Formed by bonding small molecules monomers together into

![Formed by bonding small molecules [monomers] together into chains called polymers Formed by bonding small molecules [monomers] together into chains called polymers](http://slidetodoc.com/presentation_image/60c84706931351718e21d4c28e2cde86/image-2.jpg)
Formed by bonding small molecules [monomers] together into chains called polymers
These larger molecules, macromolecules, may be composed of thousands of atoms. The 4 major classes of macromolecules are: carbohydrates, lipids, proteins, & nucleic acids.
Carbohydrates – Carbohydrates are compounds composed of carbon, hydrogen, and oxygen, with the general molecular formula CH 2 O.
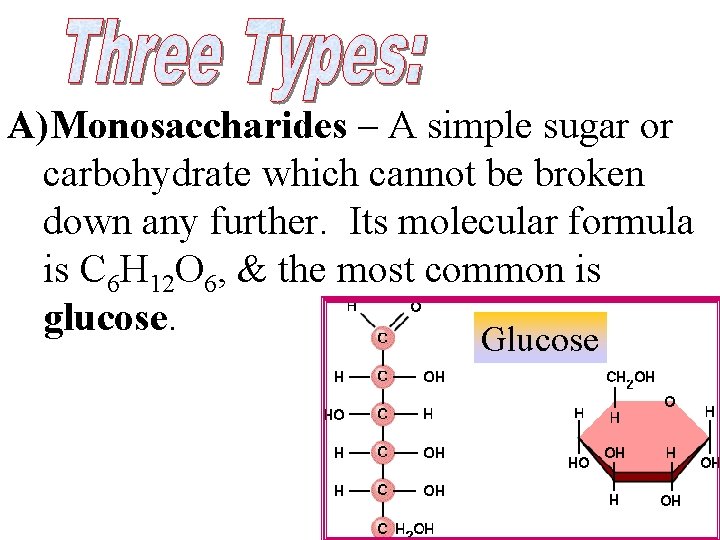
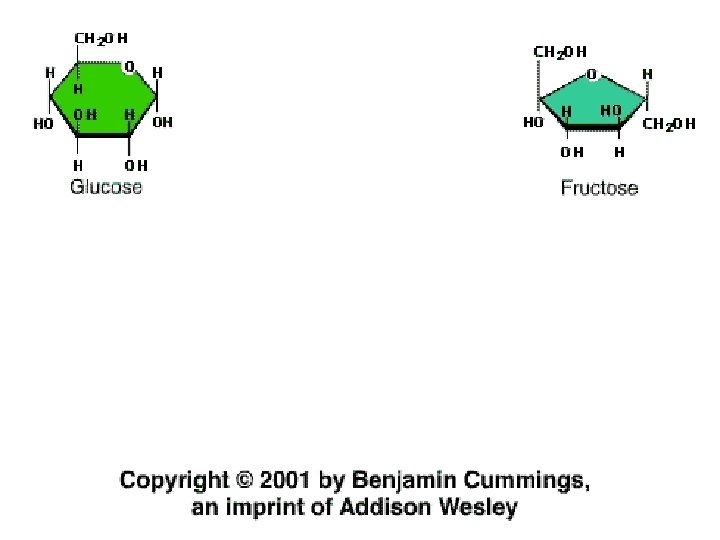
A)Monosaccharides – A simple sugar or carbohydrate which cannot be broken down any further. Its molecular formula is C 6 H 12 O 6, & the most common is glucose. Glucose
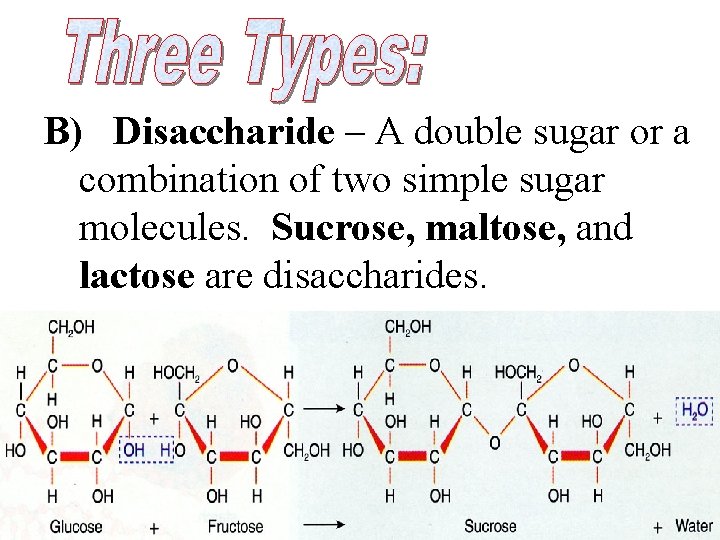
B) Disaccharide – A double sugar or a combination of two simple sugar molecules. Sucrose, maltose, and lactose are disaccharides.
