Form Revision and Development Tiffany Hunt CCRP Rachael






















- Slides: 41

Form Revision and Development Tiffany Hunt, CCRP Rachael Latchana, MPH Stephanie Meyers February 20, 2019 The CIBMTR® (Center for International Blood and Marrow Transplant Research®) is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin (MCW). TRAINING & DEVELOPMENT |.

Objectives Understand the Form Revision Process Review 2019 Revisions TRAINING & DEVELOPMENT | 2.

The Form The. Revision Form Revision Process TRAINING & DEVELOPMENT | 3.

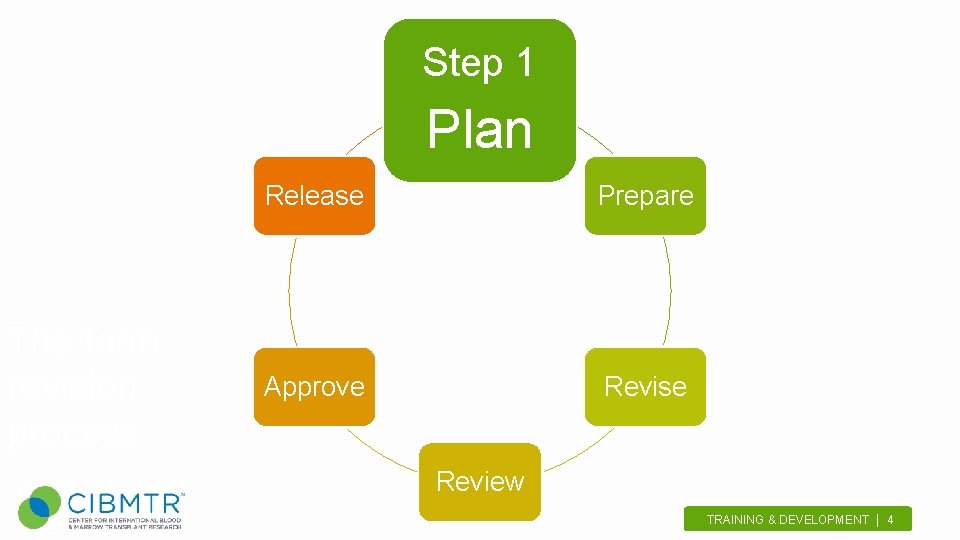
Step 1 Plan The form revision process Release Prepare Approve Revise Review TRAINING & DEVELOPMENT | 4.

Plan Why revise a form? • • • Time since last revision Upcoming studies Updates or resolving issues Updates from WHO Requests from staff or centers TRAINING & DEVELOPMENT | 5.

Plan Create a Roadmap • Form Revision Core Team • Draft proposed schedule • Obtain approval • Scientific directors and senior leaders review and approve • Be flexible! • allow wiggle room for critical revisions, or new priority forms TRAINING & DEVELOPMENT | 6.

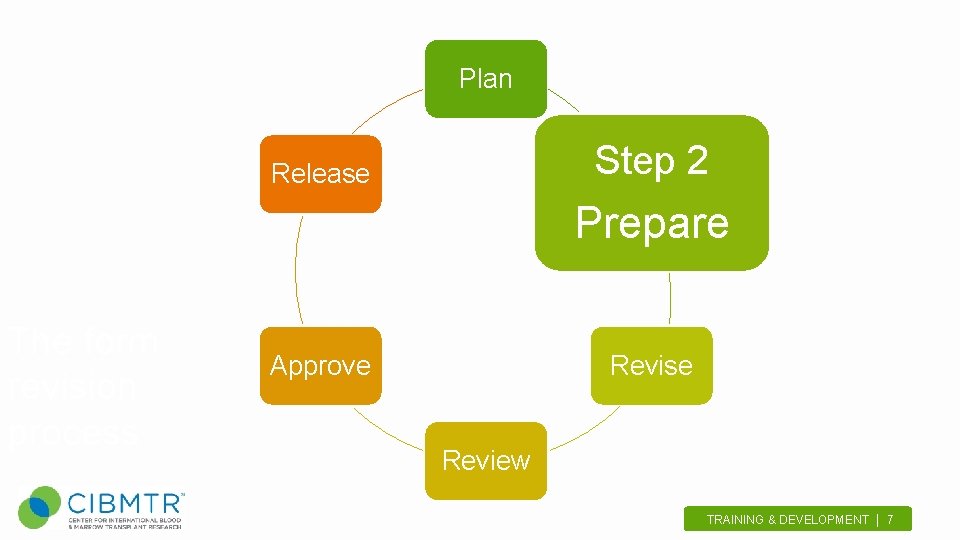
Plan Step 2 Release Prepare The form revision process Approve Revise Review TRAINING & DEVELOPMENT | 7.

