FORMS of CORROSION Uniform Corrosion Galvanic Corrosion Dealloying




- Slides: 22

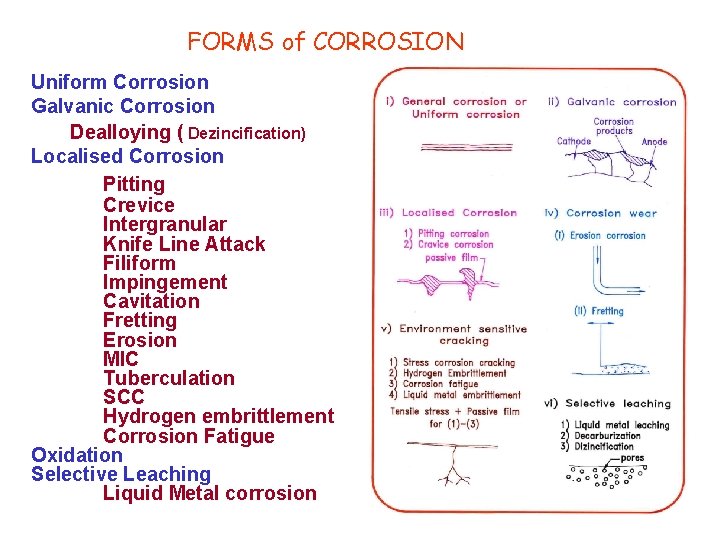
FORMS of CORROSION Uniform Corrosion Galvanic Corrosion Dealloying ( Dezincification) Localised Corrosion Pitting Crevice Intergranular Knife Line Attack Filiform Impingement Cavitation Fretting Erosion MIC Tuberculation SCC Hydrogen embrittlement Corrosion Fatigue Oxidation Selective Leaching Liquid Metal corrosion

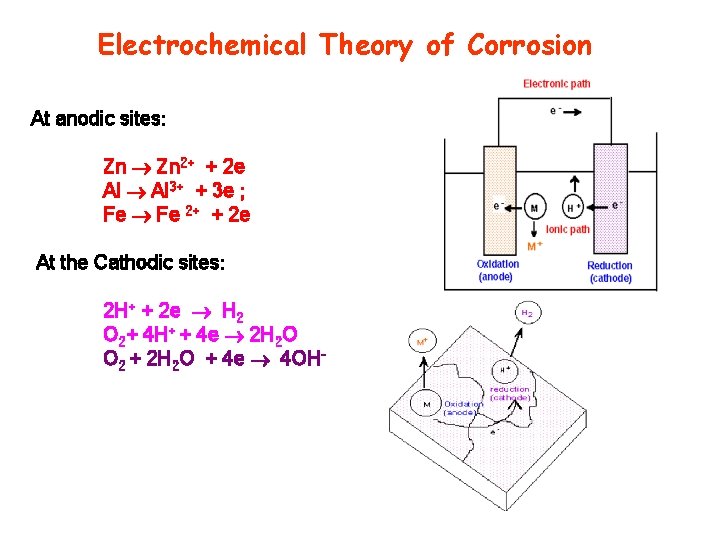
Electrochemical Theory of Corrosion At anodic sites: Zn 2+ + 2 e Al 3+ + 3 e ; Fe 2+ + 2 e At the Cathodic sites: 2 H+ + 2 e H 2 O 2+ 4 H+ + 4 e 2 H 2 O O 2 + 2 H 2 O + 4 e 4 OH-

