Forming new substances Matter and Changes in matter

Forming new substances

Matter and Changes in matter can be described in terms of physical changes and chemical changes Physical property – characteristic of a substance that can be observed w/out changing the substance into another substance • Color, hardness, texture, shine, flexibility, conducting heat and electricity Chemical property – characteristic of a substance that describes its ability to change into another substance • Flammability, rust, reativity

Physical change – any change that alters the form or appearance of a substance (can get back what you started with) Chemical change – change in matter that produces one or more new substances Chemical changes occur when bonds break and new bonds form

Chemical Reactions Involve changes in properties and changes in energy that you can observe.

Evidence to a Chemical Rxn • Gas formation • Solid formation – called a precipitate • Color change • Temperature/Energy change (heat) • Light is emitted • Change in smell or taste

Chemical Formulas Shorthand way of using chemical symbols and numbers to represent a compound or group of elements. Shows the number of atoms of each element present in the formula.

i. e. - chemical formula for water = H 2 O tells us that it is composed of 2 hydrogen atoms and 1 oxygen atom Ex. O 2 C 6 H 12 O 6 Na 2 SO 4

The small numbers are referred to as subscripts Common Prefixes: Mono = 1 Tri =3 Penta = 5 Hepta = 7 Nona = 9 Di = 2 Tetra=4 Hexa= 6 Octa = 8 Deca = 10

Writing Formulas Covalent compounds made of 2 nonmetals CO 2 = Carbon “di” oxide N 2 O = Dinitrogen monoxide N 3 O 5 = ? ?

Ionic compounds made with a metal and a non-metal The compounds overall charge = zero Na. Cl = Sodium chloride (Na is a +ion and Cl is a –ion) Mg. Cl 2 = Magnesium chloride (Mg is a 2+ion, so we need 2 –ions of Cl)
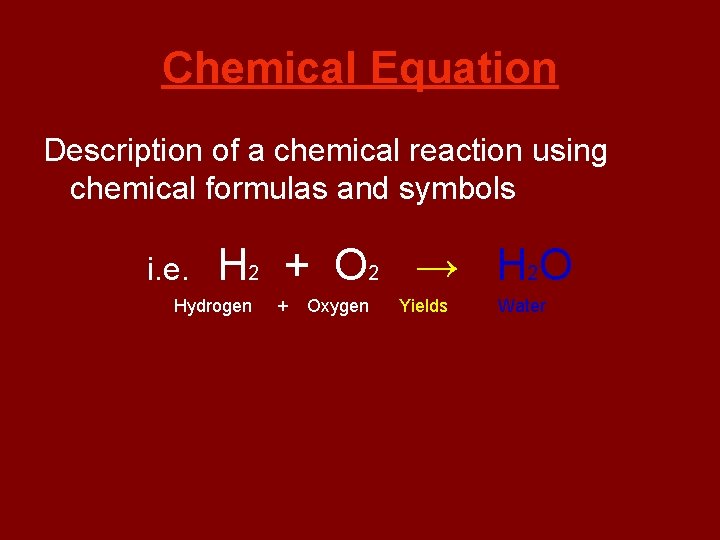
Chemical Equation Description of a chemical reaction using chemical formulas and symbols i. e. H 2 + O 2 → H 2 O Hydrogen + Oxygen Yields Water

Equation Terms Reactants starting materials & ALWAYS on left Products formed materials & ALWAYS on right Coefficient a number placed in front of a chemical symbol or formula ( a regular size number ) Used to BALANCE equations, has distributed propery
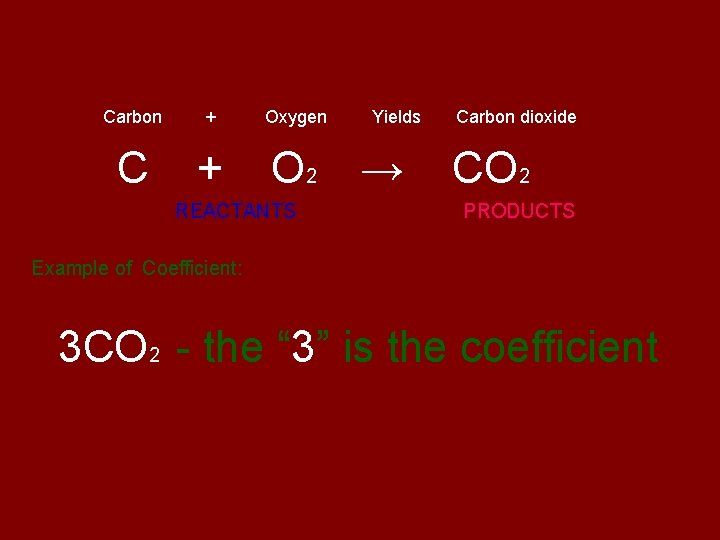
Carbon + C + Oxygen Yields O 2 → REACTANTS Carbon dioxide CO 2 PRODUCTS Example of Coefficient: 3 CO 2 - the “ 3” is the coefficient

