Formation of Compounds Basic Concepts Forming Chemical Bonds

Formation of Compounds: Basic Concepts Forming Chemical Bonds • Elements become stable by achieving a noble gas configuration. • Valence electrons of atoms rearrange to give each atom an octet by: 1. transferring from one atom to another 2. being attracted by the nuclei of 2 atoms
Formation of Compounds: Basic Concepts Forming Chemical Bonds • The force that holds two atoms together is called a chemical bond. • Chemical bonds form because of ELECTROSTATIC ATTRACTIONS 1. between oppositely charged atoms, called ions, or 2. between electrons and 2 nuclei.
Formation of Compounds: Basic Concepts Electrons Can Be Transferred • Now that each atom has an octet of outerlevel electrons, they are no longer neutral atoms; they are charged particles called ions. • An ion is an atom or group of combined atoms that has a charge • A cation, or positive ion, is formed when an atom loses one or more electrons. • An anion, or negative ion, is formed when an atom gains one or more electrons.
Formation of Compounds: Basic Concepts Electrons Can Be Transferred • The strong attractive force between ions of opposite charge is called an ionic bond. • A compound that is composed of ions is called an ionic compound. • Note that only the arrangement of electrons has changed. Nothing about the atom’s nucleus has changed.

Types of Compounds: Basic Concepts Properties of Ionic Compounds • In a solid ionic compound, the positive ions are surrounded by negative ions, and the negative ions by positive ions. • The resulting structure is called a crystal lattice and contains a regular, repeating, three -dimensional arrangement of ions.

Types of Compounds: Basic Concepts Properties of Ionic Compounds • This arrangement, which involves strong attraction between oppositely charged ions, tends almost always to produce certain properties, such as high melting and boiling points and brittleness.

Types of Compounds: Basic Concepts Properties of Ionic Compounds • Ionic compounds are always nonconductors of electricity when solid but good conductors when melted. • They also act as electrolytes, substances that conduct electric current when dissolved in water.

Types of Compounds: Basic Concepts Ionic Compounds • Because of their structure, they usually are hard solids at room temperature and are difficult to melt. Look at the structure of magnesium oxide.
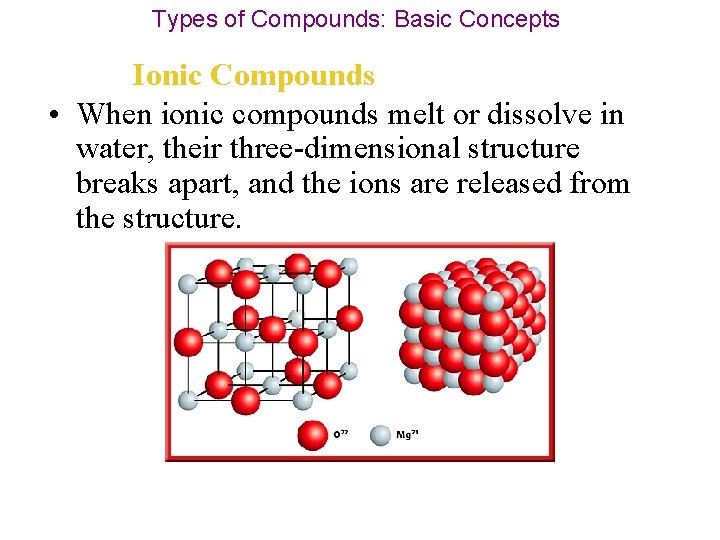
Types of Compounds: Basic Concepts Ionic Compounds • When ionic compounds melt or dissolve in water, their three-dimensional structure breaks apart, and the ions are released from the structure.
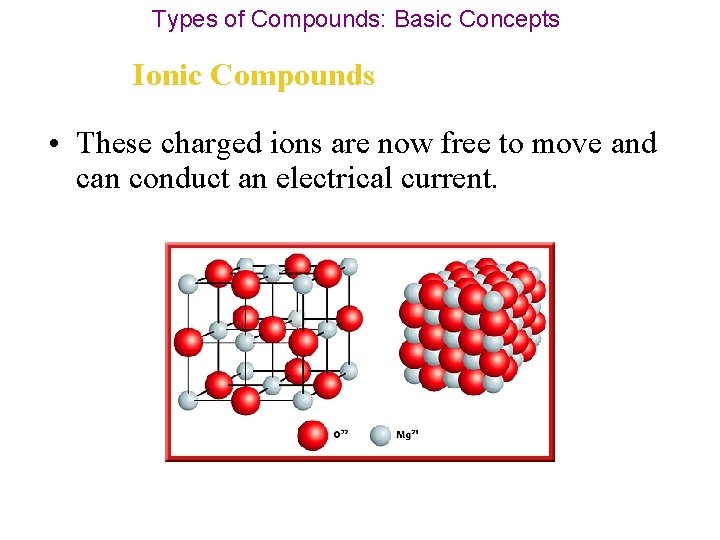
Types of Compounds: Basic Concepts Ionic Compounds • These charged ions are now free to move and can conduct an electrical current.