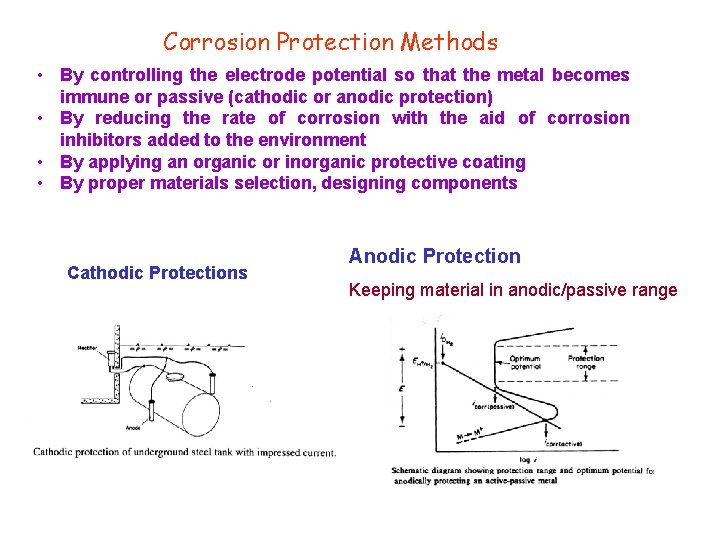
Corrosion Protection Methods • By controlling the electrode potential so that the metal becomes immune or passive (cathodic or anodic protection) • By reducing the rate of corrosion with the aid of corrosion inhibitors added to the environment • By applying an organic or inorganic protective coating • By proper materials selection, designing components Cathodic Protections Anodic Protection Keeping material in anodic/passive range

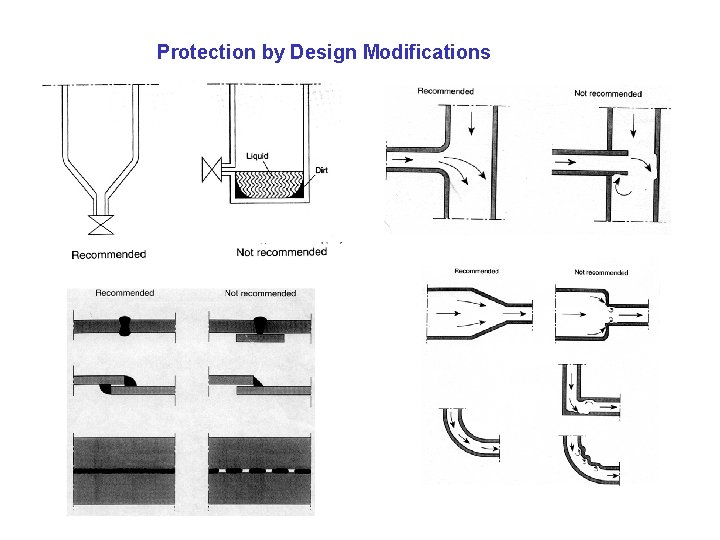
Protection by Design Modifications

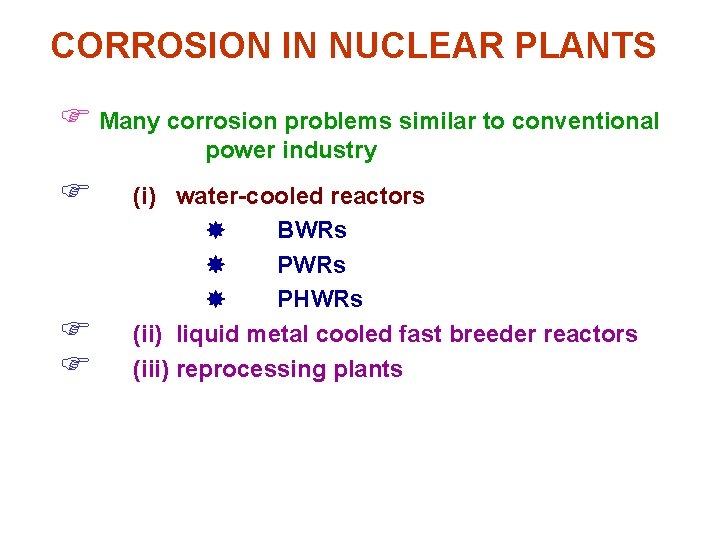
CORROSION IN NUCLEAR PLANTS F Many corrosion problems similar to conventional power industry F F F (i) water-cooled reactors BWRs PHWRs (ii) liquid metal cooled fast breeder reactors (iii) reprocessing plants

