Forming Ions Why do Ions form All elements
















- Slides: 18

Forming Ions

Why do Ions form? • All elements want to be like their closest noble gas and have 8 electrons in their last energy level • Some elements have to lose electrons while others gain electrons to get this “stable state”

Positively Charged Ions (Cations) • When an atom loses an electron, you get a POSITIVELY charged ion • Cations are usually metals (less than 4 electrons in their outer shell) • Examples: Na+, Mg 2+, Al 3+

Negatively Charged Ions (Anions) • When an atom gains an electron, you get a NEGATIVELY charged ion. • Anions are usually non-metals ( 4 or more than 4 electrons in their outer shell) • Examples: O 2 -, F-, N 3 -

Calculating Charge (copy into notes) • Each proton has a charge of +1, each electron has a charge of -1 • Sulfur has 16 electrons (6 valence electrons) • To reach a stable state, it wants 8 valence electrons • It gains TWO electrons, it now has 18 e- (8 valence e) ▫ Therefore, these have a charge of -18 (18 x -1) • It also has 16 p+ ▫ therefore, these have a charge of +16 (16 x +1) • Total Charge = -18 + (+16) = -2

Drawing an Ion Bohr Rutherford Diagram 1) Determine the charge and how many electrons are gained or lost 2) Draw a Bohr Rutherford diagram ▫ Make sure to draw the diagram with electrons gained or electrons lost to form an ion 3) Put a big square bracket around the diagram 4) Write the ion charge on the top right of the square brackets

• Practice by drawing a Ion Bohr Rutherford Diagram for Sulfur • Check your answer on the next slide

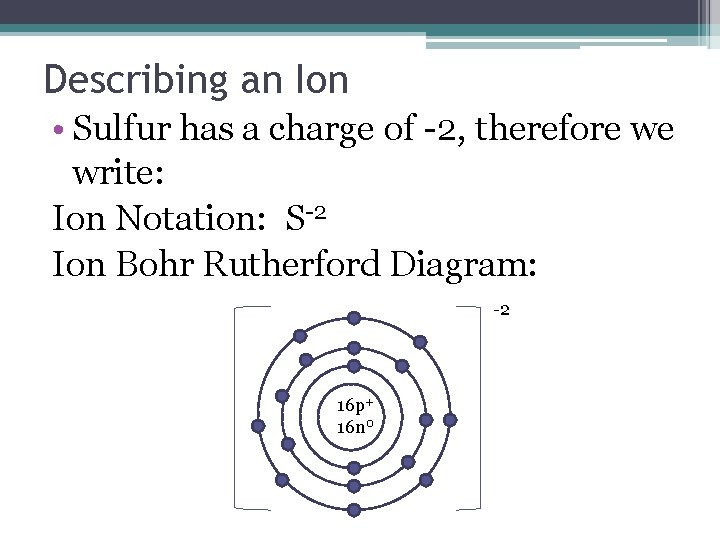
Describing an Ion • Sulfur has a charge of -2, therefore we write: Ion Notation: S-2 Ion Bohr Rutherford Diagram: -2 16 p+ 16 n 0

Chemical Bond • An attraction between two or more elements that allow the formation of a new substance • Only the valence electrons are involved in creating a chemical bond

Forming Ionic Compounds • Videos – Dogs and ions

Ionic Bonds • Between a metal and a non-metal • Electrons are transferred from the metal to the non-metal • From the video, why are the metal and non-metal attracted?

Creating an Ionic Formula 1) Determine the charge on the metal and the non -metal 2) Write them on the top right of each substance (the metal should always be written first) 3) Use the cross-over method to create the chemical formula 4) If there is a common multiple, divide each subscript by the common multiple to simplify

Example • Pick a metal and a non-metal

Naming an Ionic Compound • Write the name of the metal • Write the name of the non-metal only replace the ending with ‘ide’ ▫ Oxygen becomes Oxide ▫ Sulphur becomes Sulphide

Practice!

Multivalent Ions • A multivalent ion can have several charges ▫ Look at the back of your periodic table • Most of these are transition metals ▫ Iron has 2 possible charges - +2, +3 ▫ Which one should we use? �We use roman numerals to let people know which one was used. Ex: Iron (II) Oxide or Iron (III) Oxide

To determine which charge • Reverse Criss Cross Cu Br 2 Cu 2 Br 1 Since the charge on Bromine is -1, the charge on copper must be +2 Therefore, Copper (II) Bromide

Polyatomic Ions • Polyatomic ions are chemical best friends that are bonded together and go looking for electrons • We treat each polyatomic ion, the same way we would treat a regular ion ▫ Still use criss cross method but they have their own special names (see back of periodic table)