Formation and Dissipation of Trihalomethanes during Aquifer Storage











- Slides: 21

Formation and Dissipation of Trihalomethanes during Aquifer Storage and Recovery Operations Jason Pulley City of Salem Public Works

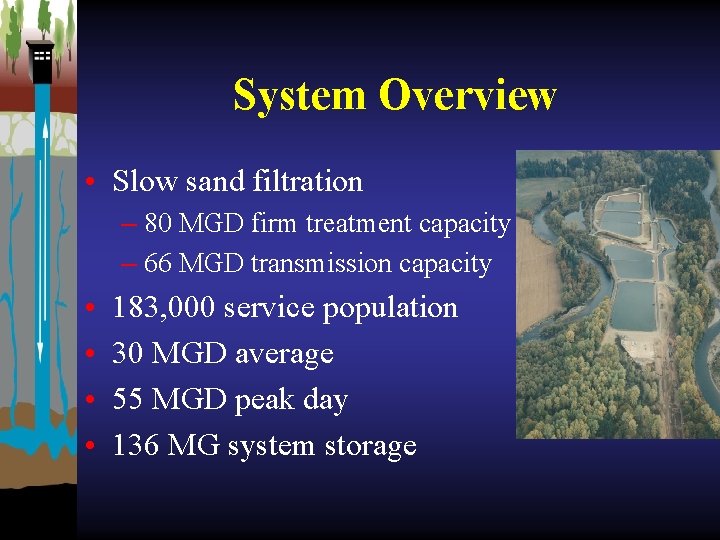
System Overview • Slow sand filtration – 80 MGD firm treatment capacity – 66 MGD transmission capacity • • 183, 000 service population 30 MGD average 55 MGD peak day 136 MG system storage

ASR Operations • ASR used to supplement treatment plant production during high-use periods – Used in winter during high turbidity events • Injection typically from Nov-March – Inject at two of four wells ≈ 3. 5 mgd • Storage goal of 500 mg – Recovery from 3 of 4 wells ≈ 6. 7 mgd – 100% recovery with use of groundwater rights

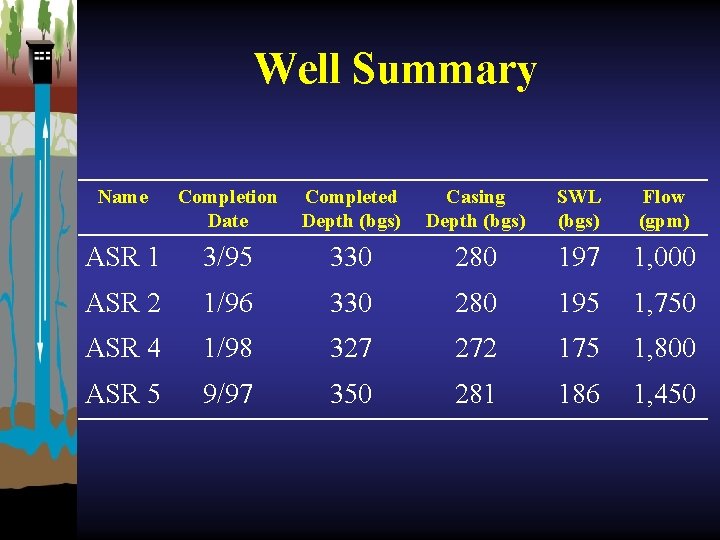
Well Summary Name Completion Date Completed Depth (bgs) Casing Depth (bgs) SWL (bgs) Flow (gpm) ASR 1 3/95 330 280 197 1, 000 ASR 2 1/96 330 280 195 1, 750 ASR 4 1/98 327 272 175 1, 800 ASR 5 9/97 350 281 186 1, 450