SPECIFICITY OF NUCLEAR REACTOR RELATED CORROSION PROBLEMS e

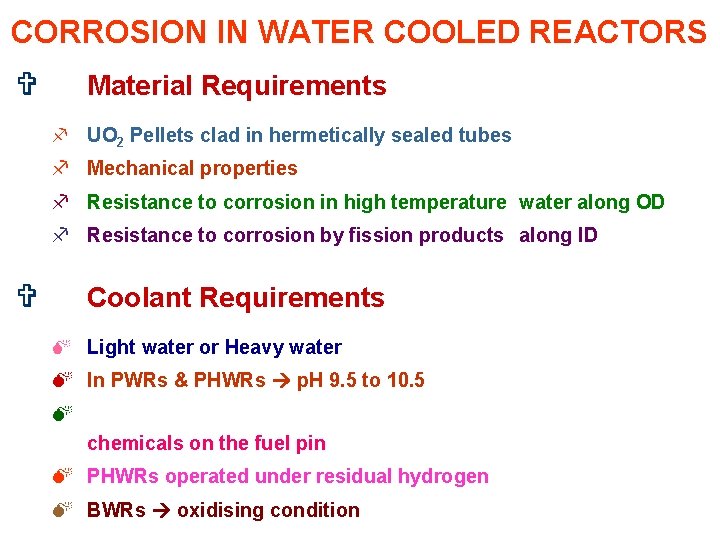
CORROSION IN WATER COOLED REACTORS V Material Requirements f UO 2 Pellets clad in hermetically sealed tubes f Mechanical properties f Resistance to corrosion in high temperature water along OD f Resistance to corrosion by fission products along ID V Coolant Requirements M Light water or Heavy water M In PWRs & PHWRs p. H 9. 5 to 10. 5 M chemicals on the fuel pin M PHWRs operated under residual hydrogen M BWRs oxidising condition

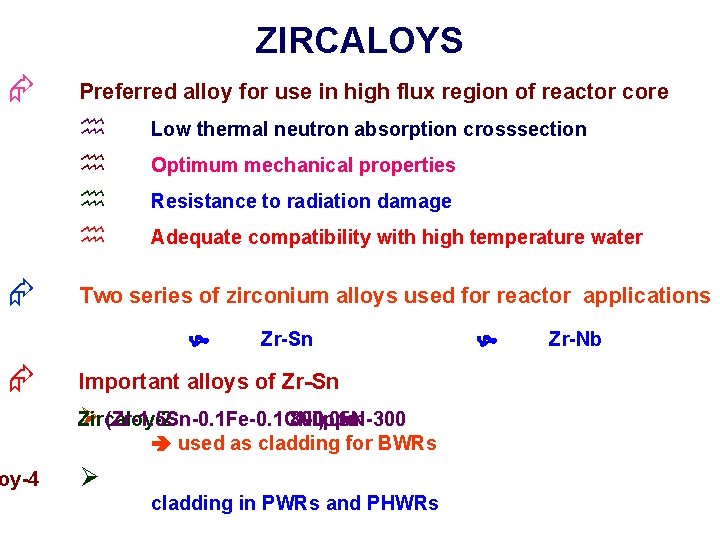
ZIRCALOYS Æ Preferred alloy for use in high flux region of reactor core h h Æ Low thermal neutron absorption crosssection Optimum mechanical properties Resistance to radiation damage Adequate compatibility with high temperature water Two series of zirconium alloys used for reactor applications Æ oy-4 Zr-Sn Important alloys of Zr-Sn Zircaloy-2 Ø (Zr-1. 5 Sn-0. 1 Fe-0. 1 Cr-0. 05 N-300 800 ppm Ni) to used as cladding for BWRs Ø cladding in PWRs and PHWRs Zr-Nb

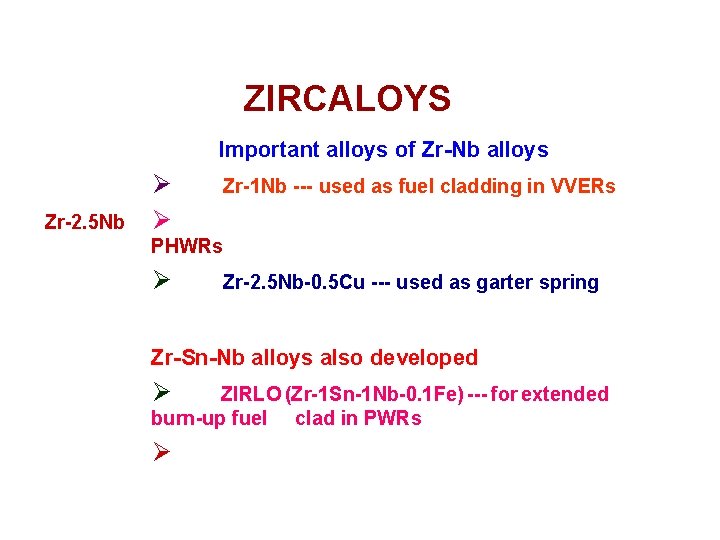
ZIRCALOYS Important alloys of Zr-Nb alloys Zr-2. 5 Nb Ø Ø Zr-1 Nb --- used as fuel cladding in VVERs PHWRs Ø Zr-2. 5 Nb-0. 5 Cu --- used as garter spring Zr-Sn-Nb alloys also developed Ø ZIRLO (Zr-1 Sn-1 Nb-0. 1 Fe) --- for extended burn-up fuel clad in PWRs Ø