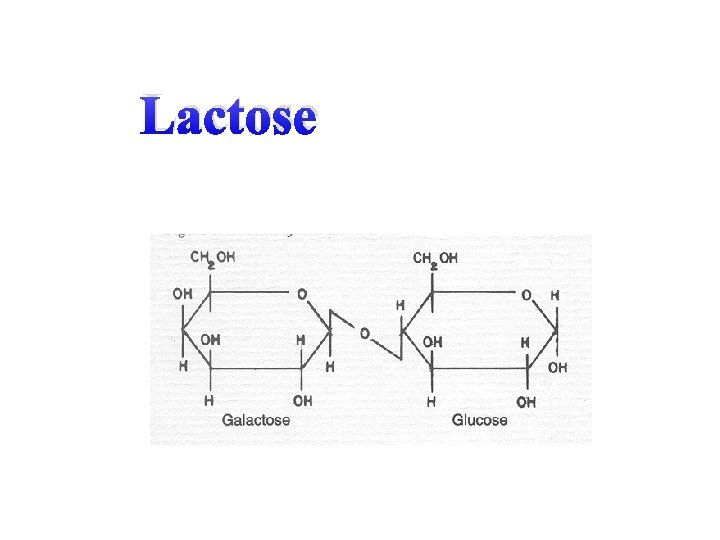
Lactose
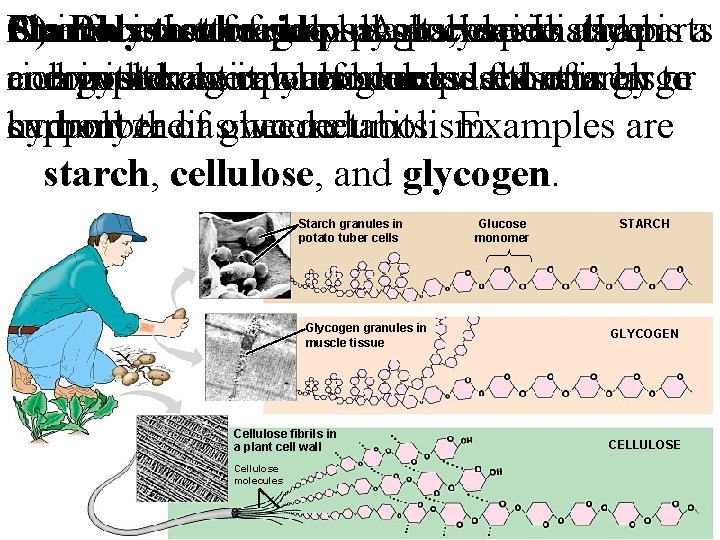
Animals C) One Starch Plants Polysaccharide function can isthat astore storage feed of surplus polysaccharides on polysaccharide –plants, Aglucose polysaccharide especially in is starch as anparts is a energy composed and rich complex withdraw in starch, storage entirely compound can itmacromolecule when also of glucose needed composed access monomers. this for that of energy starch isa large to or hydrolyzed carbon. support number their ofasglucose own needed. metabolism. units. Examples are starch, cellulose, and glycogen. Starch granules in potato tuber cells Glycogen granules in muscle tissue Cellulose fibrils in a plant cell wall Cellulose molecules Glucose monomer STARCH GLYCOGEN CELLULOSE
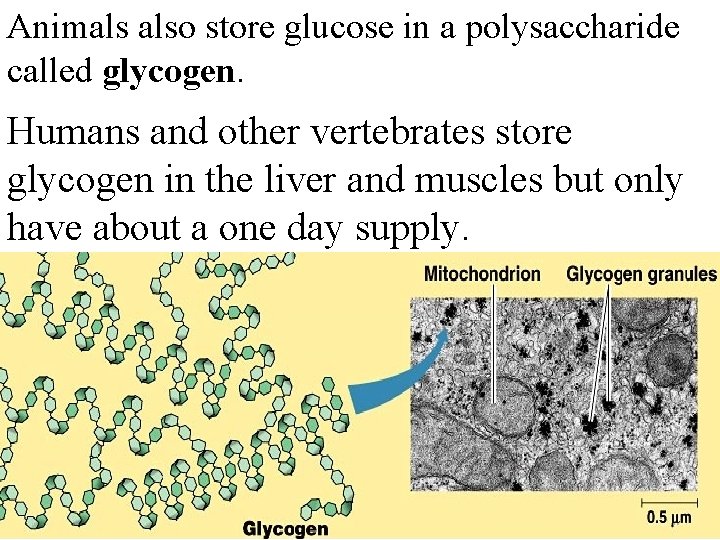
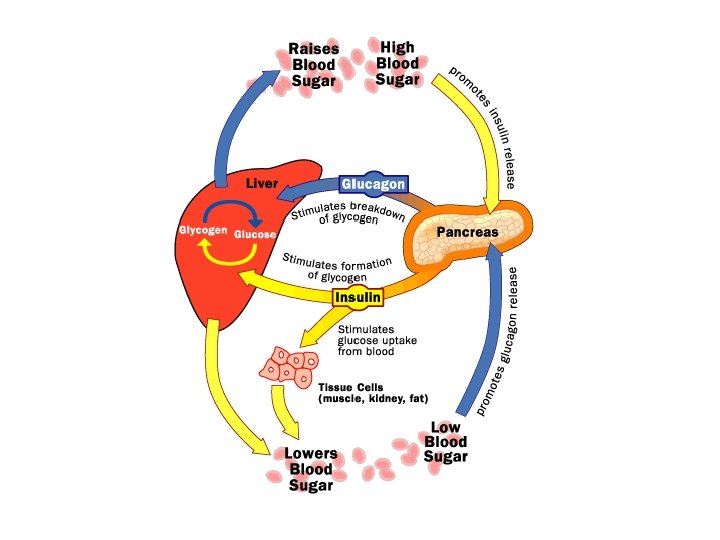
Animals also store glucose in a polysaccharide called glycogen. Humans and other vertebrates store glycogen in the liver and muscles but only have about a one day supply.
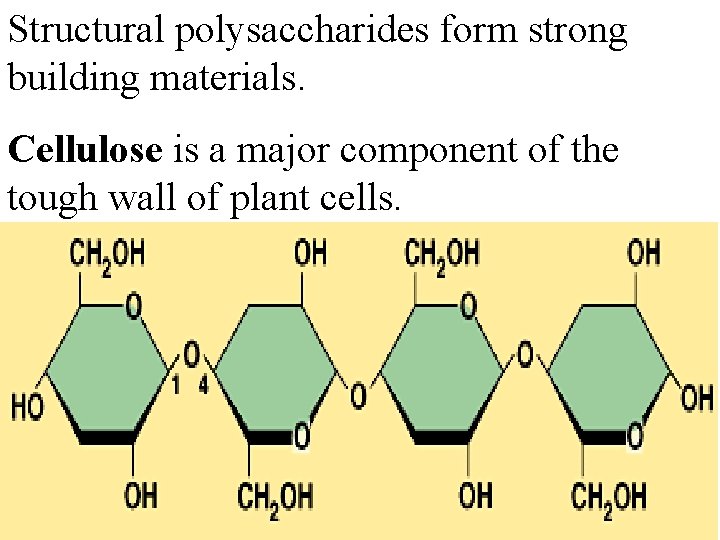
Structural polysaccharides form strong building materials. Cellulose is a major component of the tough wall of plant cells.
