Formic Sulfuric Anhydride A new chemical species with

- Slides: 20

Formic Sulfuric Anhydride: A new chemical species with possible implications for atmospheric aerosol Rebecca B. Mackenzie, Christopher T. Dewberry, and Kenneth R. Leopold Department of Chemistry, University of Minnesota 70 th International Symposium on Molecular Spectroscopy 1

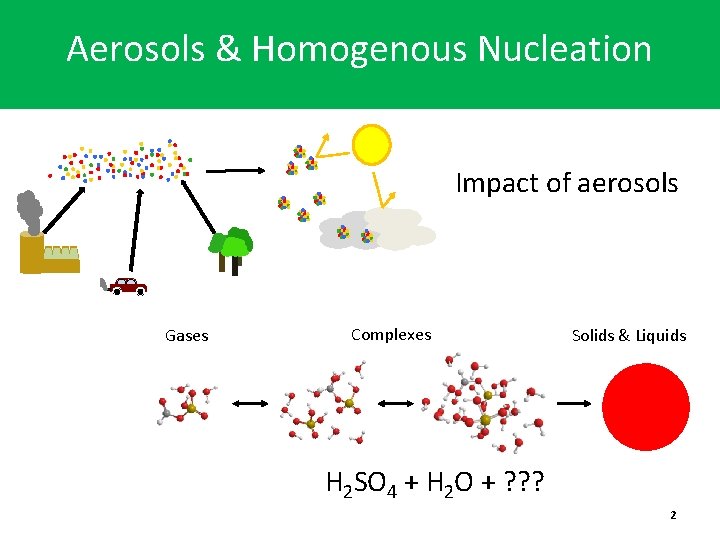
Aerosols & Homogenous Nucleation Impact of aerosols Gases Complexes Solids & Liquids H 2 SO 4 + H 2 O + ? ? ? 2

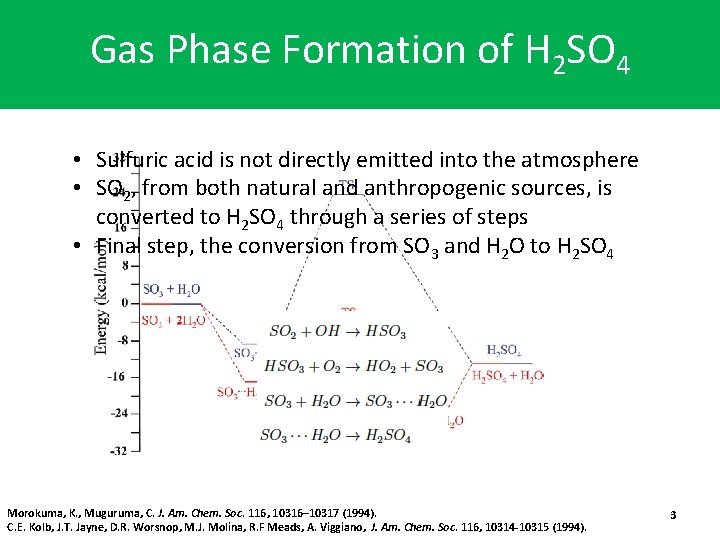
Gas Phase Formation of H 2 SO 4 • Sulfuric acid is not directly emitted into the atmosphere • SO 2, from both natural and anthropogenic sources, is converted to H 2 SO 4 through a series of steps • Final step, the conversion from SO 3 and H 2 O to H 2 SO 4 Morokuma, K. , Muguruma, C. J. Am. Chem. Soc. 116, 10316– 10317 (1994). C. E. Kolb, J. T. Jayne, D. R. Worsnop, M. J. Molina, R. F Meads, A. Viggiano, J. Am. Chem. Soc. 116, 10314 -10315 (1994). 3