CORROSION OF ZIRCALOYS AQUEOUS CORROSION OF ZIRCALOYS e Oxidation Hydriding Nodular Corrosion SCC e Crud-Induced Localised Corrosion e Hydride Blistering CORROSION OF ZIRCALOYS IN HIGH TEMPERATURE WATER Ó Uniform Corrosion Zr + H 2 O Zr. O 2+ H+ H+ enters zircaloy lattice or recombines to give H 2

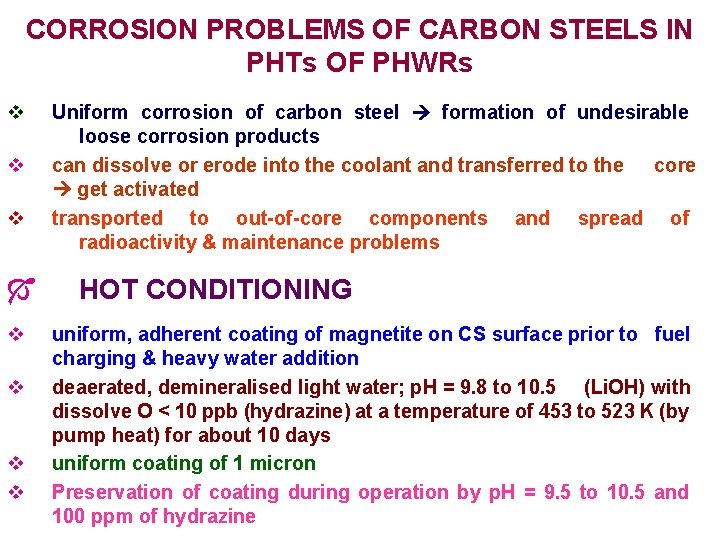
CORROSION PROBLEMS OF CARBON STEELS IN PHTs OF PHWRs v v v Ó v v Uniform corrosion of carbon steel formation of undesirable loose corrosion products can dissolve or erode into the coolant and transferred to the core get activated transported to out-of-core components and spread of radioactivity & maintenance problems HOT CONDITIONING uniform, adherent coating of magnetite on CS surface prior to fuel charging & heavy water addition deaerated, demineralised light water; p. H = 9. 8 to 10. 5 (Li. OH) with dissolve O < 10 ppb (hydrazine) at a temperature of 453 to 523 K (by pump heat) for about 10 days uniform coating of 1 micron Preservation of coating during operation by p. H = 9. 5 to 10. 5 and 100 ppm of hydrazine

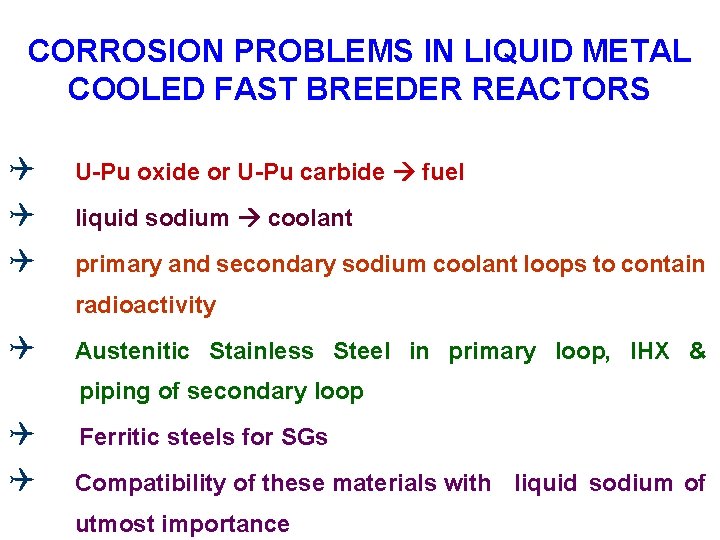
CORROSION PROBLEMS IN LIQUID METAL COOLED FAST BREEDER REACTORS Q Q Q U-Pu oxide or U-Pu carbide fuel liquid sodium coolant primary and secondary sodium coolant loops to contain radioactivity Q Austenitic Stainless Steel in primary loop, IHX & piping of secondary loop Q Q Ferritic steels for SGs Compatibility of these materials with liquid sodium of utmost importance