Types of Compounds: Basic Concepts Naming and Writing Formulas for Ionic Compounds • This simplest ratio of ions in a compound is called a formula unit. • Each formula unit of calcium fluoride consists of one calcium ion and two fluoride ions. • Each of the three ions has a stable octet configuration of electrons, and the formula unit has no overall charge.
Types of Compounds: Basic Concepts Predicting Charge on Ions • Metals have few outer-level electrons so they tend to lose them and become positive ions. • Most nonmetals, on the other hand, have outer-energy levels that contain four to seven electrons, so they tend to gain electrons and become negative ions.
Types of Compounds: Basic Concepts Predicting Charge on Ions • Because all elements in a given group have the same number of electrons in their outerenergy level, they must lose or gain the same number of electrons to achieve a noble-gas electron configuration. • Metals always lose electrons and nonmetals always gain electrons when they form ions. • The charge on the ion is known as the oxidation number of the atom.
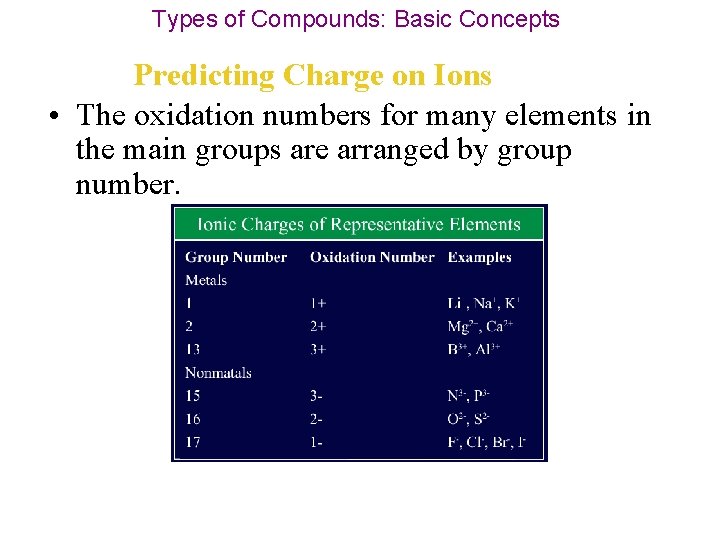
Types of Compounds: Basic Concepts Predicting Charge on Ions • The oxidation numbers for many elements in the main groups are arranged by group number.
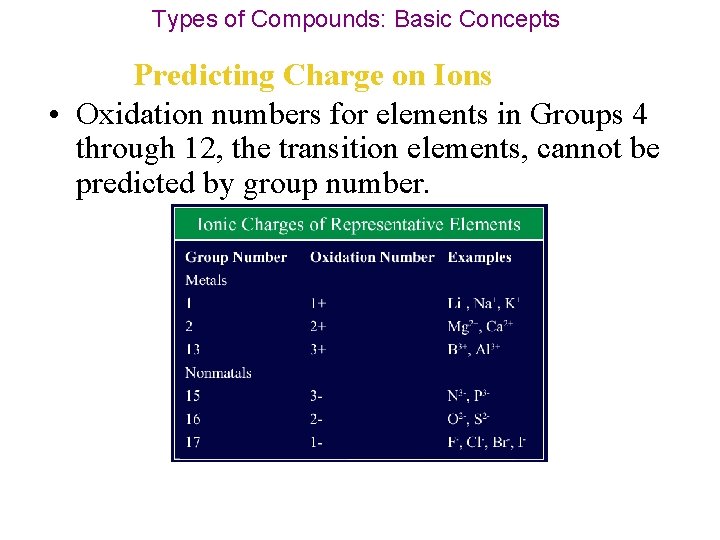
Types of Compounds: Basic Concepts Predicting Charge on Ions • Oxidation numbers for elements in Groups 4 through 12, the transition elements, cannot be predicted by group number.
Types of Compounds: Basic Concepts Compounds Containing Polyatomic Ions • An ion that has only one element is called a monatomic ion. • An ion that has two or more different elements is called a polyatomic ion. 1. Although the individual atoms have no charge, the group as a whole has an overall charge.
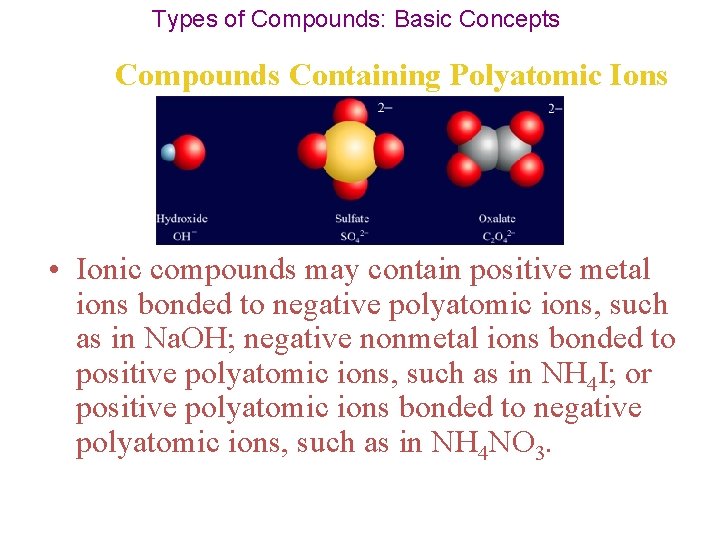