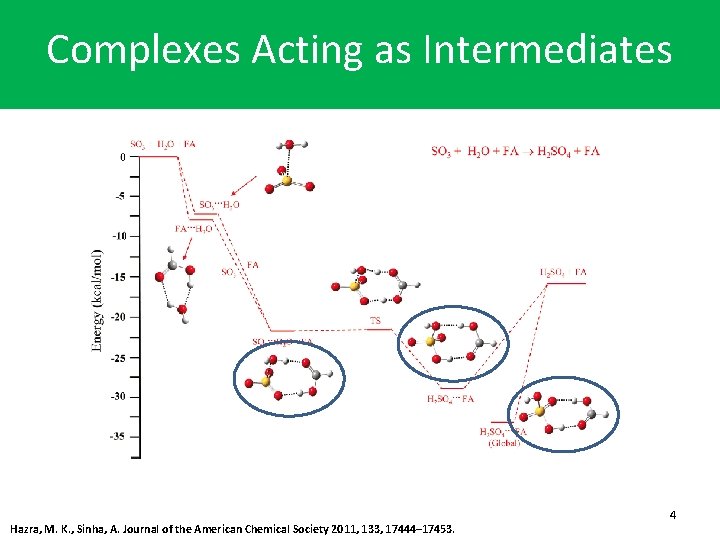
Complexes Acting as Intermediates Hazra, M. K. , Sinha, A. Journal of the American Chemical Society 2011, 133, 17444– 17453. 4

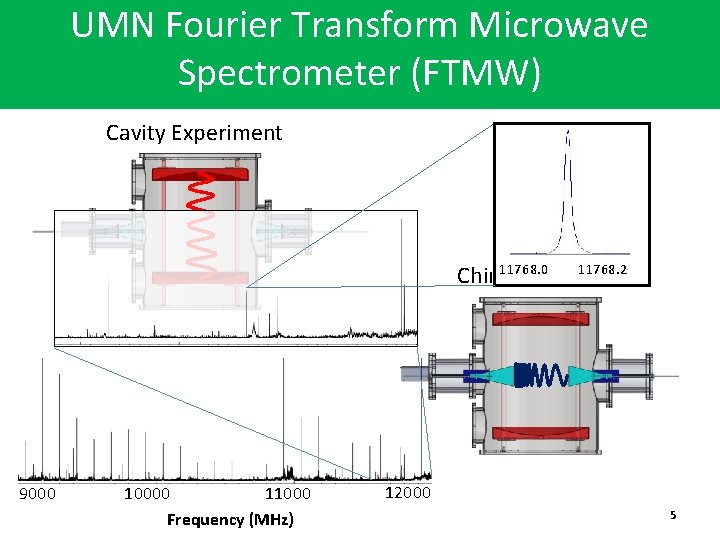
UMN Fourier Transform Microwave Spectrometer (FTMW) Cavity Experiment 11768. 2 11768. 0 Chirp Experiment 9000 10000 11000 Frequency (MHz) 12000 5

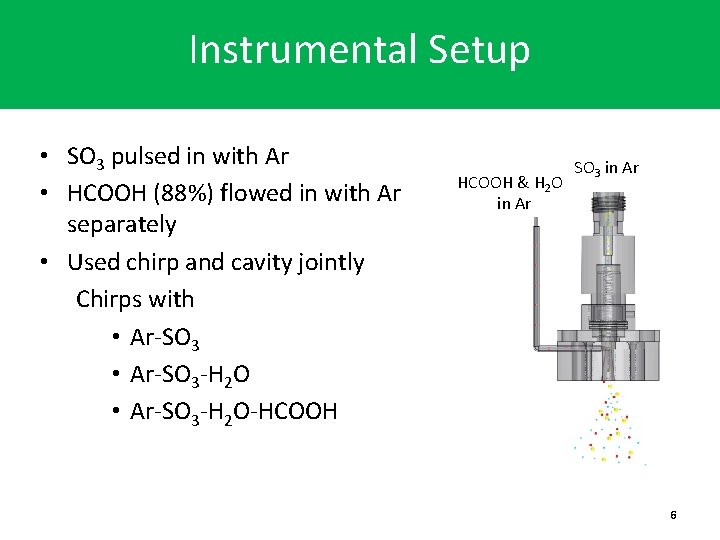
Instrumental Setup • SO 3 pulsed in with Ar • HCOOH (88%) flowed in with Ar separately • Used chirp and cavity jointly Chirps with • Ar-SO 3 -H 2 O-HCOOH & H 2 O in Ar SO 3 in Ar 6