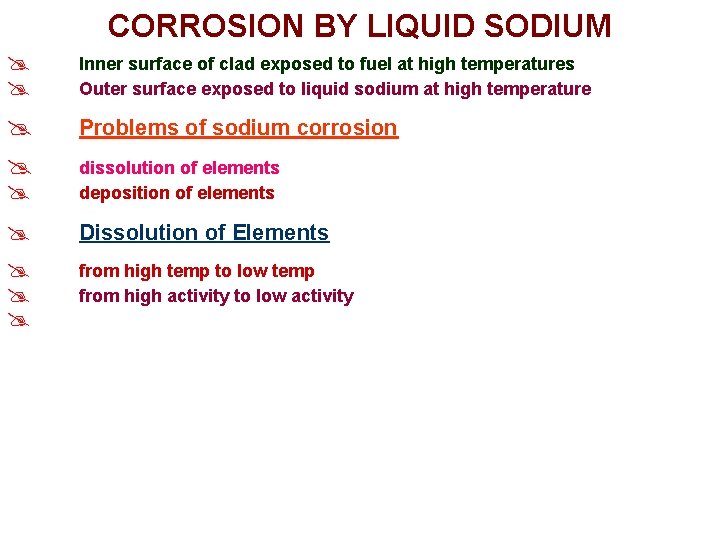
CORROSION BY LIQUID SODIUM @ @ Inner surface of clad exposed to fuel at high temperatures Outer surface exposed to liquid sodium at high temperature @ @ Problems of sodium corrosion @ @ Dissolution of Elements @ dissolution of elements deposition of elements from high temp to low temp from high activity to low activity

CORROSION BY LIQUID SODIUM V V Depth of penetration follows a parabolic law: P = k * t 0. 5 V Region of IG attack below ferrite layer V ferrite layer (4 -10 m)

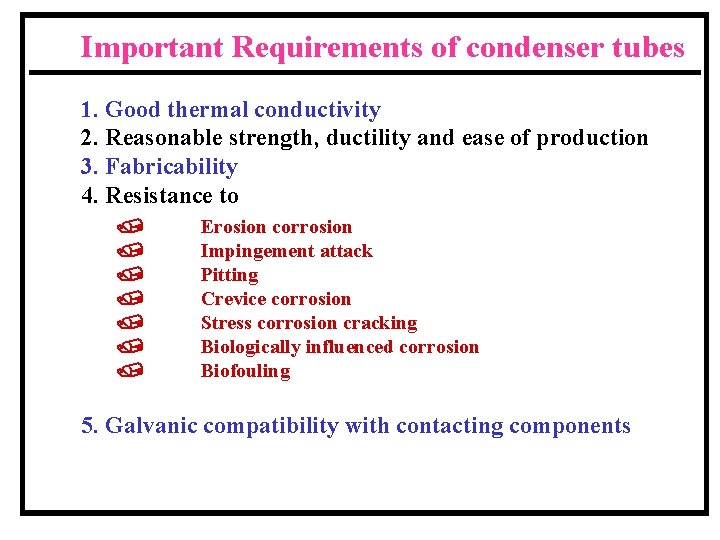
Important Requirements of condenser tubes 1. Good thermal conductivity 2. Reasonable strength, ductility and ease of production 3. Fabricability 4. Resistance to / / / / Erosion corrosion Impingement attack Pitting Crevice corrosion Stress corrosion cracking Biologically influenced corrosion Biofouling 5. Galvanic compatibility with contacting components