Prepare • Announce revisions to the network – Includes scientific directors and centers • Volunteer! – Send form change suggestions • Meet with scientific director of working committee TRAINING & DEVELOPMENT | 8.

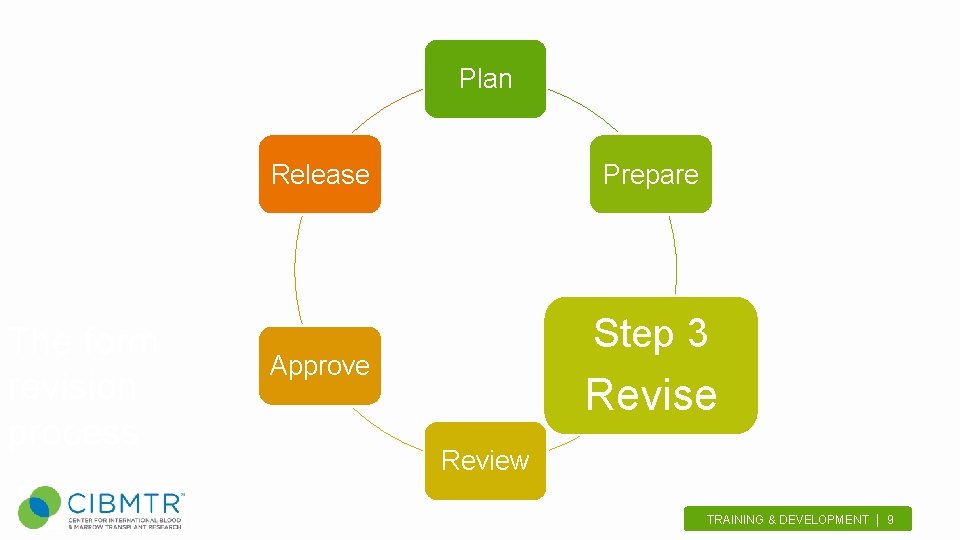
Plan Release The form revision process Prepare Step 3 Approve Revise Review TRAINING & DEVELOPMENT | 9.

Revise Initial Review Committee (IRC) • Consists of field expert physicians, subject matter experts (SME), data managers, CIBMTR staff and other individuals • Weekly Meetings (4 weeks, 2 hours) – review the form(s) top to bottom, question by question – remove outdated questions, add new questions based on new technology/treatments, clarify problematic sections • Q&A Meeting – allow for data managers to pilot the forms – ensure source documents exist for requested data TRAINING & DEVELOPMENT | 10.

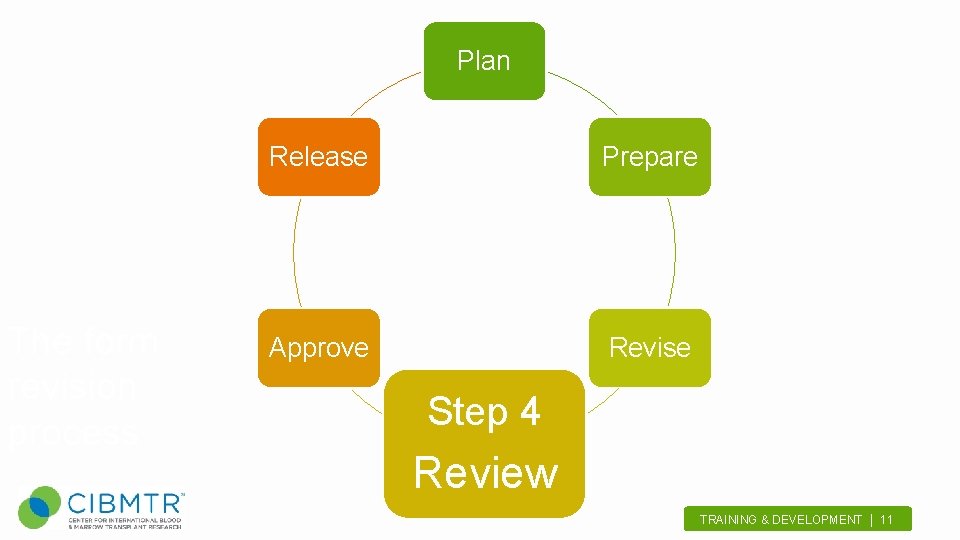
Plan The form revision process Release Prepare Approve Revise Step 4 Review TRAINING & DEVELOPMENT | 11.