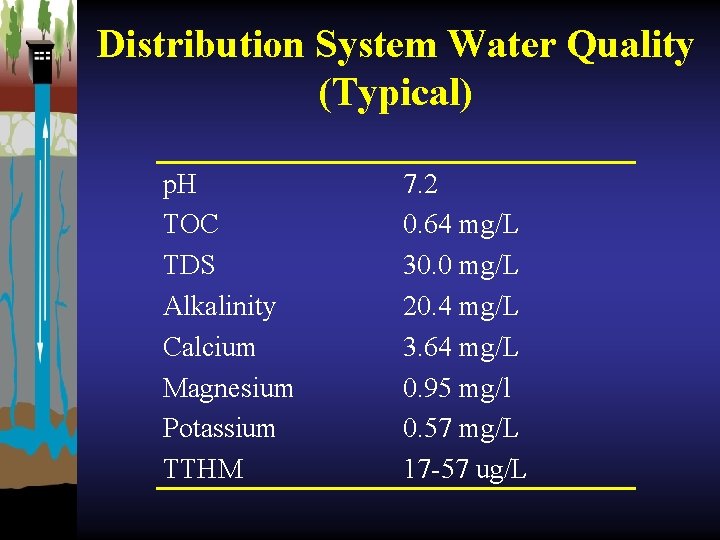
Distribution System Water Quality (Typical) p. H TOC TDS Alkalinity Calcium Magnesium Potassium TTHM 7. 2 0. 64 mg/L 30. 0 mg/L 20. 4 mg/L 3. 64 mg/L 0. 95 mg/l 0. 57 mg/L 17 -57 ug/L

Production History Million Gallons 56 mg native groundwater 2, 571 mg injected 2, 245 mg recovered

Observations • Elevated THM concentrations noticed in 2005 – Isolated at ASR 5 – Quickly dissipated during recovery operations • Observed again in 2006 at ASR 5 and ASR 4 – Possibly related to storage volume – 350 mg in 2005; 400 mg in 2006 – Had only stored 350 mg once (2000) and never 400 mg – Historic levels had fluctuated but never at the levels observed

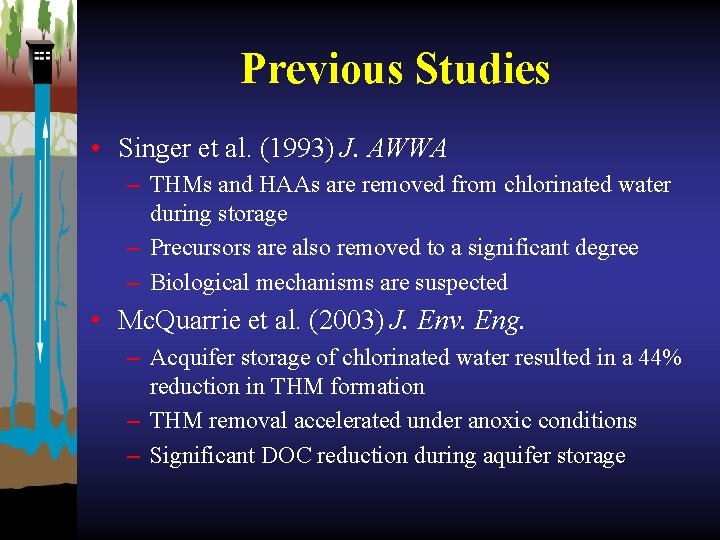
Previous Studies • Singer et al. (1993) J. AWWA – THMs and HAAs are removed from chlorinated water during storage – Precursors are also removed to a significant degree – Biological mechanisms are suspected • Mc. Quarrie et al. (2003) J. Env. Eng. – Acquifer storage of chlorinated water resulted in a 44% reduction in THM formation – THM removal accelerated under anoxic conditions – Significant DOC reduction during aquifer storage

Previous Studies • Pyne et al. (1996) AWWARF – Focused on five sites with injected treated drinking water – Storage periods from 36 -127 d – THM reductions of 25 -100% – Some loss attributed to dilution/mixing; biodegradation plays a significant role – Also reported reduction in THM precursors