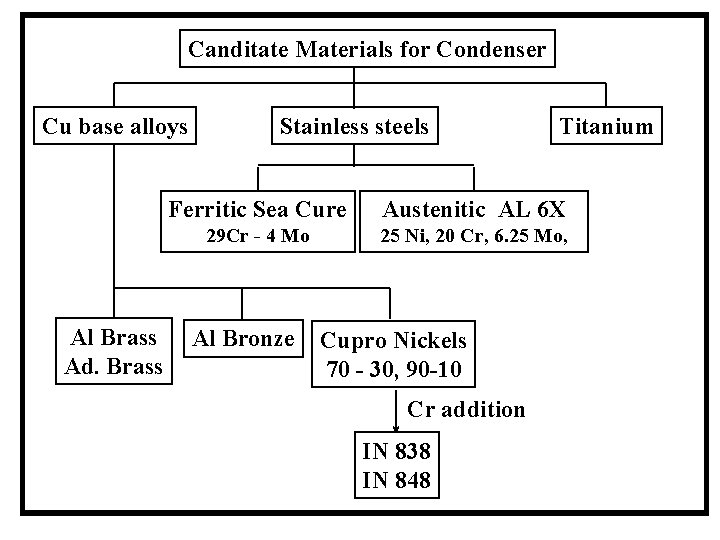
Canditate Materials for Condenser Cu base alloys Al Brass Ad. Brass Stainless steels Titanium Ferritic Sea Cure Austenitic AL 6 X 29 Cr - 4 Mo 25 Ni, 20 Cr, 6. 25 Mo, Al Bronze Cupro Nickels 70 - 30, 90 -10 Cr addition IN 838 IN 848

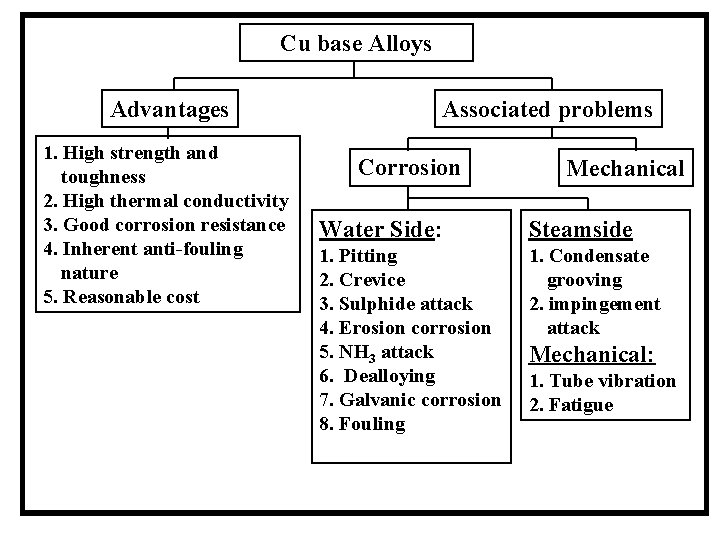
Cu base Alloys Advantages 1. High strength and toughness 2. High thermal conductivity 3. Good corrosion resistance 4. Inherent anti-fouling nature 5. Reasonable cost Associated problems Corrosion Mechanical Water Side: Steamside 1. Pitting 2. Crevice 3. Sulphide attack 4. Erosion corrosion 5. NH 3 attack 6. Dealloying 7. Galvanic corrosion 8. Fouling 1. Condensate grooving 2. impingement attack Mechanical: 1. Tube vibration 2. Fatigue