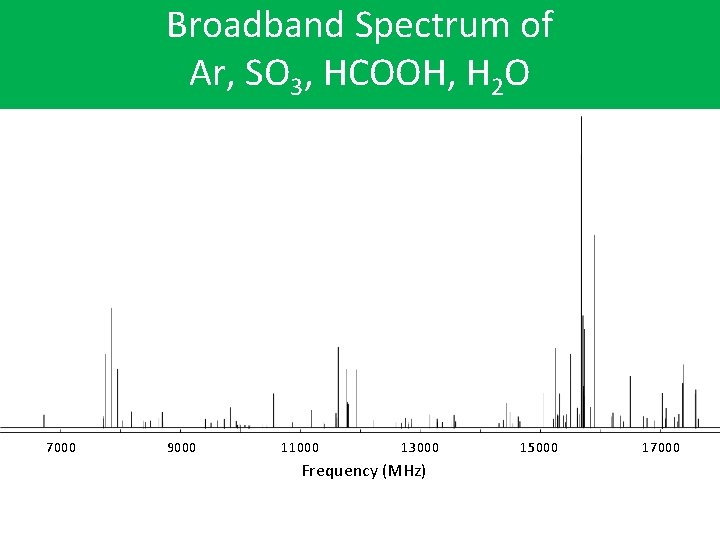
Broadband Spectrum of Ar, SO 3, HCOOH, H 2 O 7000 9000 11000 13000 Frequency (MHz) 15000 17000

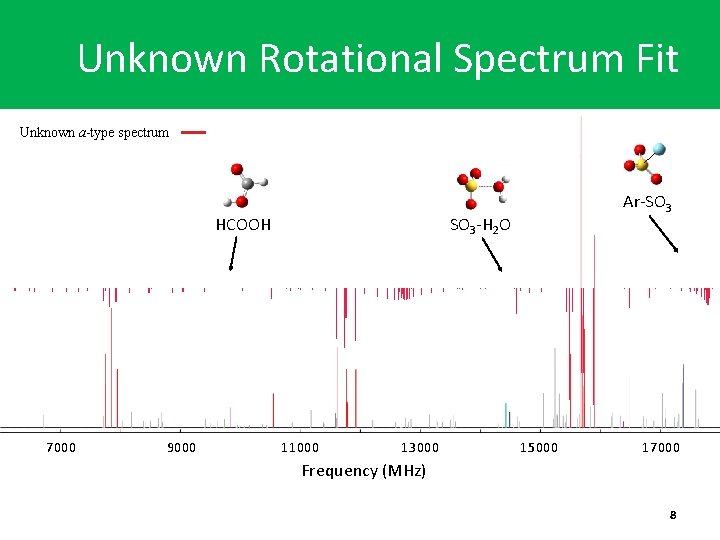
Unknown Rotational Spectrum Fit Unknown a-type spectrum SO 3 -H 2 O HCOOH 7000 9000 Ar-SO 3 11000 13000 15000 17000 Frequency (MHz) 8

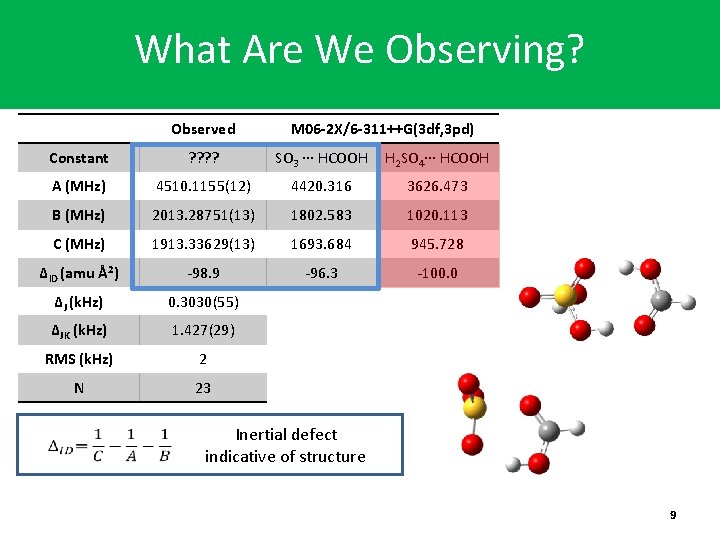
What Are We Observing? Observed M 06 -2 X/6 -311++G(3 df, 3 pd) Constant ? ? SO 3 ∙∙∙ HCOOH H 2 SO 4∙∙∙ HCOOH A (MHz) 4510. 1155(12) 4420. 316 3626. 473 B (MHz) 2013. 28751(13) 1802. 583 1020. 113 C (MHz) 1913. 33629(13) 1693. 684 945. 728 ΔID (amu Å2) -98. 9 -96. 3 -100. 0 ΔJ (k. Hz) 0. 3030(55) ΔJK (k. Hz) 1. 427(29) RMS (k. Hz) 2 N 23 Inertial defect indicative of structure 9