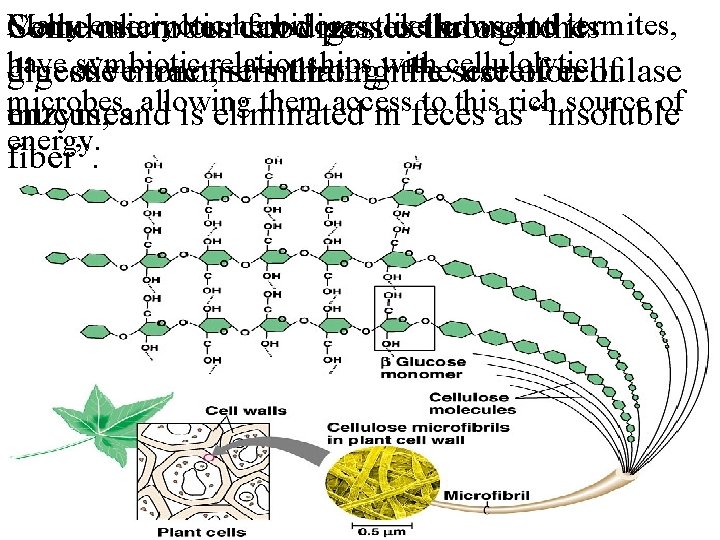
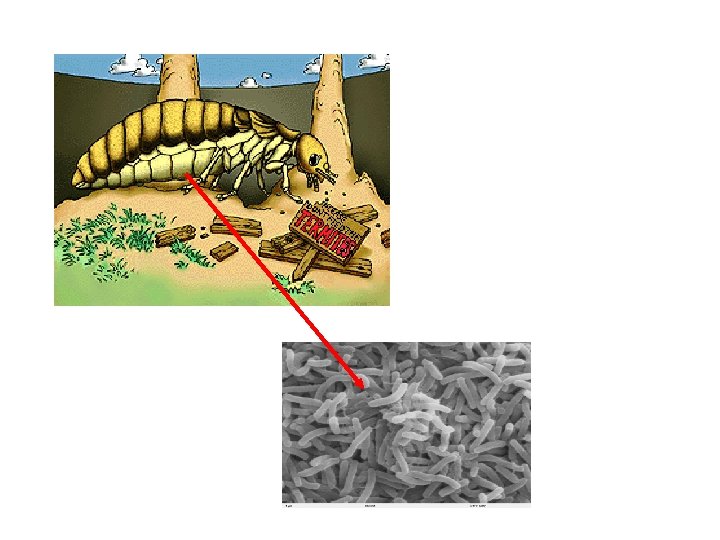
Many eukaryotic like cows and termites, Cellulose Some microbes in ourherbivores, can fooddigest passes cellulose through tothe its have symbiotic digestive glucose monomers tract, relationships stimulating throughwith thecellulolytic secretion use of cellulase of microbes, allowing them access to this rich source of mucus, and is eliminated in feces as “insoluble enzymes. energy. fiber”.
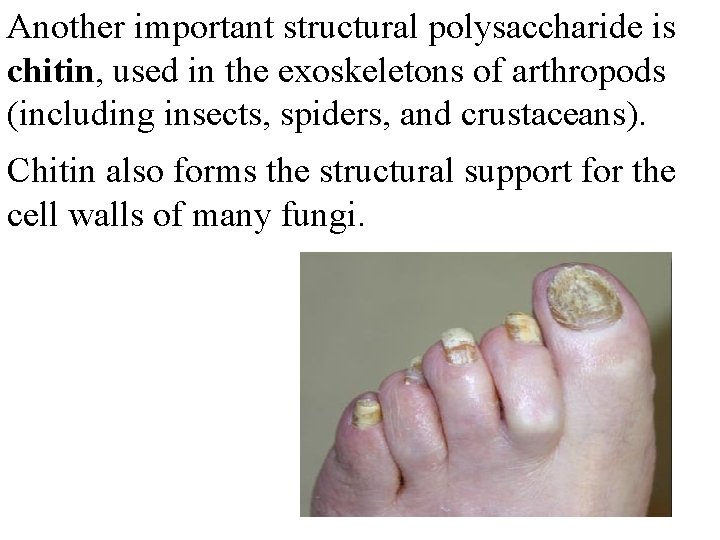
Another important structural polysaccharide is chitin, used in the exoskeletons of arthropods (including insects, spiders, and crustaceans). Chitin also forms the structural support for the cell walls of many fungi.

