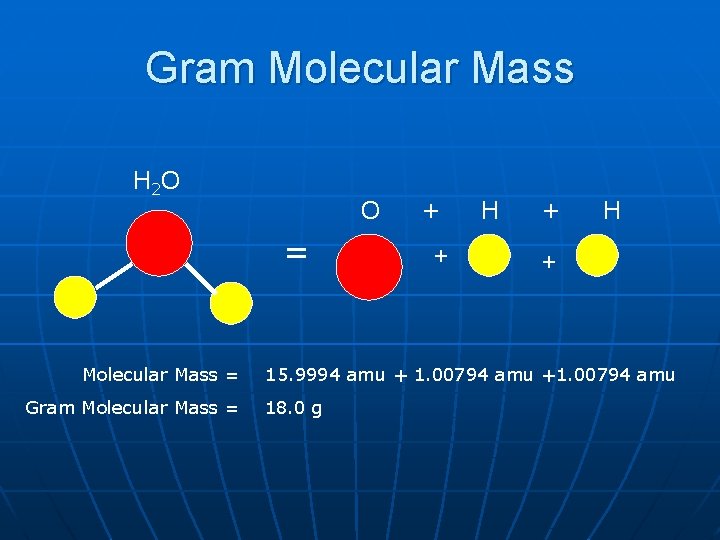
Gram Molecular Mass H 2 O O = Molecular Mass = Gram Molecular Mass = + + H + 15. 9994 amu + 1. 00794 amu +1. 00794 amu 18. 0 g

How do I calculate molecular or formula mass? n n First: Identify and count atoms in the compound. Second: Locate atomic mass of each element. Third: Multiply mass x number of atoms and total all elements. Voila!

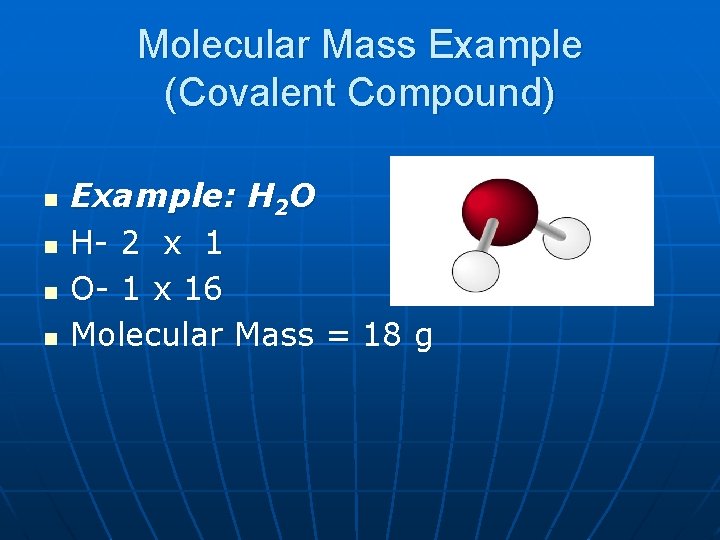
Molecular Mass Example (Covalent Compound) n n Example: H 2 O H- 2 x 1 O- 1 x 16 Molecular Mass = 18 g

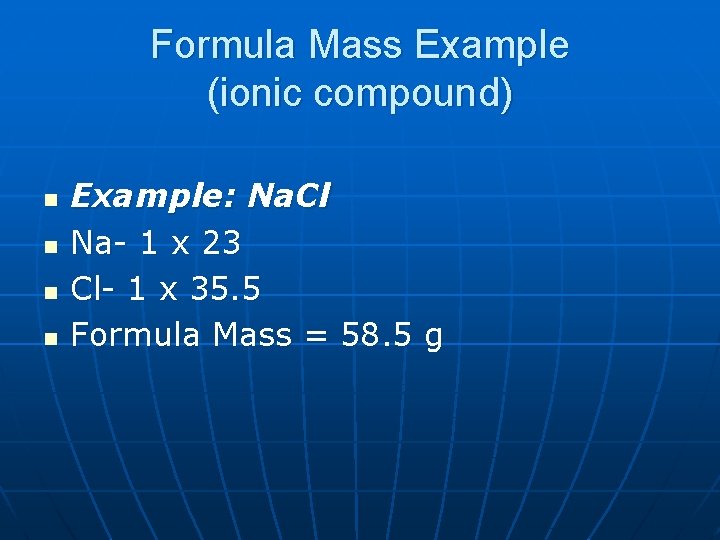
Formula Mass Example (ionic compound) n n Example: Na. Cl Na- 1 x 23 Cl- 1 x 35. 5 Formula Mass = 58. 5 g