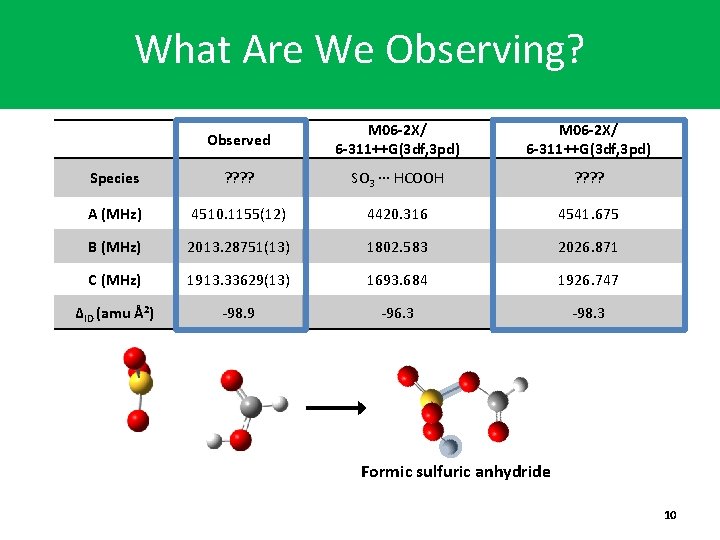
What Are We Observing? Observed M 06 -2 X/ 6 -311++G(3 df, 3 pd) Species ? ? SO 3 ∙∙∙ HCOOH ? ? A (MHz) 4510. 1155(12) 4420. 316 4541. 675 B (MHz) 2013. 28751(13) 1802. 583 2026. 871 C (MHz) 1913. 33629(13) 1693. 684 1926. 747 ΔID (amu Å2) -98. 9 -96. 3 -98. 3 Formic sulfuric anhydride 10

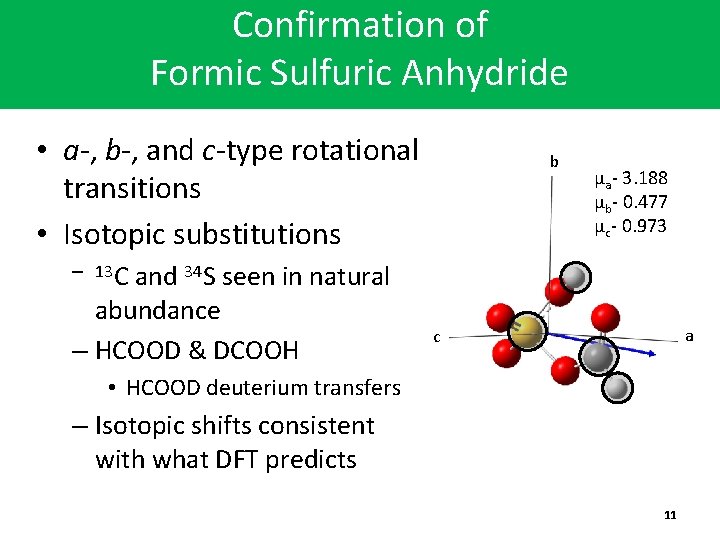
Confirmation of Formic Sulfuric Anhydride • a-, b-, and c-type rotational transitions • Isotopic substitutions b µa- 3. 188 µb- 0. 477 µc- 0. 973 – 13 C and 34 S seen in natural abundance – HCOOD & DCOOH a c • HCOOD deuterium transfers – Isotopic shifts consistent with what DFT predicts 11