Lipids are organic compounds that dissolve The unifying feature of lipids is that they all poorly, if at all, in water (hydrophobic). have little or no affinity for water. This is because their structures are dominated by nonpolar covalent bonds. Saturated Mono. Unsaturated Poly. Unsaturated
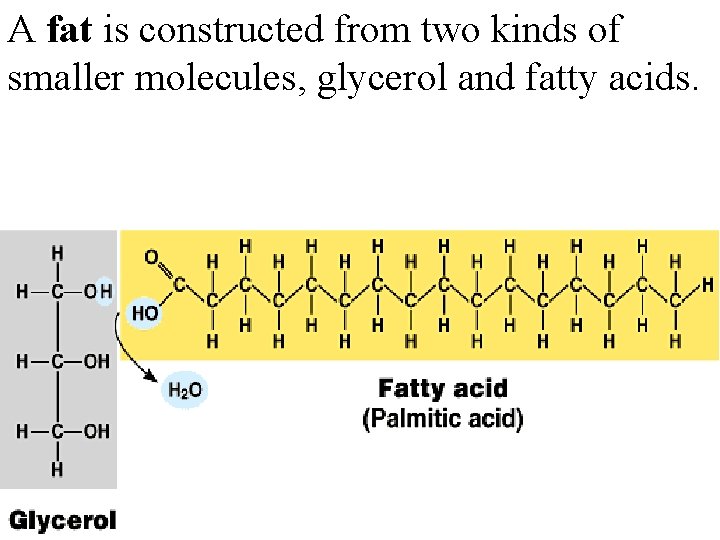
A fat is constructed from two kinds of smaller molecules, glycerol and fatty acids.
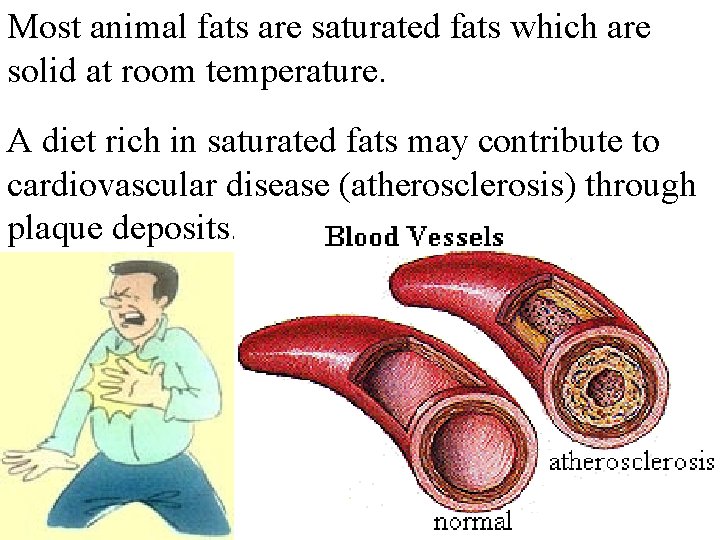
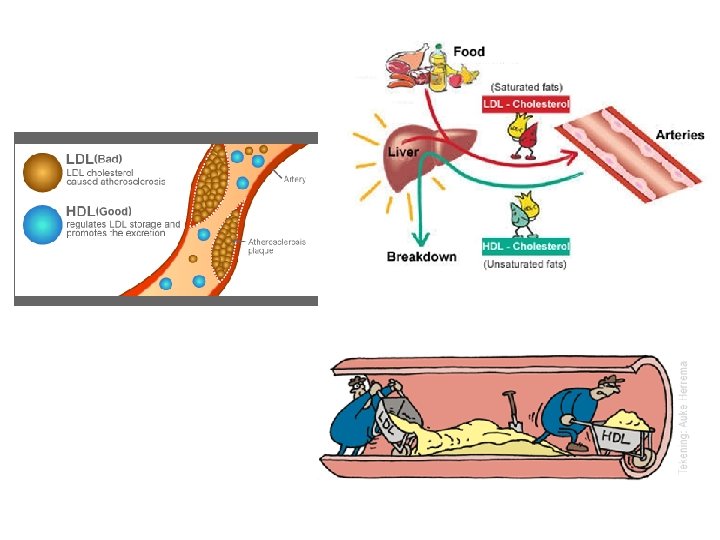
Most animal fats are saturated fats which are solid at room temperature. A diet rich in saturated fats may contribute to cardiovascular disease (atherosclerosis) through plaque deposits.
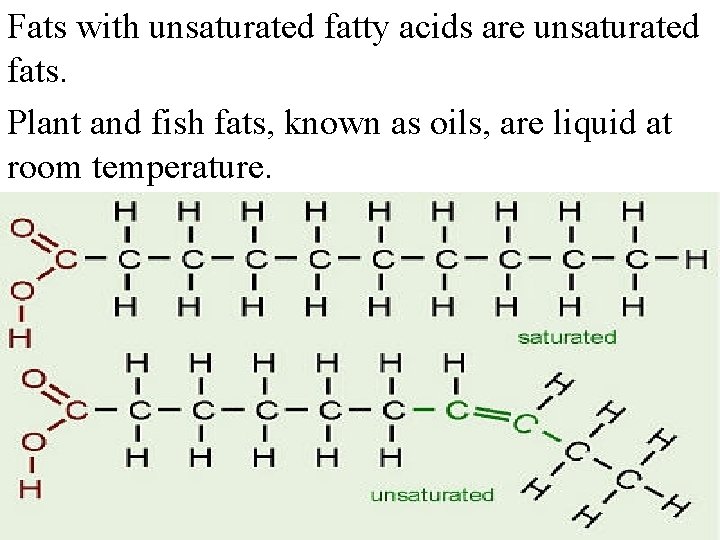
Fats with unsaturated fatty acids are unsaturated fats. Plant and fish fats, known as oils, are liquid at room temperature.
The major function of fats is energy storage. A gram of fat stores more than twice as much energy as a gram of a polysaccharide. Humans and other mammals store fats as long-term energy reserves in special cells. Fat also functions to cushion vital organs.

A layer of fats can also function as insulation. This subcutaneous layer is especially thick in whales, seals, and most other marine mammals.