Formula Mass Practice

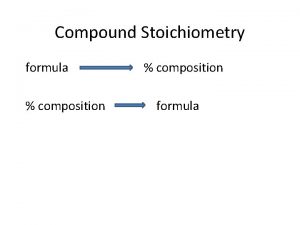
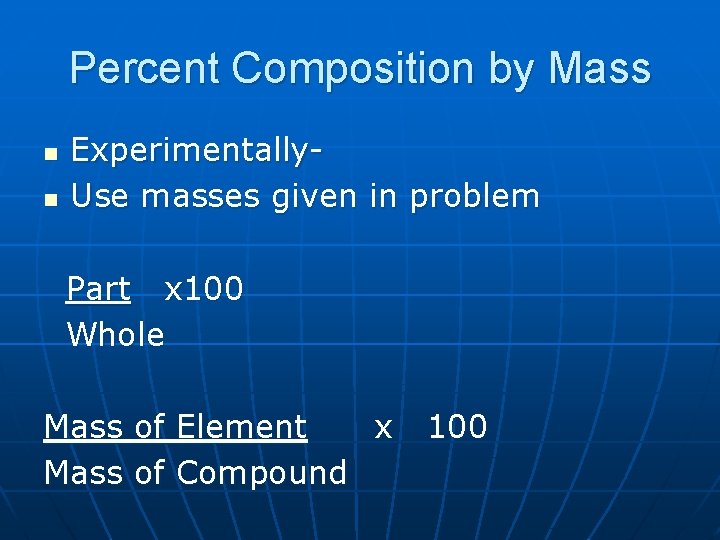
Percent Composition by Mass n n Experimentally. Use masses given in problem Part x 100 Whole Mass of Element x Mass of Compound 100

Example: n A compound containing carbon and hydrogen has a mass of 16 grams. When decomposed, 12. 0 grams are found to be carbon. What is the percent by mass of carbon in the compound?

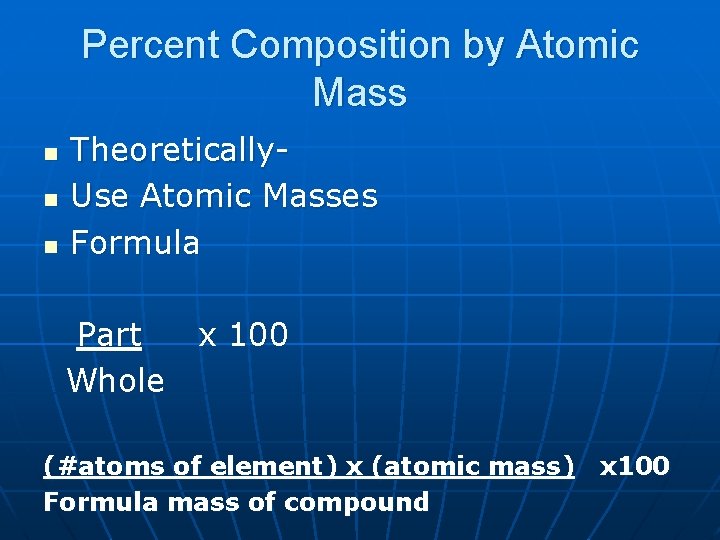
Percent Composition by Atomic Mass n n n Theoretically. Use Atomic Masses Formula Part x 100 Whole (#atoms of element) x (atomic mass) x 100 Formula mass of compound

Hydrated Crystals Some ionic compounds are found to have surrounding water molecules. n These compounds are called Hydrated Crystals. n The percent water hydration can be found. Example Cu. SO 4. 5 H 2 O n The dot shows that 5 H 2 O molecules are attached to 1 Cu. SO 4 molecule. n