Review Internal Review – CIT review – Statistical review – Database review • Develop validations, identify potential problem areas, and discuss form development TRAINING & DEVELOPMENT | 12.

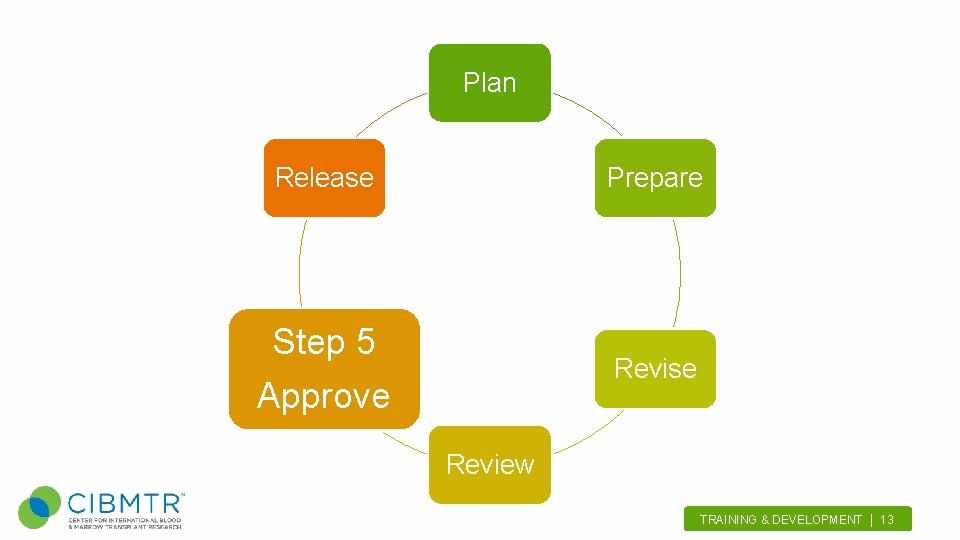
Plan Release Prepare Step 5 Revise Approve Review TRAINING & DEVELOPMENT | 13.

Approve • Scientific Director(s) – Review form drafts and change summaries – Review occurs at standing weekly meeting • Request volunteers for time studies – Assess form completion burdens TRAINING & DEVELOPMENT | 14.

Approve OMB Approval • Form 2400, 2402, 2004, 2005, 2006, and 2450 • Long process (6+ months) • Post to the Federal Register: 60 day notice, 30 day notice • OMB review and approval TRAINING & DEVELOPMENT | 15.

Post- Approval • Forms. Net 3 Process – Build new forms – Write/test validations – Define Events and Actions (logic for how forms come due) – Metadata work – AGNIS – Mappings TRAINING & DEVELOPMENT | 16.

Plan Step 5 Release Prepare Approval Revise Review TRAINING & DEVELOPMENT | 17.

Release • New Forms – Announce to Network – Release forms prior to “Go Live” date • Forms Instruction Manual – Announce to Network – Release updated manuals prior to “Go Live” date • Update / develop e. Learnings TRAINING & DEVELOPMENT | 18.

Finally…How can YOU help? • Submit form change suggestions – Submit at any time – Send to your CRC – Suggestions reviewed during the next revision • Volunteer – Participate on a form revision committee! – Important to have data managers involved TRAINING & DEVELOPMENT | 19.

Finally…How can YOU help? • Time studies – We need your input on the length of time it takes to complete the forms – Important information for OMB process but also any revised forms TRAINING & DEVELOPMENT | 20.

Upcoming Revisions 2 1 TRAINING & DEVELOPMENT | 21.

The Core Forms • 2400, 2402, 2000, 2004, 2005, 2006, 2450, 2804 – Revision committees met May – July 2018 for 2400/2000, 2006 – Internal review July – October 2018 – Requires OMB approval (in process) • Scheduled for release in Summer 2019 – Will be posted 2 months in advance for preview TRAINING & DEVELOPMENT | 2222.

Pre-TED (Form 2400) Revision Highlights *not a complete list Fun Fact: Decreased total number of questions (357 to 142) • ‘Check all that apply’ capability: • Race, race detail, country of residence, comorbidities, planned post-HCT therapy • Recipient Information section – Ethnicity, race detail • Collected on CRID assignment (Form 2804) • Auto-populated on Pre-TED TRAINING & DEVELOPMENT | 2323.