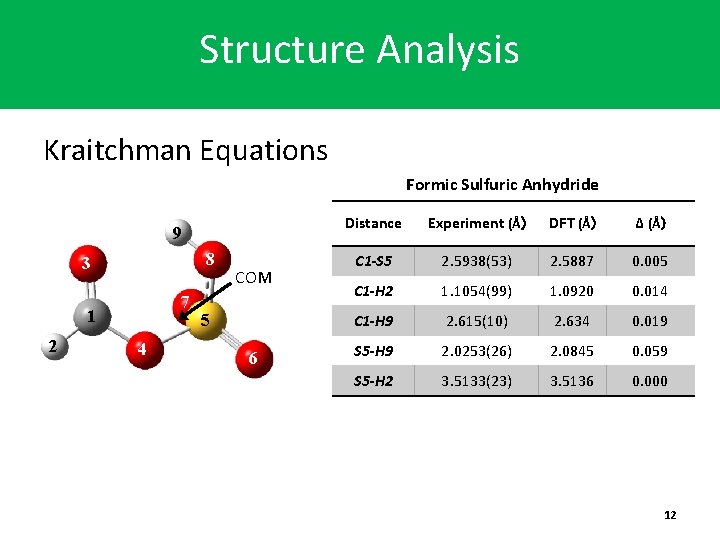
Structure Analysis Kraitchman Equations Formic Sulfuric Anhydride 9 8 3 7 1 2 4 COM 5 6 Distance Experiment (Å) DFT (Å) Δ (Å) C 1 -S 5 2. 5938(53) 2. 5887 0. 005 C 1 -H 2 1. 1054(99) 1. 0920 0. 014 C 1 -H 9 2. 615(10) 2. 634 0. 019 S 5 -H 9 2. 0253(26) 2. 0845 0. 059 S 5 -H 2 3. 5133(23) 3. 5136 0. 000 12

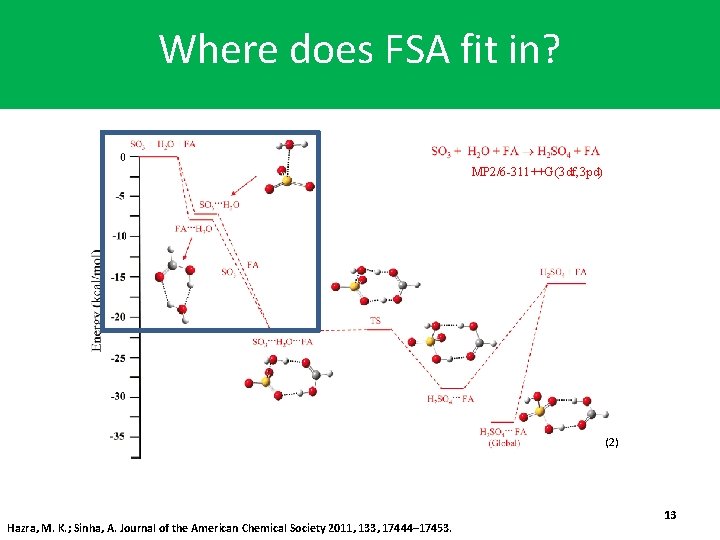
Where does FSA fit in? MP 2/6 -311++G(3 df, 3 pd) (2) Hazra, M. K. ; Sinha, A. Journal of the American Chemical Society 2011, 133, 17444– 17453. 13