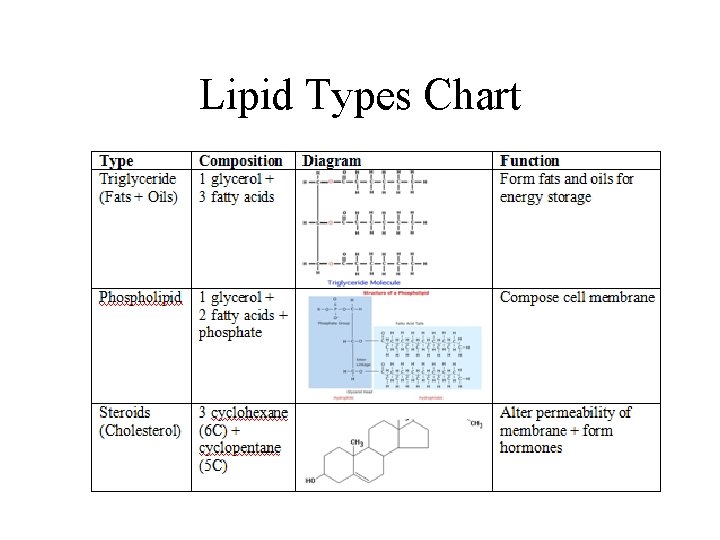
Lipid Types Chart

Phospholipids have two fatty acids attached to glycerol and a phosphate group.
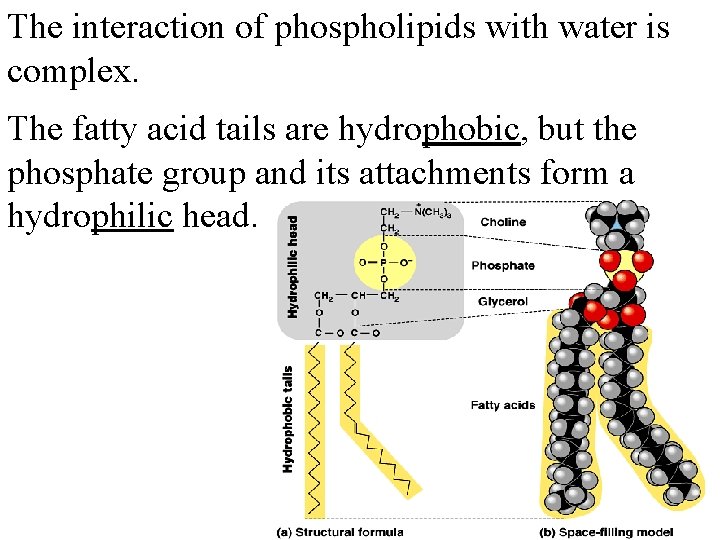
The interaction of phospholipids with water is complex. The fatty acid tails are hydrophobic, but the phosphate group and its attachments form a hydrophilic head.
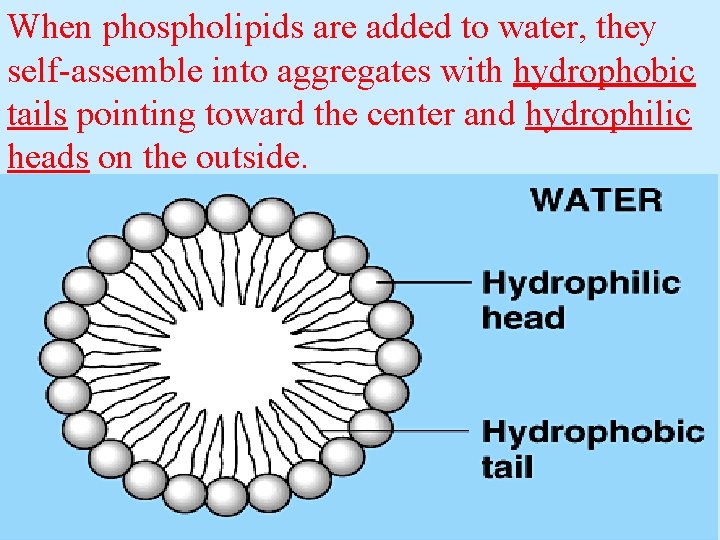
When phospholipids are added to water, they self-assemble into aggregates with hydrophobic tails pointing toward the center and hydrophilic heads on the outside.
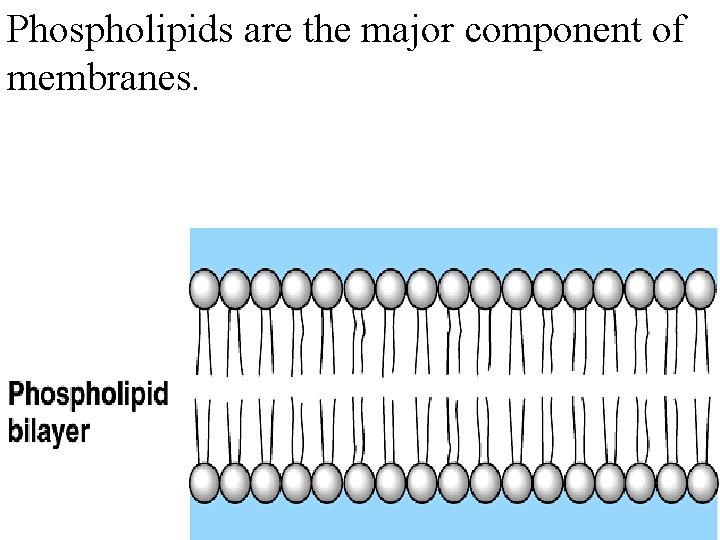
Phospholipids are the major component of membranes.