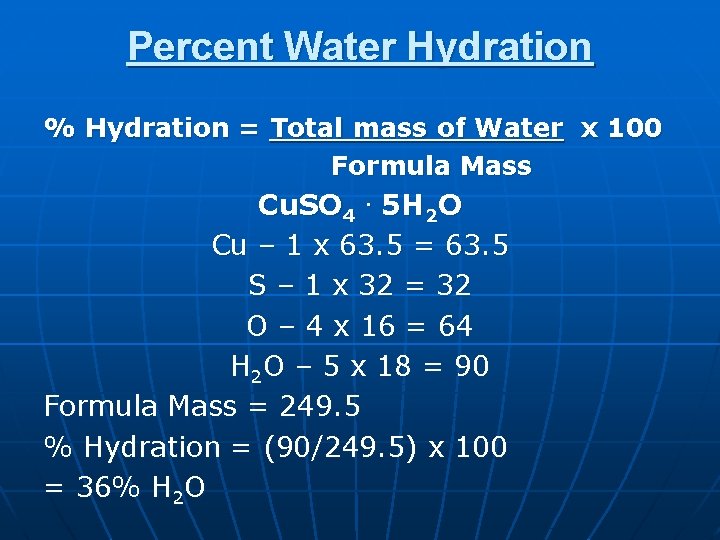
Percent Water Hydration % Hydration = Total mass of Water x 100 Formula Mass Cu. SO 4. 5 H 2 O Cu – 1 x 63. 5 = 63. 5 S – 1 x 32 = 32 O – 4 x 16 = 64 H 2 O – 5 x 18 = 90 Formula Mass = 249. 5 % Hydration = (90/249. 5) x 100 = 36% H 2 O

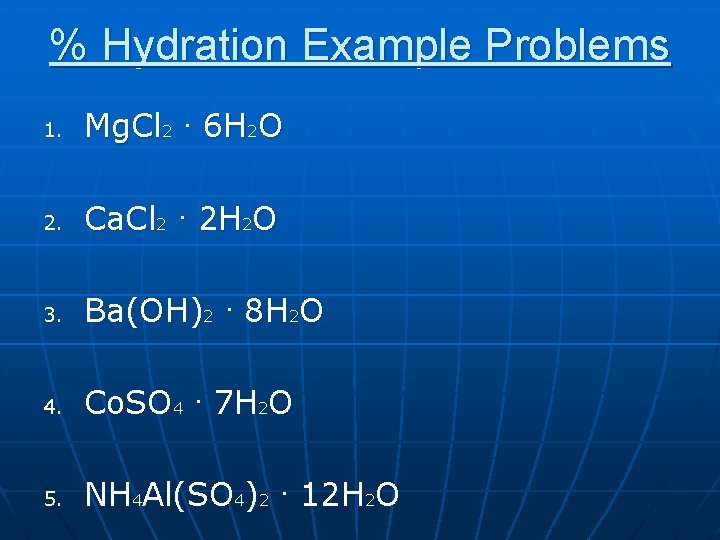
% Hydration Example Problems 1. Mg. Cl 2. 6 H 2 O 2. Ca. Cl 2. 2 H 2 O 3. Ba(OH)2. 8 H 2 O 4. Co. SO 4. 7 H 2 O 5. NH 4 Al(SO 4)2. 12 H 2 O

The Mole n n n Avagadro’s Number is the number of atoms (for monatomic elements) or molecules (for diatomic elements & compounds) in 1 mole of any substance. Avagadro’s Number is 6. 02 x 1023 That means that in 1 mole of any sample of matter there are 6. 02 x 1023 particles. (atoms or molecules depending on the substance)

The Mole n n n There are 6. 02 x 1023 particles in one mole of any substance (element or compound). One particle can be one atom or one molecule. That’s a BIG number!!!!! Even though for any substance the number of particles in 1 mole is always the same different substances have different masses so the mass of 1 mole will be different for all substances. Atomic / Formula / Molecular Mass = Molar Mass because it is the mass of 1 mole of any substance. Example: 1 mole of H 2 O = 18 g