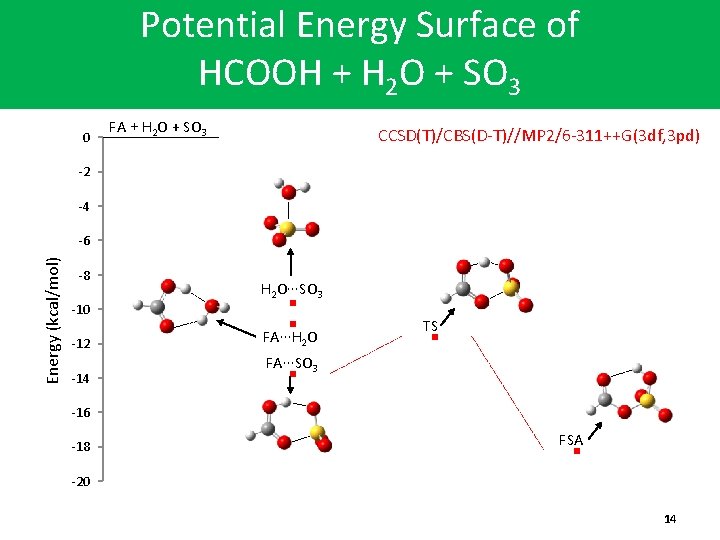
Potential Energy Surface of HCOOH + H 2 O + SO 3 0 FA + H 2 O + SO 3 CCSD(T)/CBS(D-T)//MP 2/6 -311++G(3 df, 3 pd) -2 -4 Energy (kcal/mol) -6 -8 -10 -12 -14 H 2 O∙∙∙SO 3 FA∙∙∙H 2 O TS FA∙∙∙SO 3 -16 -18 FSA -20 14

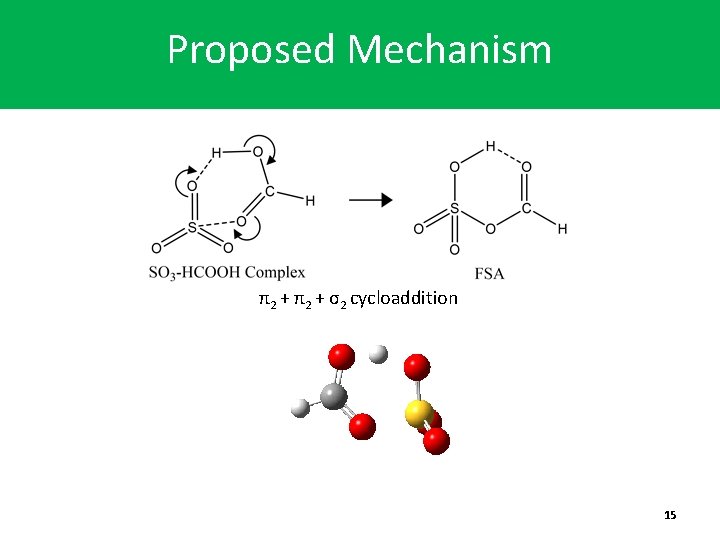
Proposed Mechanism π2 + σ2 cycloaddition 15

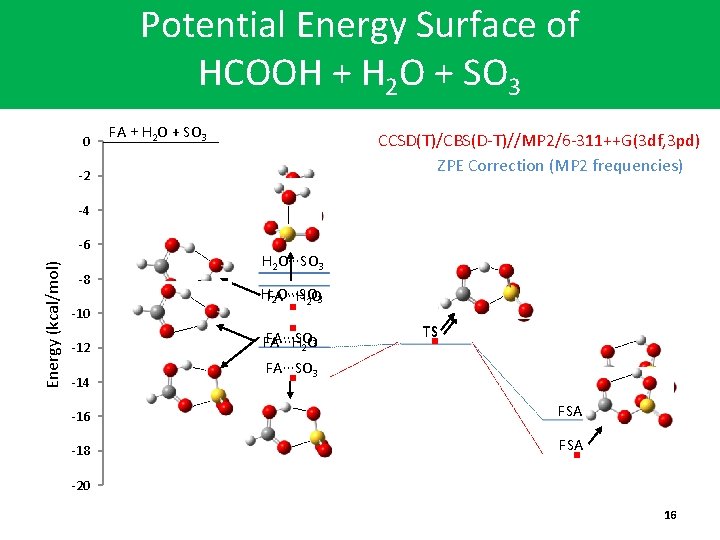
Potential Energy Surface of HCOOH + H 2 O + SO 3 0 FA + H 2 O + SO 3 CCSD(T)/CBS(D-T)//MP 2/6 -311++G(3 df, 3 pd) ZPE Correction (MP 2 frequencies) -2 -4 Energy (kcal/mol) -6 -8 -10 -12 -14 H 2 O∙∙∙SO 3 HFA∙∙∙H 2 O∙∙∙SO 2 O 3 FA∙∙∙SO FA∙∙∙H 2 O 3 TS FA∙∙∙SO 3 -16 FSA -18 FSA -20 16