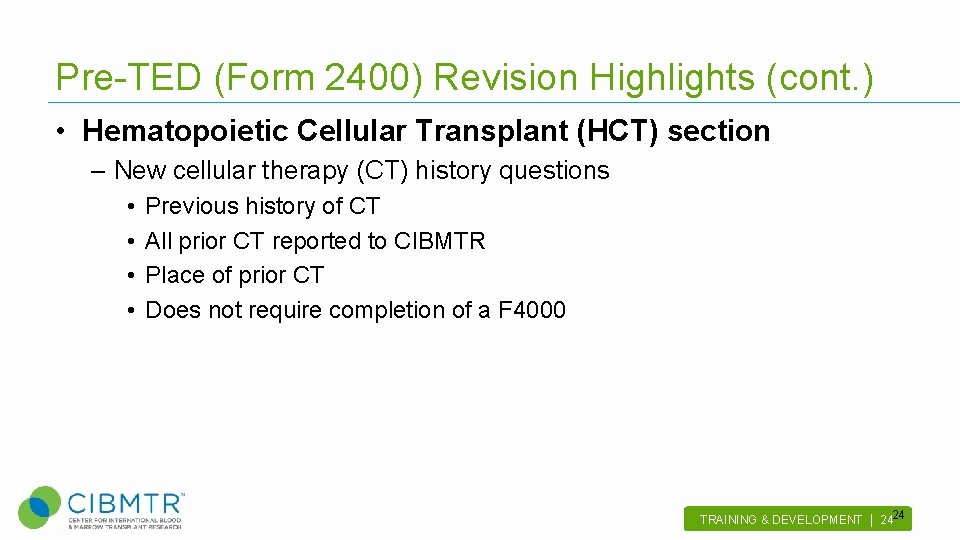
Pre-TED (Form 2400) Revision Highlights (cont. ) • Hematopoietic Cellular Transplant (HCT) section – New cellular therapy (CT) history questions • • Previous history of CT All prior CT reported to CIBMTR Place of prior CT Does not require completion of a F 4000 TRAINING & DEVELOPMENT | 2424.

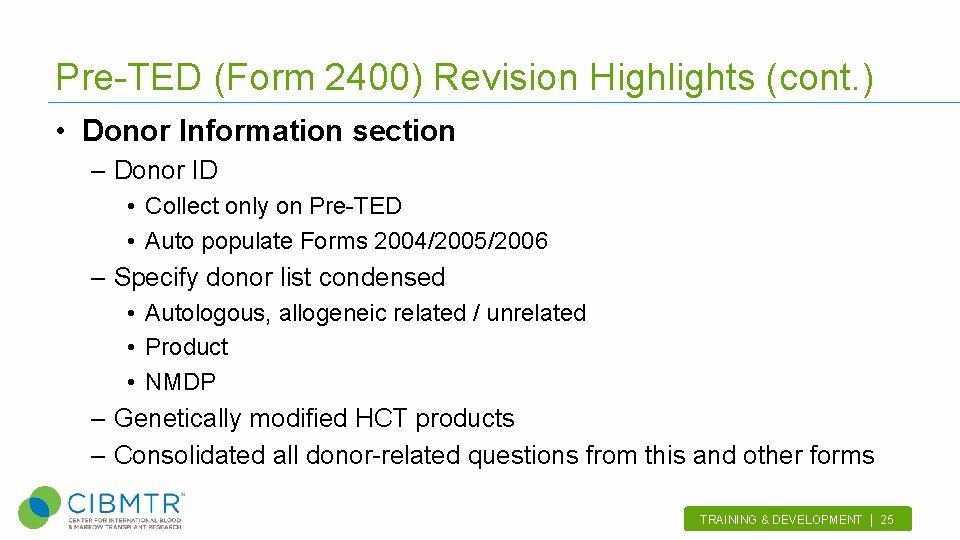
Pre-TED (Form 2400) Revision Highlights (cont. ) • Donor Information section – Donor ID • Collect only on Pre-TED • Auto populate Forms 2004/2005/2006 – Specify donor list condensed • Autologous, allogeneic related / unrelated • Product • NMDP – Genetically modified HCT products – Consolidated all donor-related questions from this and other forms TRAINING & DEVELOPMENT | 25.

Pre-TED (Form 2400) Revision Highlights (cont. ) • Pre-HCT Preparative Regimen (Conditioning) section – Chemotherapy Drugs • Added ‘multiple’ capability to eliminate Yes/No for each drug • Updated drug list • Additional Drugs Given in Peri-Transplant Period section – NEW SECTION TO FORM • Used to report drugs given for both the preparative regimen and GVHD prophylaxis TRAINING & DEVELOPMENT | 2626.