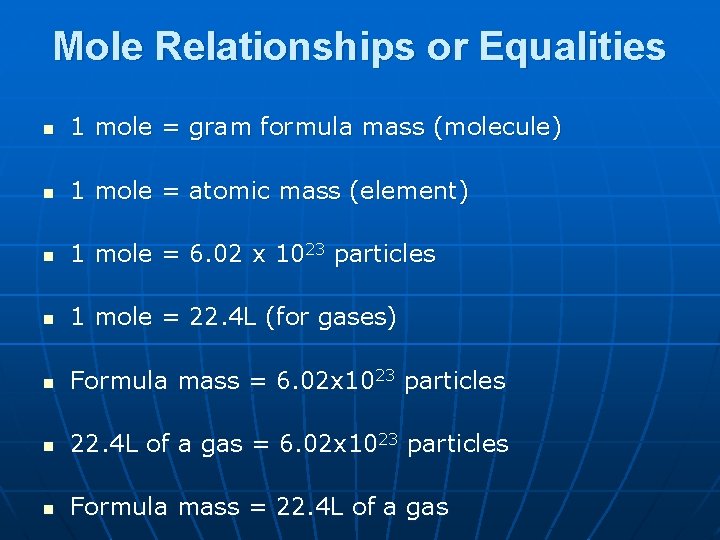
Mole Relationships or Equalities n 1 mole = gram formula mass (molecule) n 1 mole = atomic mass (element) n 1 mole = 6. 02 x 1023 particles n 1 mole = 22. 4 L (for gases) n Formula mass = 6. 02 x 1023 particles n 22. 4 L of a gas = 6. 02 x 1023 particles n Formula mass = 22. 4 L of a gas

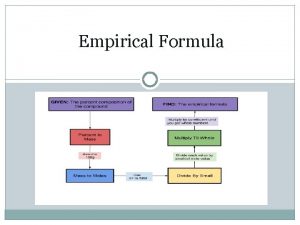
Table T : Mole Calculations n n n For questions that involve mass (gram) to mole conversions use the “Mole Calculation” formula on Table T. In order to use this formula you will need to plug in what is given and solve for the unknown value. If solving for moles then plug in given mass and gram formula mass (GFM) If solving for a mass then plug in moles and GFM and solve for given mass You will usually have to calculate the gram formula mass of the substance unless it is given. See Table T

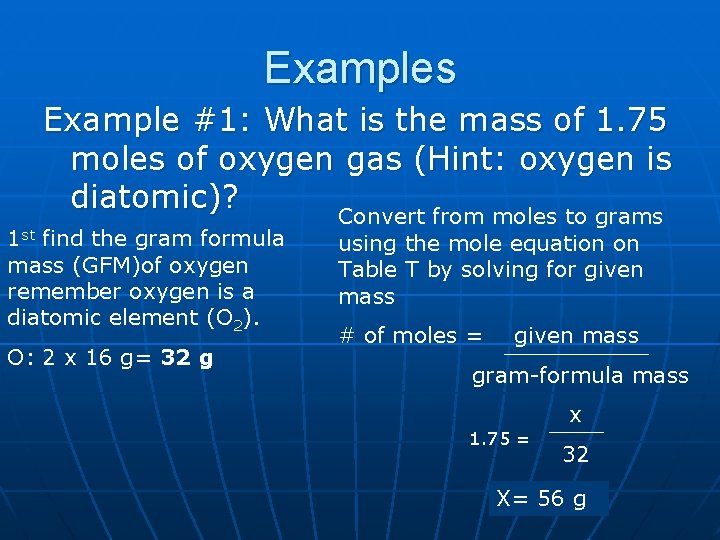
Examples Example #1: What is the mass of 1. 75 moles of oxygen gas (Hint: oxygen is diatomic)? Convert from moles to grams 1 st find the gram formula mass (GFM)of oxygen remember oxygen is a diatomic element (O 2). O: 2 x 16 g= 32 g using the mole equation on Table T by solving for given mass # of moles = given mass gram-formula mass 1. 75 = x 32 X= 56 g