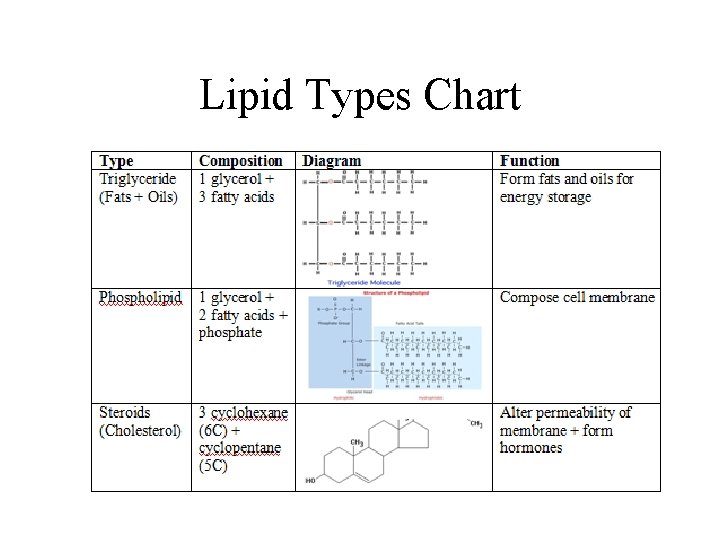
Lipid Types Chart
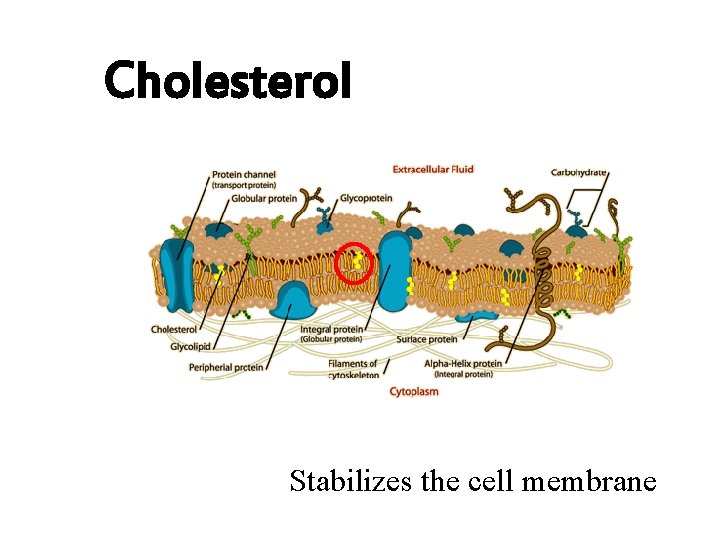
Cholesterol Stabilizes the cell membrane
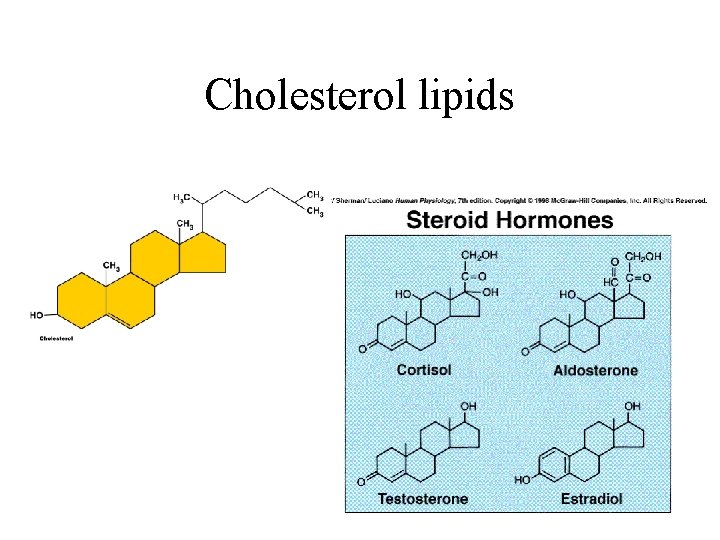
Cholesterol lipids

Lipid Digestion Animation http: //www. wiley. com/college/boyer/0470003790/animations/ fatty_acid_metabolism/fatty_acid_metabolism. htm

a. k. a. “Poly. Peptides”
Proteins are composed of carbon, hydrogen, oxygen, nitrogen, and sometimes phosphorus and sulfur. Approx. 50% of the dry weight of living matter is protein.

Proteins are instrumental in about everything that an organism does. These functions include structural support, and are the enzymes that break and construct products in our bodies

Proteins are instrumental in about everything that an organism does. Proteins are the overwhelming # of enzymes in a cell and break down and put together materials in the body.
Humans have tens of thousands of different proteins, each with their own structure and function. What are proteins made out of? I’m not sure
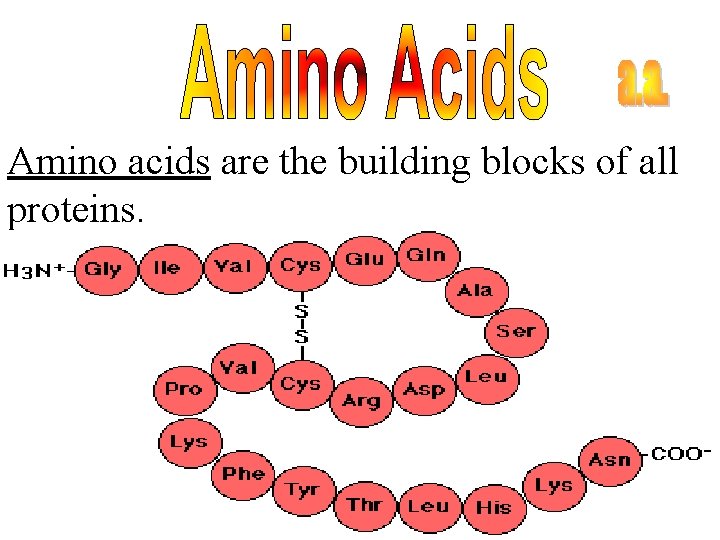
Amino acids are the building blocks of all proteins.