Law of Conservation of Matter is neither created nor destroyed in ordinary chemical/physical changes
Balancing Chemical Equations A chemical equation must show the same number of each type of atom on both sides of the equation. Steps to Balance an Equation 1. Write the equation 2. Count the atoms 3. Use coefficients to balance atoms 4. Look back and check

1. Write the equation H 2 + O 2 → H 2 O 2. Count the atoms Reactants H = 2, O = 2 Products H = 2, O = 1 3. Use Coefficients to balance atoms change H 2 O to 2 H 2 O makes products H=4, O=2 H 2 + O 2 → 2 H 2 O then change H 2 to 2 H 2 makes reactants H=4, O=2 2 H 2 + O 2 → 2 H 2 O 4. Look Back and Check Reactants: H=4, O=2 Products: H=4, O=2 Balanced Equation

4 Types of Chemical Rxns 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement

1. Synthesis Rxn Two or more substances combine to form a single compound. *To Put together* 2 Na + Cl 2 → 2 Na. Cl

2. Decomposition *To Take Apart* A single compound breaks down to form two or more simpler substances. H 2 CO 3 → H 2 O + CO 2
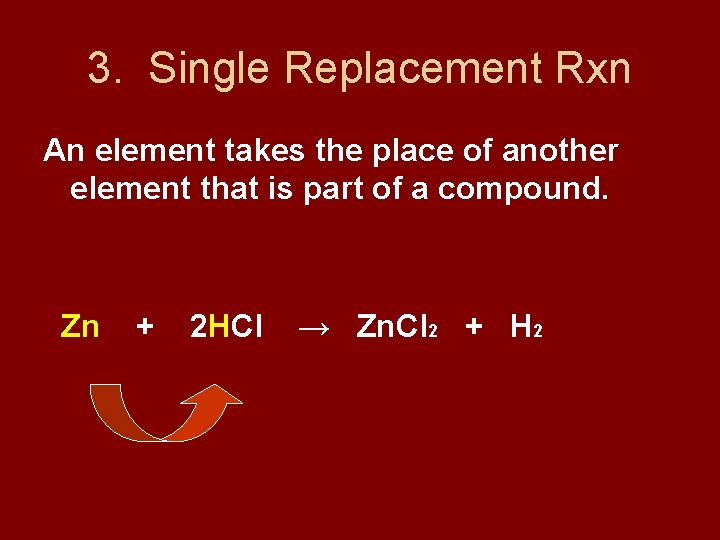
3. Single Replacement Rxn An element takes the place of another element that is part of a compound. Zn + 2 HCl → Zn. Cl 2 + H 2
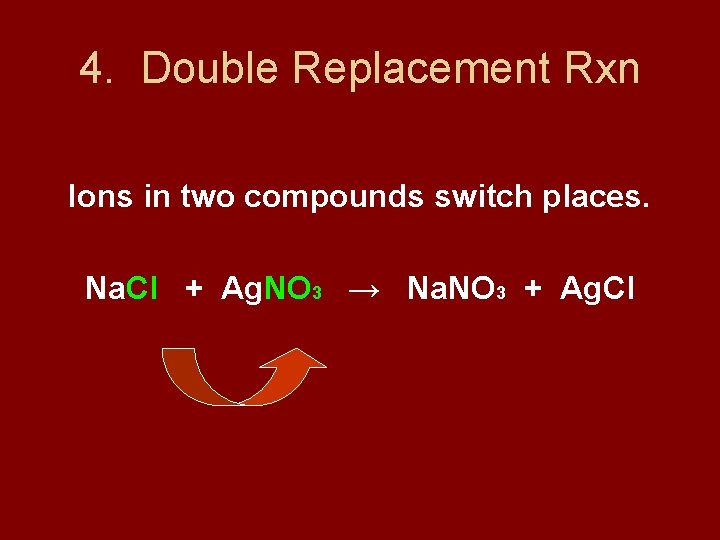
4. Double Replacement Rxn Ions in two compounds switch places. Na. Cl + Ag. NO 3 → Na. NO 3 + Ag. Cl