Types of Compounds: Basic Concepts Compounds Containing Polyatomic Ions • Ionic compounds may contain positive metal ions bonded to negative polyatomic ions, such as in Na. OH; negative nonmetal ions bonded to positive polyatomic ions, such as in NH 4 I; or positive polyatomic ions bonded to negative polyatomic ions, such as in NH 4 NO 3.
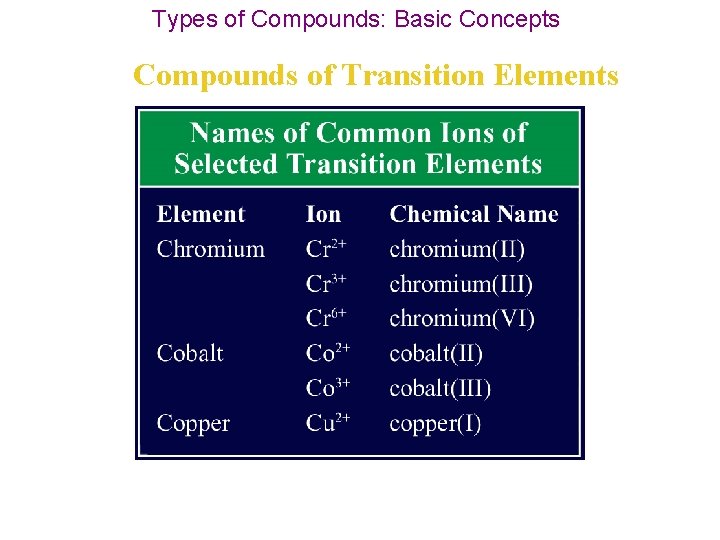
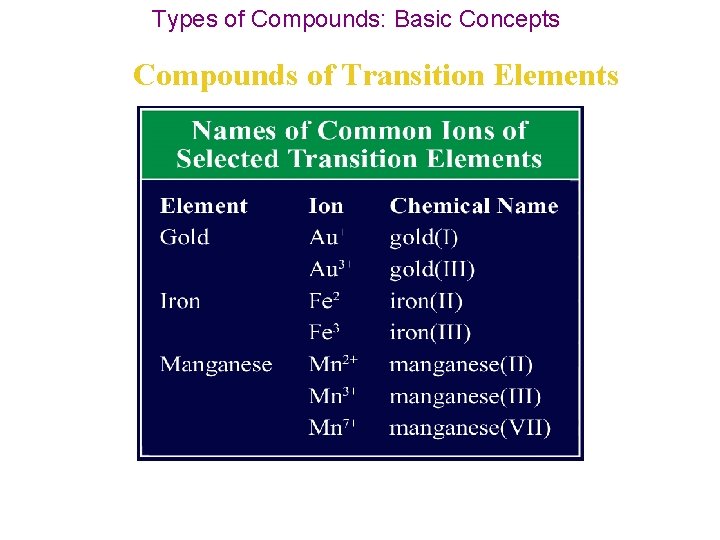
Types of Compounds: Basic Concepts Compounds of Transition Elements • Elements known as transition elements are located in Groups 3 through 12 in the periodic table. • Transition elements & p block metals form positive ions just as other metals do, but most transition elements can form more than one type of positive ion. • In other words, transition elements can have more than one oxidation number.
Types of Compounds: Basic Concepts Compounds of Transition Elements • For example, copper can form both Cu+ and Cu 2+ ions, and lead can form both Pb 2+ and Pb 4+ ions. • Exceptions to the variability of transition elements: 1. silver ion is Ag+, 2. zinc ion is Zn 2+, 3. aluminum ion is Al 3+.
Types of Compounds: Basic Concepts Compounds of Transition Elements • Chemists must have a way to distinguish the names of compounds formed from the different ions of a transition element. • They do this by using a Roman numeral to indicate the oxidation number of a transition element ion.
Types of Compounds: Basic Concepts Compounds of Transition Elements
Types of Compounds: Basic Concepts Compounds of Transition Elements
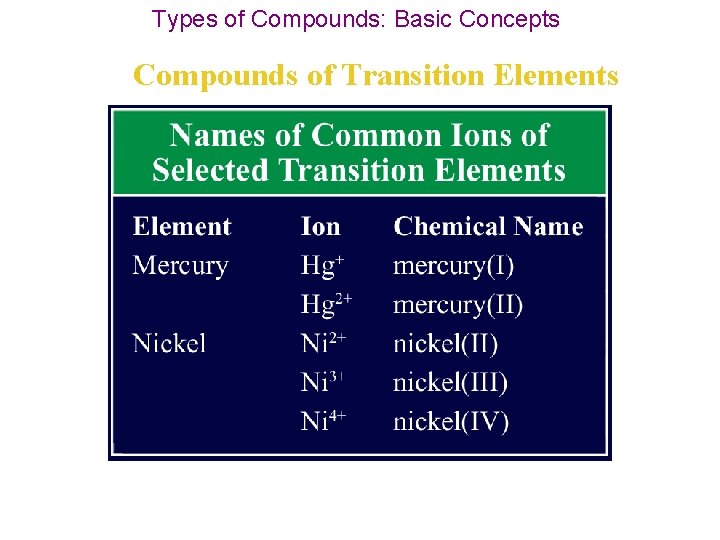
Types of Compounds: Basic Concepts Compounds of Transition Elements
Formation of Compounds: Additional Concepts Metallic Bonds and Properties of Metals • The bonding in metals is explained by the electron sea model, which proposes that the atoms in a metallic solid contribute their valence electrons to form a “sea” of electrons that surrounds metallic cations. • These delocalized electrons are not held by any specific atom and can move easily throughout the solid. • A metallic bond is the attraction between these electrons and a metallic cation.