Previous Studies • Landmeyer et al. (2000) J. AWRA – Las Vegas Valley Water District ASR – Observed increases in THM concentrations during recovery – Conc. decreased with continued pumping • Adsorption • Mixing • Microbial degradation – Lab studies show no significant CHCl 3 biodegradation (aerobic or anaerobic) • Low organic carbon content restricts microbial attenuation – CHCL 3 entrained in water or formed in situ will tend to persist

Initial Investigations • Monitor THM concentrations over a 30 -day storage period – Weekly measurements of THM at each of four wells • Collect samples from each well and finished water from TP – 7 -d THMFP, DOC, SUVA on all samples – 30 -d THMFP on finished water – 7 & 30 -d SDS on finished water • Time series analysis after 30 -d storage (every 10 min for 1 h)

Analytical Methodology • EPA Method 524. 2 – Purgeable organic compounds by capillary column GC/MS – Alternate methods 551. 1 & 552. 2 (liquid extraction with ECD) – All provide full speciation • Hach Procedure 10132 – Colorimetric read on spectrophotometer – All results reported as chloroform (CHCl 3) – Estimated detection limit of 6 g L-1

Hach Procedure 10132 • Provides “screening level” data – Low-cost quantitative data – Internal comparisons within +/- 10% – Prep and analysis time < 30 min • Hach validation – vs 524. 2, 551. 1, 552. 2 – R 2 values of 0. 906, 0. 938, and 0. 959, respectively

NOM & SUVA • NOM – a mixture of humic and nonhumic organic substances – Contributes to DBP precursor levels and speciation • Humic substances have higher SUVA and formation potential than nonhumic – SUVA = UV @ 254 / DOC • SUVA provides an indicator for DBP formation • SUVA > 2 L/mg-m generally considered high formation potential

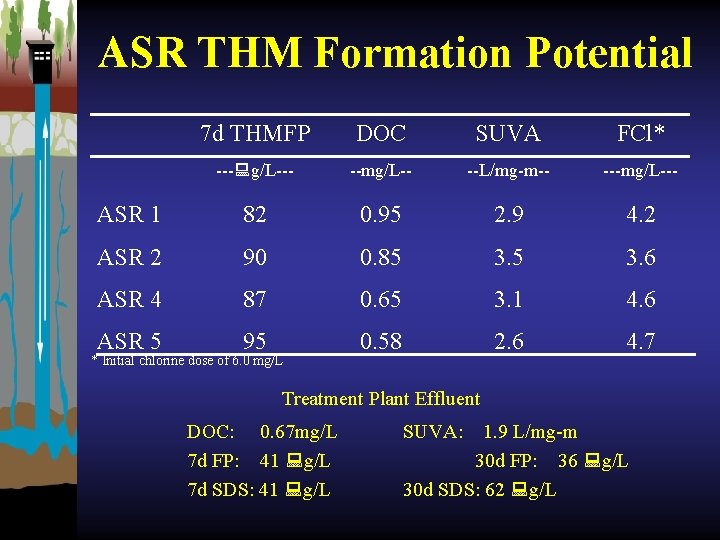
ASR THM Formation Potential 7 d THMFP DOC SUVA FCl* --- g/L--- --mg/L-- --L/mg-m-- ---mg/L--- ASR 1 82 0. 95 2. 9 4. 2 ASR 2 90 0. 85 3. 6 ASR 4 87 0. 65 3. 1 4. 6 ASR 5 95 0. 58 2. 6 4. 7 * Initial chlorine dose of 6. 0 mg/L Treatment Plant Effluent DOC: 0. 67 mg/L 7 d FP: 41 g/L 7 d SDS: 41 g/L SUVA: 1. 9 L/mg-m 30 d FP: 36 g/L 30 d SDS: 62 g/L