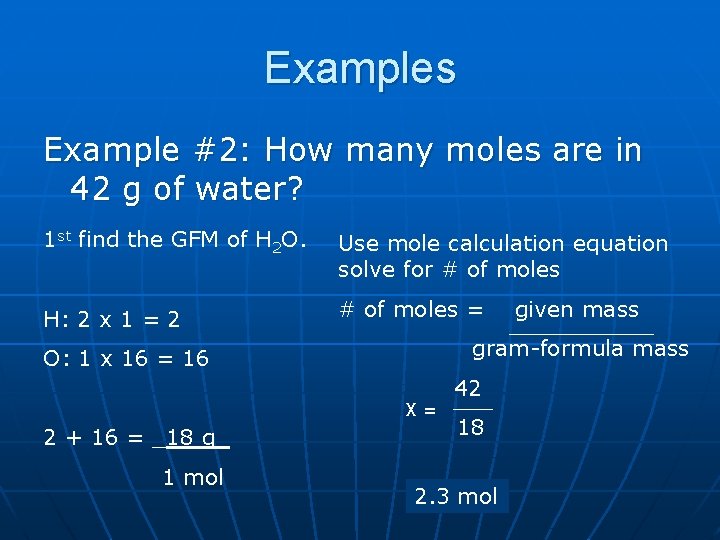
Examples Example #2: How many moles are in 42 g of water? 1 st find the GFM of H 2 O. Use mole calculation equation solve for # of moles H: 2 x 1 = 2 # of moles = gram-formula mass O: 1 x 16 = 16 X= 2 + 16 = _18 g_ 1 mol given mass 42 18 2. 3 mol

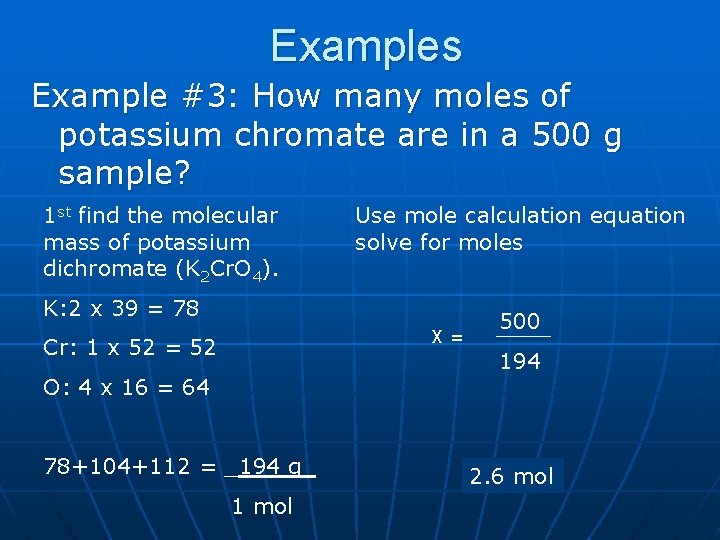
Examples Example #3: How many moles of potassium chromate are in a 500 g sample? 1 st find the molecular mass of potassium dichromate (K 2 Cr. O 4). Use mole calculation equation solve for moles K: 2 x 39 = 78 X= Cr: 1 x 52 = 52 O: 4 x 16 = 64 78+104+112 = _194 g_ 1 mol 500 194 2. 6 mol

Example 4 How much does 2. 0 moles of carbon dioxide weigh?

Example 5 What is the mass of 0. 50 moles of sulfur dioxide at STP?

Example 6 How many moles of helium gas are in 20. 0 grams of helium? Try the Practice problems

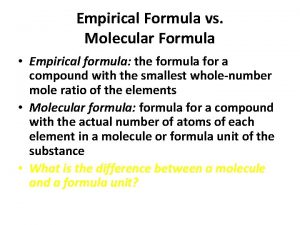
Empirical & Molecular Formulas Empirical Formula: formula that contains the lowest whole number ratio of atoms. n Molecular Formula: the actual formula for a compound (does not have to be empirical but it can) n The subscripts in any formula represents the number of moles of atoms in a 1 mole sample. n The subscripts in an empirical formula can tell you the mole ratio of atoms in a compound. Example: In 1 mole of H 2 O there are 2 moles of Hydrogen atoms and 1 mole of oxygen atoms. n

Calculating the Molecular Formula Given Formula Mass and Empirical Formula n Since the empirical formula is the lowest mole ratio of atoms in a compound the molecular formula mass must be a multiple of the empirical formula mass.