Formation of Compounds: Additional Concepts Metallic Bonds and Properties of Metals • Metals generally have extremely high boiling points because it is difficult to pull metal atoms completely away from the group of cations and attracting electrons.
Formation of Compounds: Additional Concepts Metallic Bonds and Properties of Metals • Metals are also malleable (able to be hammered into sheets) and ductile (able to be drawn into wire) because of the mobility of the particles. • The delocalized electrons make metals good conductors of electricity.

Formation of Compounds: Additional Concepts Metallic Bonds and Properties of Metals • A mixture of elements that has metallic properties is called an alloy. • Alloys can be of two basic types. 1. A substitutional alloy is one in which atoms of the original metal are replaced by other atoms of similar size. 2. An interstitial alloy is one in which the small holes in a metallic crystal are filled by other smaller atoms.

Additional Assessment Questions Question 1 What is the electron configuration, in abbreviated form, for nickel? Answer [Ar]4 s 23 d 8

Additional Assessment Questions Question 2 What structure has positive ions surrounded by negative ions, and the negative ions surrounded by positive ions? Answer a crystal lattice

Additional Assessment Questions Question 3 What is the correct formula for the ionic compound aluminum sulfite? Answer Al 2(SO 3)3

Practice Problems Question 4 Determine the correct formula for the ionic compound composed of the following pairs of ions.

Practice Problems Question 4 a aluminum and bicarbonate Answer 4 a Al(HCO 3)3

Practice Problems Question 4 b magnesium and carbonate Answer 4 b Mg. CO 3

Practice Problems Question 4 c calcium and perchlorate Answer 4 c Ca(Cl. O 4 )2

Practice Problems Question 5 Name the following compounds.

Practice Problems Question 5 a Co(OH)2 Answer 5 a cobalt(II) hydroxide

Practice Problems Question 5 b Ag 2 Cr. O 4 Answer 5 b silver chromate

Practice Problems Question 5 c Na 3 PO 2 Answer 5 c sodium hypophosphite
- Slides: 38