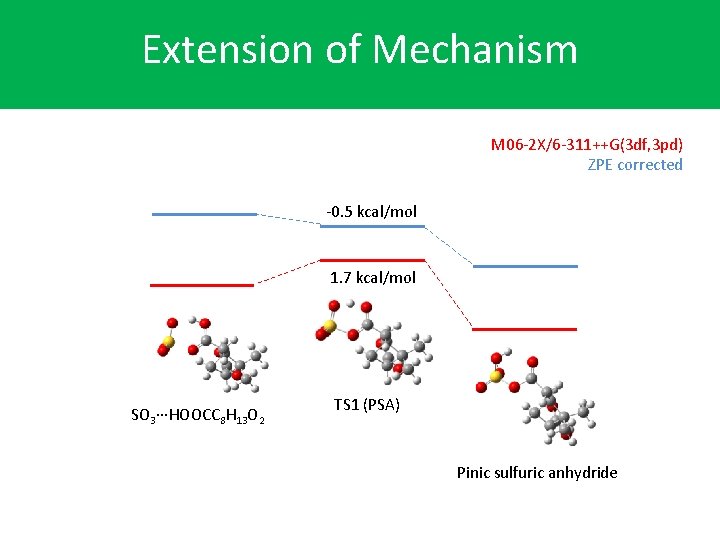
Extension of Mechanism M 06 -2 X/6 -311++G(3 df, 3 pd) ZPE corrected -0. 5 kcal/mol 1. 7 kcal/mol SO 3∙∙∙HOOCC 8 H 13 O 2 TS 1 (PSA) Pinic sulfuric anhydride

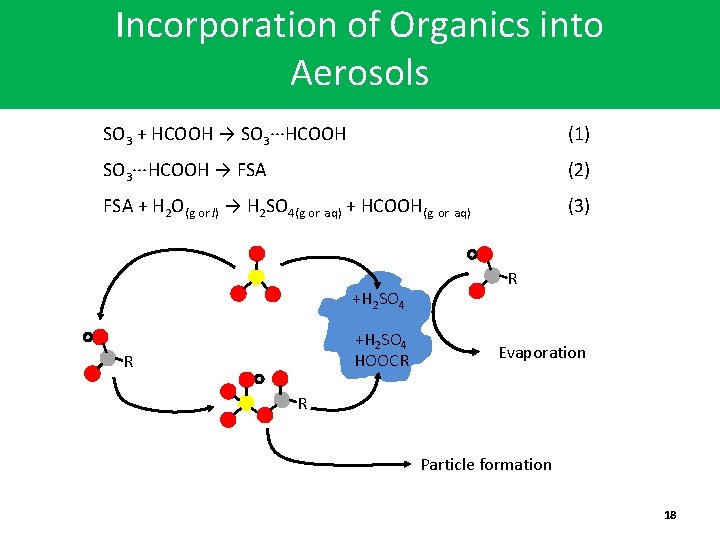
Incorporation of Organics into Aerosols SO 3 + HCOOH → SO 3∙∙∙HCOOH (1) SO 3∙∙∙HCOOH → FSA (2) FSA + H 2 O(g or l) → H 2 SO 4(g or aq) + HCOOH(g or aq) (3) +H 2 SO 4 HOOCR R R Evaporation R Particle formation 18

Conclusions • FSA forms readily from SO 3 + HCOOH – Computational work suggests a barrierless cycloaddition reaction – Requires the formation of only a dimer not a trimer • Mechanism appears applicable to other carboxylic acids • Hydrolysis could incorporate sulfuric acid and a carboxylic acid in prenucleation clusters 19

Funding & Acknowledgements • Leopold Group Dr. Chris Dewberry • Dr. Pete Mc. Murry • Dr. Tom Hoye • Will Isley 20