Formative Assessment Finding and Defining a Molar Mass








- Slides: 18

Formative Assessment: Finding and Defining a Molar Mass to Calculate the Number of Atoms Richard Schneeberger Michigan State University 2013

Learning Goals • At the end of this unit, students will be able to: 1) Use the term “mole” correctly as a unit of measurement 2) Use Avogadro’s Number to convert between grams, moles, and number of atoms 3) Apply the Factor Label Method to derive their answer 4) Calculate the molar mass of a compound 5) Use a triple beam balance 6) Articulate the strategy used to another student

Setting the stage • Osborn Evergreen is situated in Detroit, Michigan at the intersection of Seven Mile and Hoover Unfortunately, students math skills ranged from 3 rd grade to 11 th grade, and this prompted several “mini math lessons”

What’s the run down? Students spent a period of three weeks (nine class periods in all due to block scheduling) to learn about the value of the mole. This included: • Revisiting scientific notation • Applying the factor label method learned at the beginning of the year • Using the Periodic Table to find the molar mass • Using a triple beam balance to measure mass • Articulating correctly to the class the strategy for obtaining their answer • Appropriately using units • Multiplying and dividing exponents • Dividing fractions

Misconceptions • I found there to be few preconceived misconceptions on the mole, as this was a new term for all but 3 of 75 chemistry students. • Some examples showed students reporting Avogadro’s Number as “ 6. 022”, leaving out the base and exponent. • Students were placing values in the wrong spot of the fraction, resulting in strange units such as the: “mole 2 / atoms” (resulting from: moles x (moles / atoms) • The link between multiplication and division was not well understood

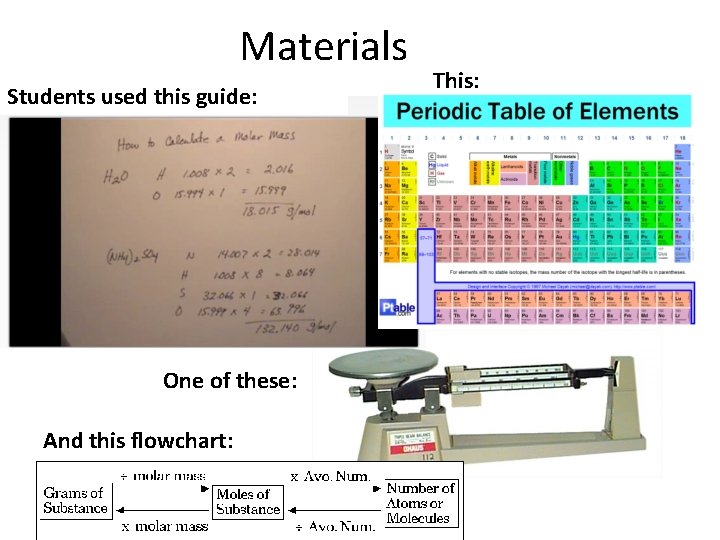
Materials Students used this guide: One of these: And this flowchart: This:

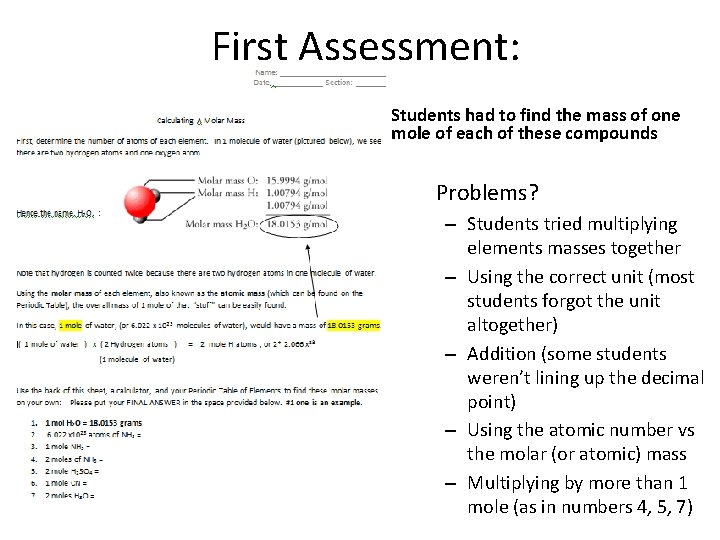
First Assessment: Students had to find the mass of one mole of each of these compounds • Problems? – Students tried multiplying elements masses together – Using the correct unit (most students forgot the unit altogether) – Addition (some students weren’t lining up the decimal point) – Using the atomic number vs the molar (or atomic) mass – Multiplying by more than 1 mole (as in numbers 4, 5, 7)

Goal of the 1 st assessment: • Get comfortable multiplying the molar mass (in grams / mole) by some number of moles • Recognize the connection between the number of atoms and the weight • Develop good habits to use for stoichiometry

1 st Assessment Conclusions • After this assignment, based on their oral explanations at the board, students were beginning to remember how to calculate the mass of 1 mole. • Students were getting better at locating elements on the Periodic Table • The notion of a mole as an amount was still foreign • Students remembered to multiply the coefficient by the base from our last unit on balancing equations