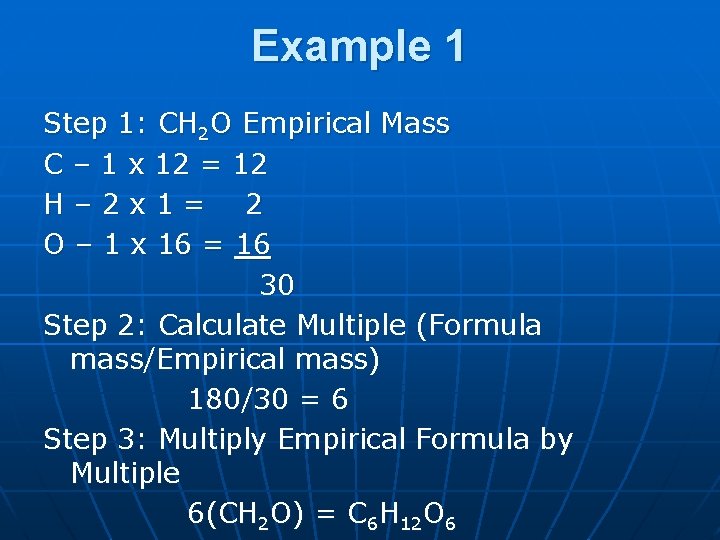
Example 1: What is the molecular formula for a compound that has an empirical formula of CH 2 O and the molecular mass is 180 g. n n n Step 1: Find the empirical formula mass. Step 2: Divide molecular mass given by the empirical mass you calculated in step 1. Step 3: Multiply all of the subscripts in the empirical formula by the multiple you calculated in step 2

Example 1 Step 1: CH 2 O Empirical Mass C – 1 x 12 = 12 H– 2 x 1= 2 O – 1 x 16 = 16 30 Step 2: Calculate Multiple (Formula mass/Empirical mass) 180/30 = 6 Step 3: Multiply Empirical Formula by Multiple 6(CH 2 O) = C 6 H 12 O 6

Example 2: A mass of a given compound is 9. 2 g. It is determined that 2. 8 g is nitrogen and 6. 4 g is oxygen. What is the empirical formula of this compound? n n Step 1: Convert the given mass of each element to moles by dividing by the atomic mass. Step 2: Plug moles values from step 1 into the formula and divide both by the lowest to get a whole number ratio. If ratio is still not whole number then multiply the whole formula by 2.

Example 2: A mass of a given compound is 9. 2 g. It is determined that 2. 8 g is nitrogen and 6. 4 g is oxygen. What is the empirical formula of this compound? n N: 2. 8/14 =. 2 mol. O: 6. 4/16 =. 4 mol. n N. 2/. 2 O. 4/. 2 n Answer = NO 2 n

Example 3: A compound is found to be 25. 9% nitrogen and 74. 1% oxygen. What is the emeprical formula of this compound? n n Step 1: Treat the percentage values as mass values (assume sample size is 100 g) and convert to moles. Step 2: Plug mole values into the equation and divide both by the lowest to get whole number ratios. If ratio is still not whole number then multiply the entire formula by 2.

Example 3: A compound is found to be 25. 9% nitrogen and 74. 1% oxygen. What is the emeprical formula of this compound? n N: 25. 9/14 = 1. 85 mol. O: 74. 1/16 = 4. 63 mol. n N 1. 85/1. 85 O 4. 63/1. 85 = 2(NO 2. 5) n Answer = N 2 O 5 n