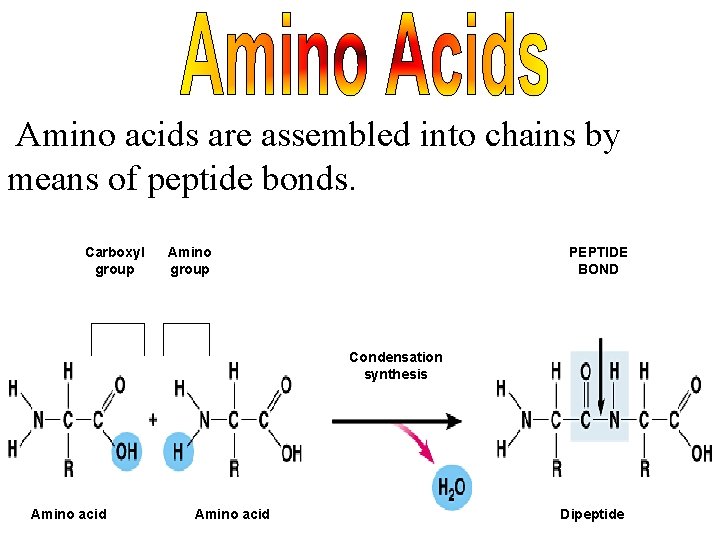
Amino acids are assembled into chains by means of peptide bonds. Carboxyl group Amino group PEPTIDE BOND Condensation synthesis Amino acid Dipeptide
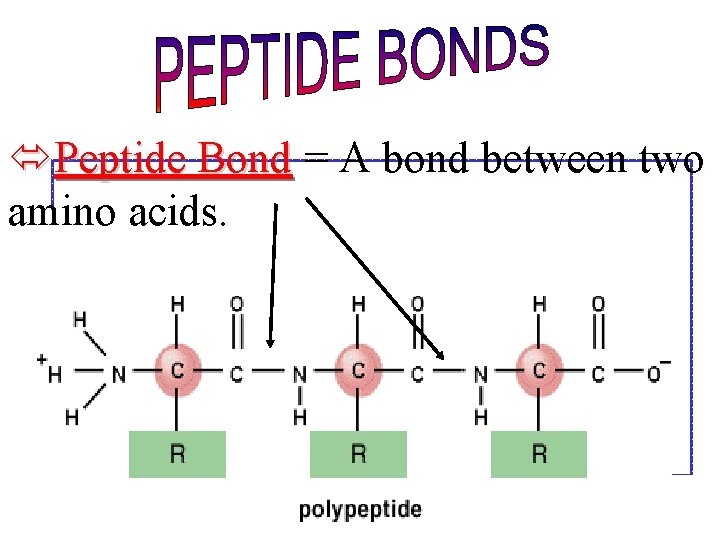
óPeptide Bond = A bond between two amino acids.
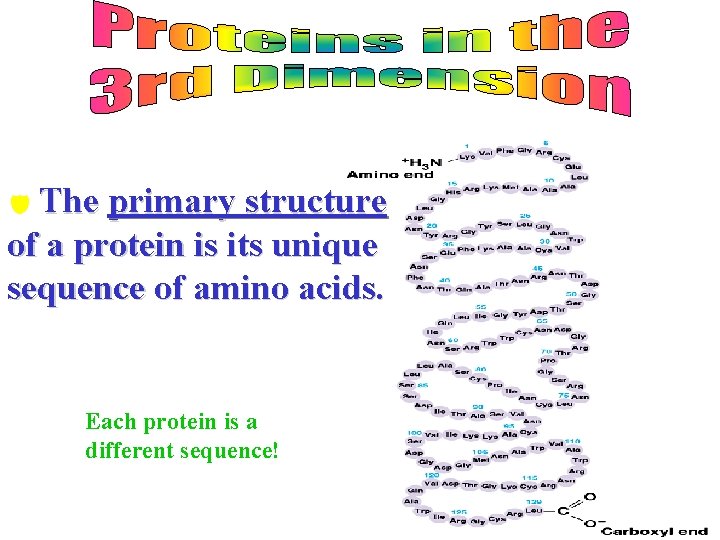
d The primary structure of a protein is its unique sequence of amino acids. Each protein is a different sequence!
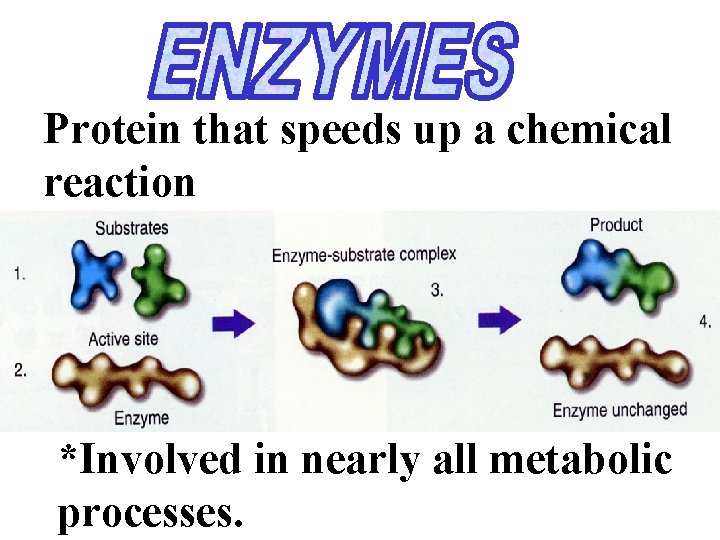
Protein that speeds up a chemical reaction *Involved in nearly all metabolic processes.