Historic Storage Period THM Concentrations

THM and Storage Volume

Geochemistry of Stored Water

Time-Series THM Concentrations During Recovery

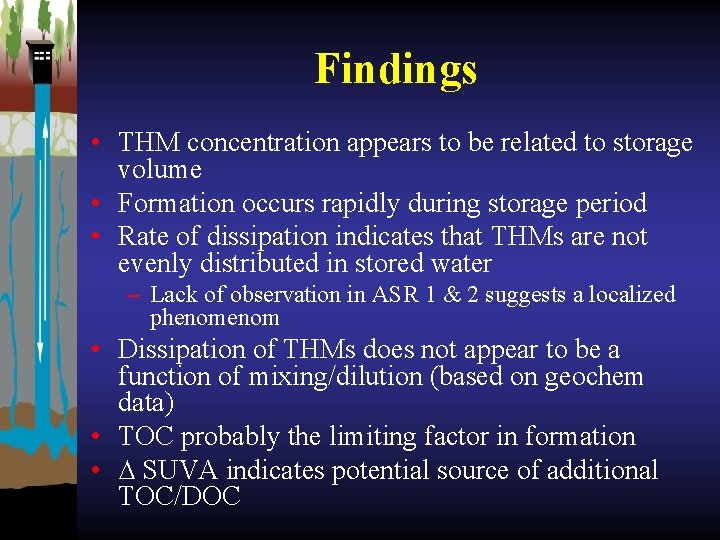
Findings • THM concentration appears to be related to storage volume • Formation occurs rapidly during storage period • Rate of dissipation indicates that THMs are not evenly distributed in stored water – Lack of observation in ASR 1 & 2 suggests a localized phenom • Dissipation of THMs does not appear to be a function of mixing/dilution (based on geochem data) • TOC probably the limiting factor in formation • ∆ SUVA indicates potential source of additional TOC/DOC

Next Steps • Continue to monitor during current storage period to see if levels increase above existing concentrations – More frequent analysis during recovery operations • Further analysis of SUVA during injection and recovery cycles at varying water elevations • Examine HAA formation characteristics • Pursue dechlorination of injection water if increasing concentration are not manageable
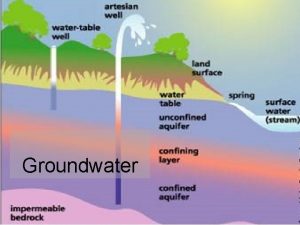
 Unconfined aquifer definition
Unconfined aquifer definition Trihalomethanes in swimming pools
Trihalomethanes in swimming pools Velocity-dependent potential and dissipation function
Velocity-dependent potential and dissipation function Transistor power dissipation
Transistor power dissipation Dissipation visqueuse
Dissipation visqueuse Nmos inverter with enhancement load
Nmos inverter with enhancement load Disipasi daya maksimum
Disipasi daya maksimum Dissipation test
Dissipation test Power dissipation
Power dissipation Eddy dissipation rate
Eddy dissipation rate Transferring of data from auxiliary storage to main storage
Transferring of data from auxiliary storage to main storage Secondary storage vs primary storage
Secondary storage vs primary storage What is the earth's atmosphere made of
What is the earth's atmosphere made of Dockum aquifer
Dockum aquifer Gulf coast aquifer
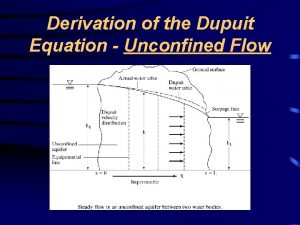
Gulf coast aquifer Dupuit's equation
Dupuit's equation Leaky aquifer definition
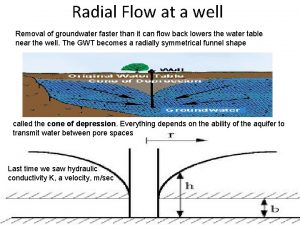
Leaky aquifer definition Radial flow
Radial flow Ogallala aquifer
Ogallala aquifer Ogallala aquifer
Ogallala aquifer Ogallala aquifer
Ogallala aquifer What is an aquifer
What is an aquifer