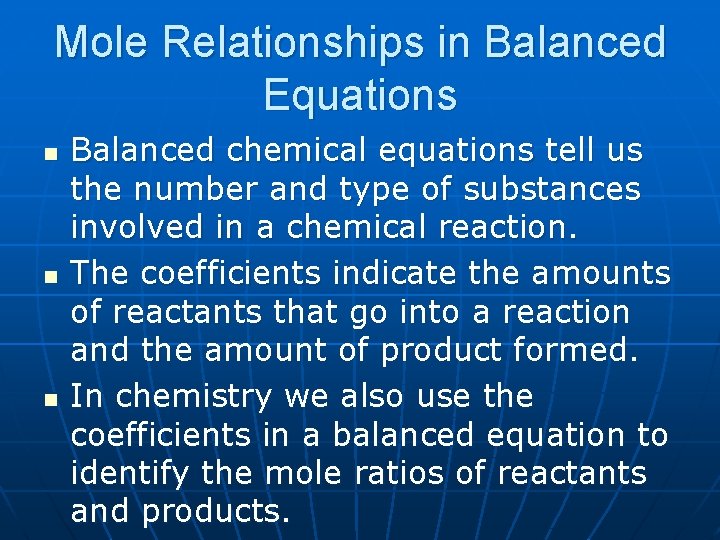
Mole Relationships in Balanced Equations n n n Balanced chemical equations tell us the number and type of substances involved in a chemical reaction. The coefficients indicate the amounts of reactants that go into a reaction and the amount of product formed. In chemistry we also use the coefficients in a balanced equation to identify the mole ratios of reactants and products.

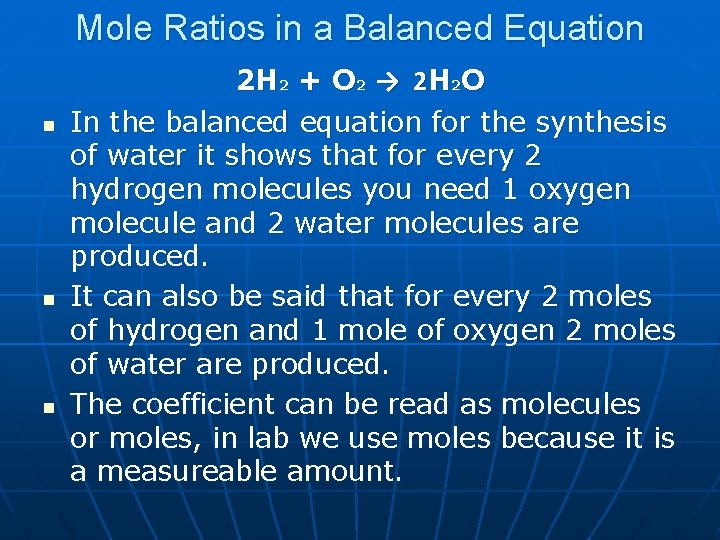
Mole Ratios in a Balanced Equation n 2 H 2 + O 2 → 2 H 2 O In the balanced equation for the synthesis of water it shows that for every 2 hydrogen molecules you need 1 oxygen molecule and 2 water molecules are produced. It can also be said that for every 2 moles of hydrogen and 1 mole of oxygen 2 moles of water are produced. The coefficient can be read as molecules or moles, in lab we use moles because it is a measureable amount.

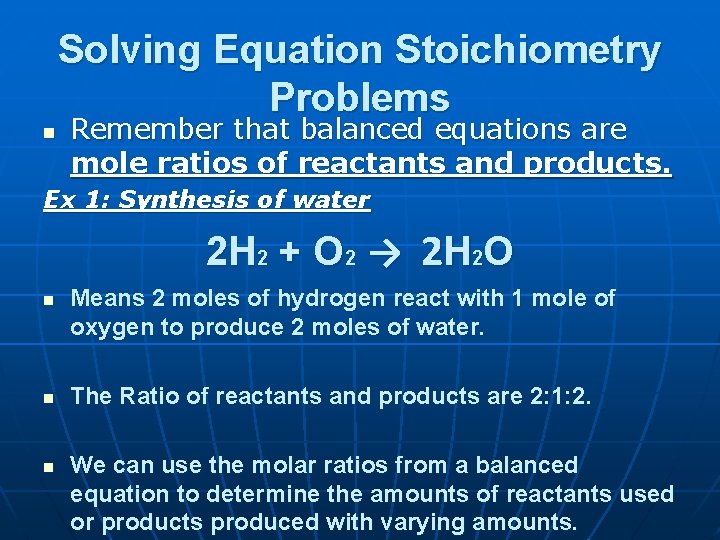
Solving Equation Stoichiometry Problems n Remember that balanced equations are mole ratios of reactants and products. Ex 1: Synthesis of water 2 H 2 + O 2 → 2 H 2 O n n n Means 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water. The Ratio of reactants and products are 2: 1: 2. We can use the molar ratios from a balanced equation to determine the amounts of reactants used or products produced with varying amounts.