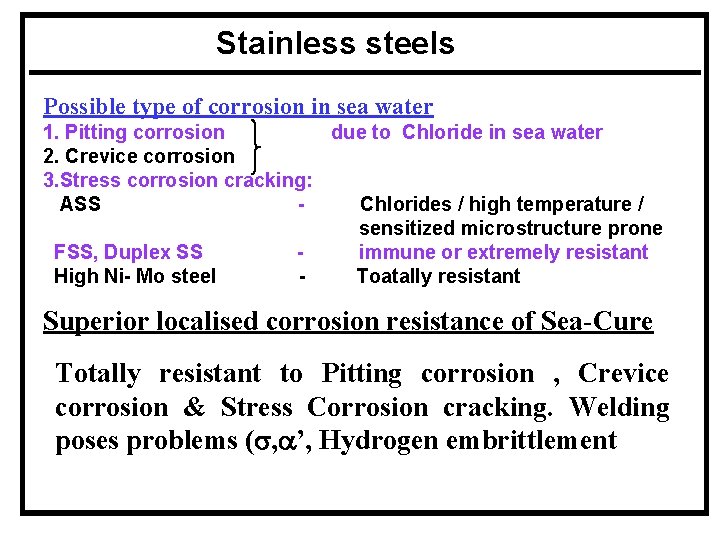
Stainless steels Possible type of corrosion in sea water 1. Pitting corrosion due to Chloride in sea water 2. Crevice corrosion 3. Stress corrosion cracking: ASS Chlorides / high temperature / sensitized microstructure prone FSS, Duplex SS immune or extremely resistant High Ni- Mo steel Toatally resistant Superior localised corrosion resistance of Sea-Cure Totally resistant to Pitting corrosion , Crevice corrosion & Stress Corrosion cracking. Welding poses problems (s, a’, Hydrogen embrittlement)

Stainless steels 4. Galvanic corrosion - being noble problems are more 5. Erosion corrosion - greater resistance than Cu base alloys 6. Biofouling - prone and less resistant than Cu base alloys 7. Tube vibration - lesser than Ti/Cu base alloys ( Higher elastic modulus ) 8. Thermal stresses - coefficient of thermal expansion more than carbon steel and therefore more thermal stresses - buckling in tube/tube sheet joints AL 6 x, AL 29 - 4 C, Sea cure are resistant in sea water conditions. But available experience & information are very less

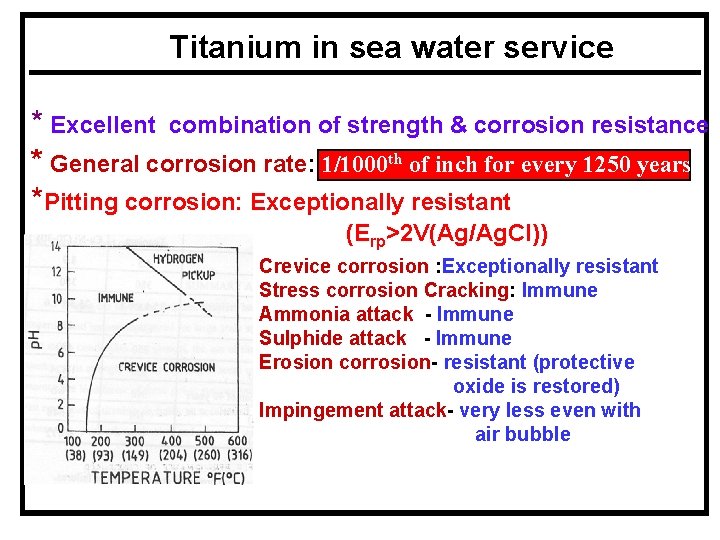
Titanium in sea water service * Excellent combination of strength & corrosion resistance * General corrosion rate: 1/1000 th of inch for every 1250 years *Pitting corrosion: Exceptionally resistant (Erp>2 V(Ag/Ag. Cl)) Crevice corrosion : Exceptionally resistant Stress corrosion Cracking: Immune Ammonia attack - Immune Sulphide attack - Immune Erosion corrosion- resistant (protective oxide is restored) Impingement attack- very less even with air bubble

Nuclear Fuel Reprocessing Nitric Acid is used in various unit operations of spent Nuclear Fuel Reprocessing plants from: â Dilute to concentrated â Room temperature to boiling condition â With redox systems â With Process impurities AISI Type 304 L SS is the common material of construction ðSevere corrosive conditions exist in: Ø Acid Dissolver Ø Acid Recovery Evaporator Ø Intercycle U+Pu Evaporator Ø Final Pu nitrate Concentrator Ø Oxalic mother liquor Evaporator

Nuclear Fuel Reprocessing ÓNew advanced materials for corrosion mitigation v Uranus 65 or AISI 310 L (high corrosion in boiling conditions) v Titanium (higher corrosion in condensate phase) v v v Zirconium ( SCC in nitric acid ) Ti-5% Ta Ti-5%Ta-2%Nb (Good at all conc. and temperature )