Law of Conservation of Energy can neither be created nor destroyed Every rxn involves energy

Energy & Reactions There is a “certain” amount of energy required to make substances react. This is called the - Activation Energy
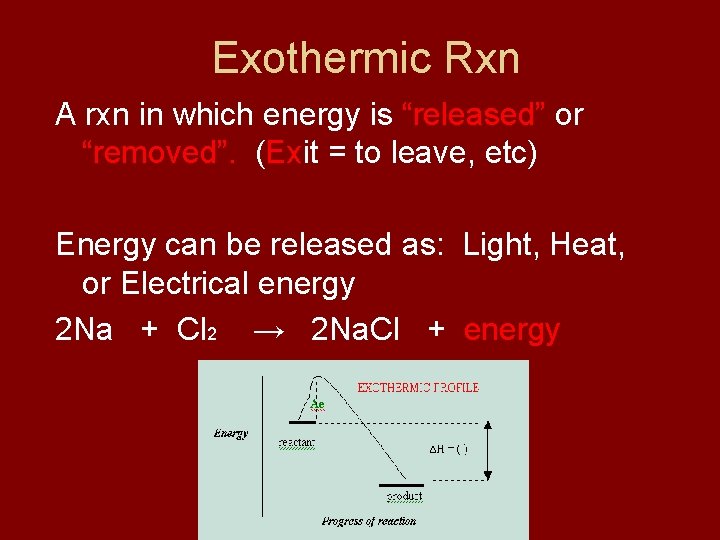
Exothermic Rxn A rxn in which energy is “released” or “removed”. (Exit = to leave, etc) Energy can be released as: Light, Heat, or Electrical energy 2 Na + Cl 2 → 2 Na. Cl + energy
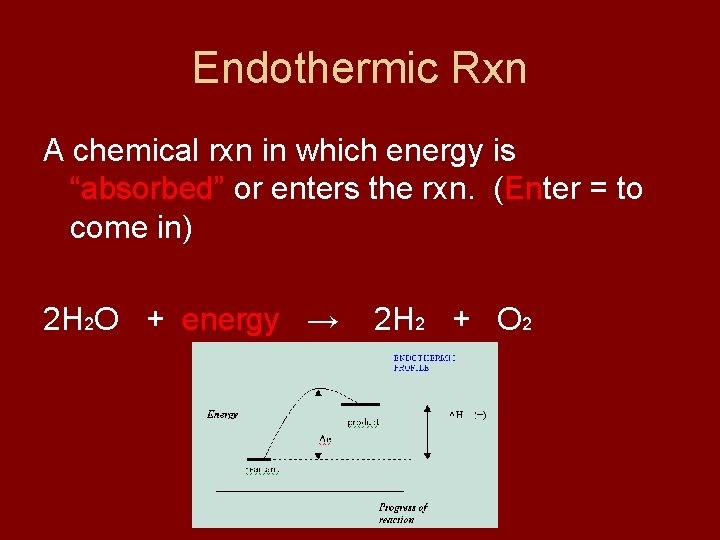
Endothermic Rxn A chemical rxn in which energy is “absorbed” or enters the rxn. (Enter = to come in) 2 H 2 O + energy → 2 H 2 + O 2
Factors that affect Rates of Rxn 1. Temperature – Increase temp = increase rxn – Decrease temp = decrease rxn 2. Concentration [ ] Inc [ ] = Inc rxn 3. Surface area Inc SA = Inc rxn

4. Catalyst Substance that speeds up a rxn Lowers the Activation Energy Biological catalyst is called an enzyme 5. Inhibitor slows down or stops rxn (i. e. preservatives and poison)
- Slides: 31