Examples Problem 2 H 2 + O 2 → 2 H 2 O Ex 2: How many moles of water are produced if 2 moles of oxygen reacted? Set up a proportion Solve for unknown

How many moles of carbon dioxide will be produced by the complete combustion of 2. 0 moles of glucose (C 6 H 12 O 6) First: write the equation and balance it. C 6 H 12 O 6 + 6 O 2 ⟶ 6 CO 2 + 6 H 2 O Next: set up a proportion Finally: solve for the number of moles

How many moles of ammonia can be produced from 9. 0 moles of hydrogen reacting with nitrogen? Try the practice!!!
 Stoichiometry deals with...
Stoichiometry deals with... Stoichiometry deals with
Stoichiometry deals with Stoichiometry deals with
Stoichiometry deals with Stoichiometry deals with...
Stoichiometry deals with... Stoichiometry deals with.
Stoichiometry deals with. Stoichiometry formulas
Stoichiometry formulas Stoichiometry equation
Stoichiometry equation How to solve stoichiometric calculations
How to solve stoichiometric calculations Stoichiometry formula
Stoichiometry formula Thermodynamics is the branch of science that deals with
Thermodynamics is the branch of science that deals with Thermodynamics deals with
Thermodynamics deals with Microtaxonomy and macrotaxonomy
Microtaxonomy and macrotaxonomy Statistics branch of mathematics
Statistics branch of mathematics Ecology deals with
Ecology deals with Morphology deals with
Morphology deals with Ano ang marketing management
Ano ang marketing management Business ethics deals primarily with
Business ethics deals primarily with Management deals with
Management deals with Karl pearson coefficient of correlation example
Karl pearson coefficient of correlation example Pelagic zone
Pelagic zone P1-p2
P1-p2 Macroeconomic deals withसमग्रलक्षी
Macroeconomic deals withसमग्रलक्षी Association clause
Association clause Fluid statics deals with fluid at rest
Fluid statics deals with fluid at rest Data link control deals with the design and procedures for
Data link control deals with the design and procedures for Articles of association deals with
Articles of association deals with Study of plants
Study of plants Unlike other characters in the play creon displays
Unlike other characters in the play creon displays Oedipus rex practice multiple choice questions answers
Oedipus rex practice multiple choice questions answers Nonfiction deals with facts.
Nonfiction deals with facts. Synchronic and diachronic linguistics
Synchronic and diachronic linguistics Fractals deals with curves that are
Fractals deals with curves that are Prosperity deals
Prosperity deals Bodabil kahulugan
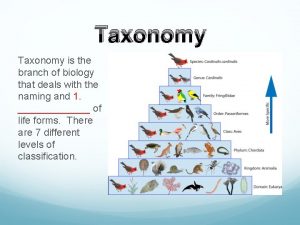
Bodabil kahulugan Taxonomy is the branch of science that deals with
Taxonomy is the branch of science that deals with Anronomasia
Anronomasia Classification of lexical stylistic devices
Classification of lexical stylistic devices Proposal penawaran perangkat lunak
Proposal penawaran perangkat lunak Marketing more than any other business function deals with
Marketing more than any other business function deals with Hydrology is science which deals with
Hydrology is science which deals with Histogram
Histogram Kepler's second law deals with
Kepler's second law deals with A 4m-long quarter-circular gate of radius 3 m
A 4m-long quarter-circular gate of radius 3 m Price off deals sales promotion
Price off deals sales promotion The last lesson ppt
The last lesson ppt Singlets needle leaves
Singlets needle leaves Taxonomy is the science that deals with
Taxonomy is the science that deals with Marketing more than any other business function deals with
Marketing more than any other business function deals with The discipline that deals with what is good and bad
The discipline that deals with what is good and bad