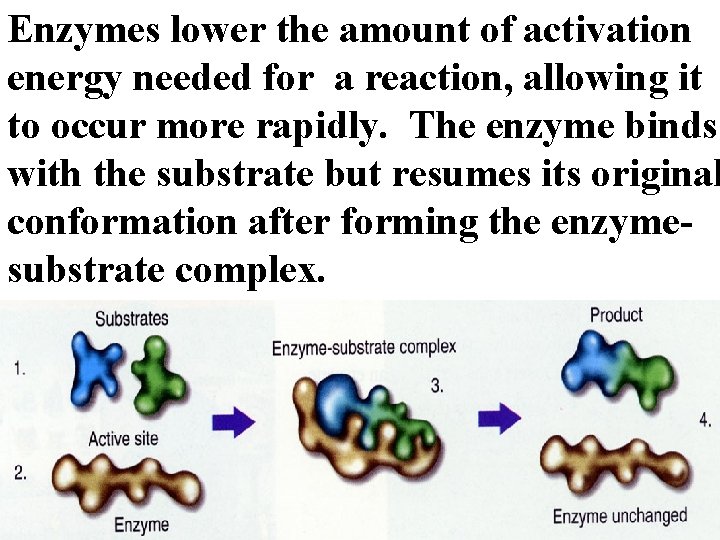
Enzymes lower the amount of activation energy needed for a reaction, allowing it to occur more rapidly. The enzyme binds with the substrate but resumes its original conformation after forming the enzymesubstrate complex.

ENz. YMe ANIMATIONS http: //www. kscience. co. uk/animations/anim_2. htm
FACTOR EFFECT EXPLANATION
Nucleic Acids – long polymers involved in heredity and in the manufacture of different kinds of proteins. The two most important nucleic acids are DNA and RNA.

Nucleotides – These are the building blocks of nucleic acids. Nucleotides are complex molecules composed of a nitrogenous base, a 5 -carbon sugar, and a phosphate group.
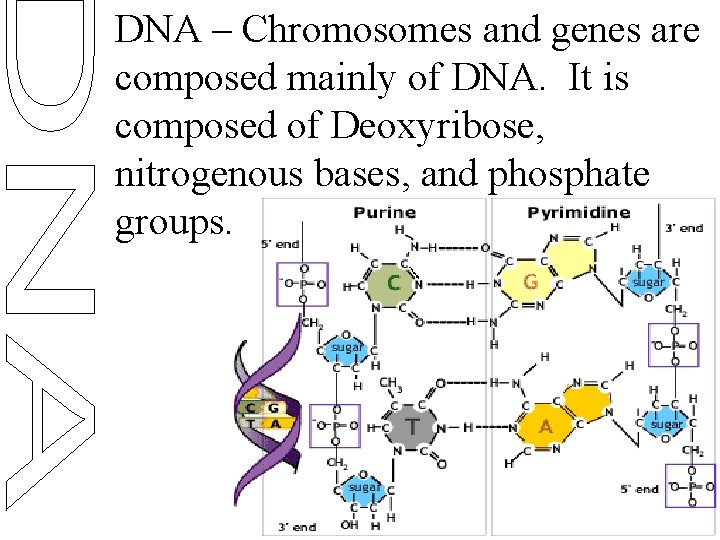
DNA – Chromosomes and genes are composed mainly of DNA. It is composed of Deoxyribose, nitrogenous bases, and phosphate groups.
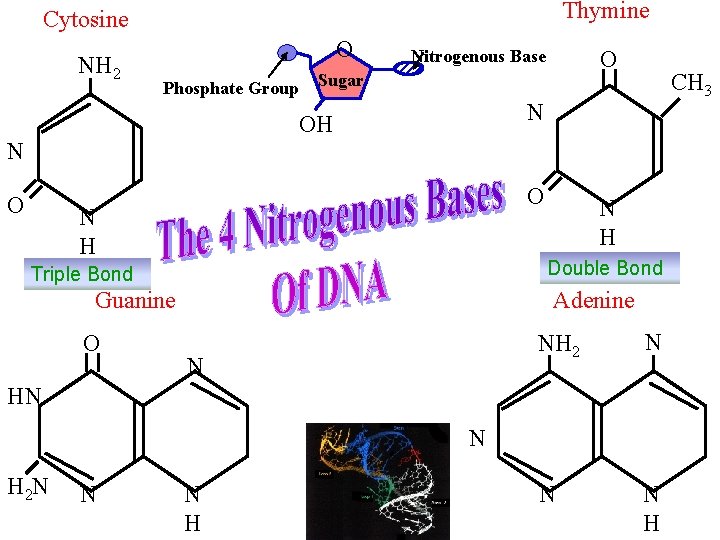
Thymine Cytosine NH 2 O Phosphate Group Sugar O O N H Double Bond Triple Bond Guanine O CH 3 N OH N O Nitrogenous Base Adenine N NH 2 N N N H HN N H 2 N N N H
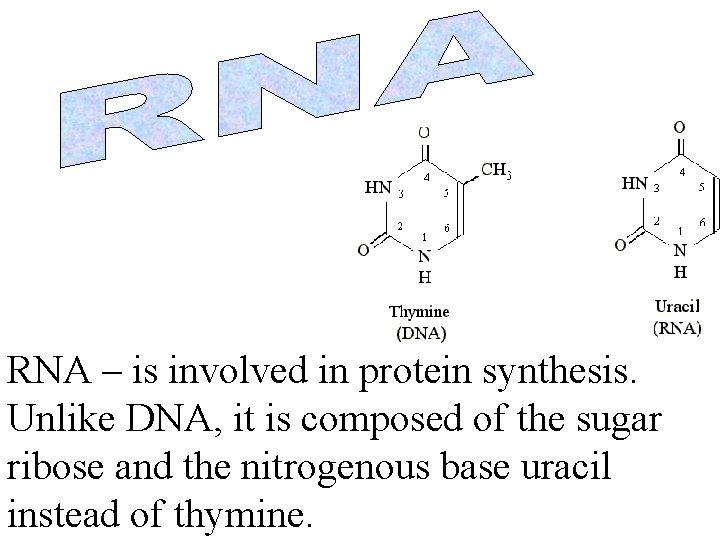
RNA – is involved in protein synthesis. Unlike DNA, it is composed of the sugar ribose and the nitrogenous base uracil instead of thymine.

Describe the four major groups of organic compounds that compose the human body. Include their chemical make-up in your essay, but focus on their function and importance to organic life forms.
- Slides: 51