Second Assessment: • This consisted of several parts – Using scientific notation – Employing the Factor Label method – Dividing Fractions with exponents (I was working in tandem with their algebra II teacher on this) – Using the correct Order of Operations while multiplying – The notion that a conversion factor is equal to one (ie, 3 feet / 1 yard)

Results from Second Assessment • Students had begun to correctly use scientific notation • We had not yet introduced this in the context of moles, but the stage was set for stoichiometry • Fractional exponents had been mastered • Students were proficient using a triple beam balance

Findings (cont’d) Students were not writing down units on the numbers, and consequently getting lost Some students were using the “cross multiplying” strategy incorrectly When dividing two values in scientific notation, students had been using the wrong order of operations (they were not comfortable using the “fraction bar” notation) It was not well understood that the numerator is equal to the denominator when using a conversion factor

Remediation • Intervention put the lesson on hold while we revisited the factor label method; starting with “How many football fields (ff) is 850 yards? ” • My TI 83+ was being passed around the classroom to see the right order of operations • Dividing exponents with the same base (as in fractions using scientific notation) proved to be helpful in diagnosing misconceptions

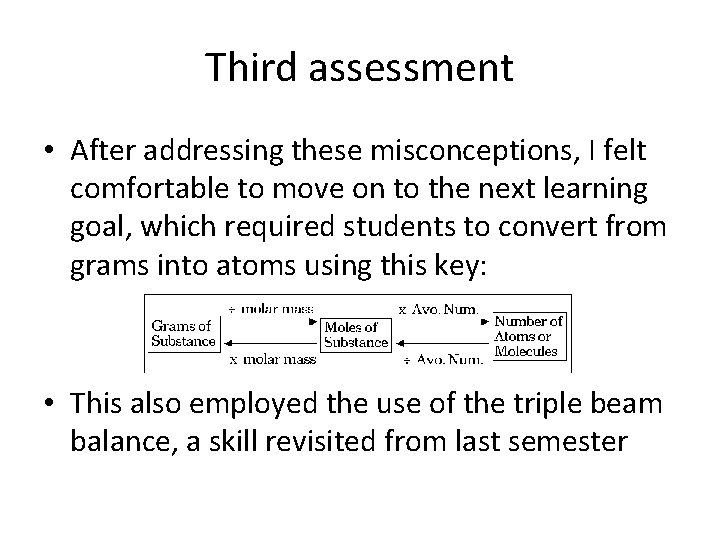
Third assessment • After addressing these misconceptions, I felt comfortable to move on to the next learning goal, which required students to convert from grams into atoms using this key: • This also employed the use of the triple beam balance, a skill revisited from last semester

Third Assessment

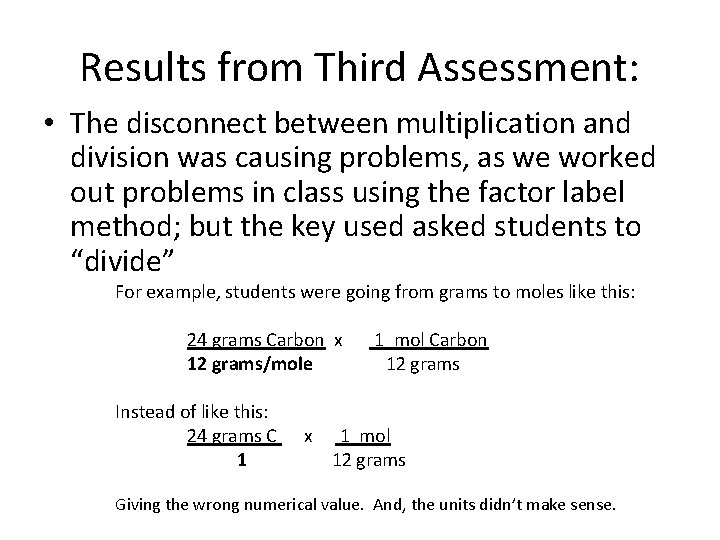
Results from Third Assessment: • The disconnect between multiplication and division was causing problems, as we worked out problems in class using the factor label method; but the key used asked students to “divide” For example, students were going from grams to moles like this: 24 grams Carbon x 12 grams/mole Instead of like this: 24 grams C 1 x 1 mol Carbon 12 grams 1 mol 12 grams Giving the wrong numerical value. And, the units didn’t make sense.

Small Victories… • This confusion was extremely rewarding, as students had finally begun to comfortably use the Factor Label Method, factoring out units. • Furthermore, they recognized the unit on their answer did not make sense. This prompted yet another mini math lesson to iterate that: “dividing is the same as multiplying by the reciprocal”

Conclusion: • Students with poor math skills don’t do well in science classes • Without advanced calculators, it becomes difficult to multiply using scientific notation • The analogy of “couple, few, dozen, mole” only works if several examples using familiar units are done first • Half of my students were able to use the Factor Label Method to get the number of grams, moles, and atoms • Poor attendance is negatively affecting performance across the board