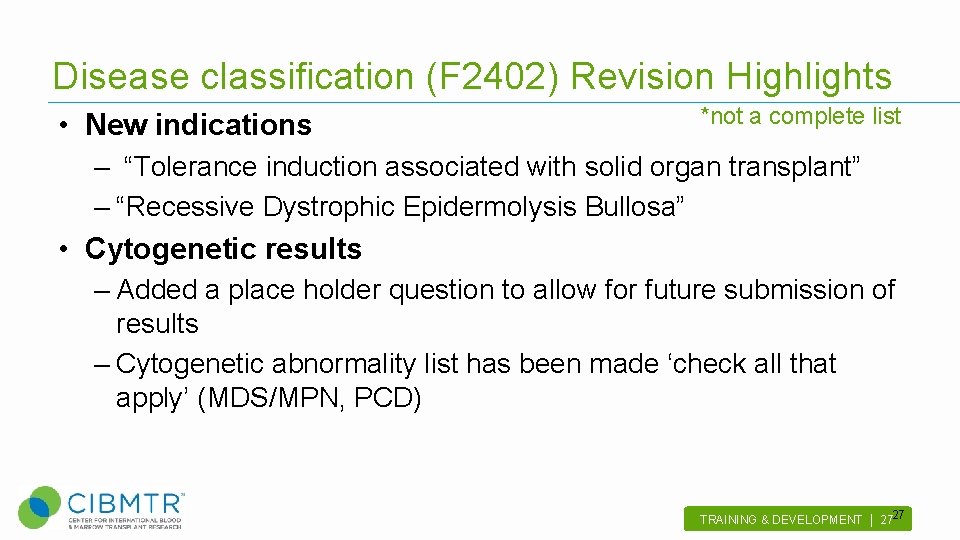
Disease classification (F 2402) Revision Highlights • New indications *not a complete list – “Tolerance induction associated with solid organ transplant” – “Recessive Dystrophic Epidermolysis Bullosa” • Cytogenetic results – Added a place holder question to allow for future submission of results – Cytogenetic abnormality list has been made ‘check all that apply’ (MDS/MPN, PCD) TRAINING & DEVELOPMENT | 2727.

Disease classification (F 2402) Revision Highlights cont. • Inherited Abnormalities of Erythrocyte Differentiation or Function section – New questions added to capture beta thalassemia and sickle cell TRAINING & DEVELOPMENT | 2828.

Baseline (F 2000) Revision Highlights*not a complete list Fun Fact: Decreased total number of questions (264 to 118) • Recipient Demographics / Race / Clinical status – Moved country of primary residence, ethnicity, race detail, recipient blood type and Rh factor to pre-TED • Infection – Collect infections in 6 months prior to infusion • Pre-HCT Preparative Regimen (Conditioning) section – Chemotherapy Drugs • Added ‘multiple’ capability to eliminate Yes/No for each drug • Updated drug list TRAINING & DEVELOPMENT | 2929.

Baseline (F 2000) Revision Highlights (cont. ) • Additional Drugs Given in Peri-Transplant Period section – NEW SECTION TO FORM • Used to report drugs given for both the preparative regimen and GVHD prophylaxis • Socioeconomic Information – Will collect most recent works status – Updated insurance list TRAINING & DEVELOPMENT | 3030.

Infusion (F 2006) Revision Highlights*not a complete list Fun Fact: Decreased total number of questions (285 to 170) • Donor/cord blood unit identification – Donor Identification to be auto-populated on form in key fields • Product processing and manipulation – Consolidated questions from pre-TED to here • Removed whole section for Autologous products TRAINING & DEVELOPMENT | 3131.

Infusion (F 2006) Revision Highlights (cont. ) • Product analysis – Consolidated timepoints to “pre-cryopreservation” and “post thaw” – Expanded cell types (TNC, CD 34, CD 3/4/8) and • collect viability per cell type instead of overall viability • Product infusion – Clarified question “was the entire volume of received product infused” TRAINING & DEVELOPMENT | 3232.

Infusion (F 2006) Revision Highlights (cont. ) • Donor/Infant demographic information – Donor blood type/Rh factor moved to pre-TED – Biological relationship of donor to recipient • Removed and consolidated to the pre-TED – Transferable genetic or clonal abnormalities • Expanded option list TRAINING & DEVELOPMENT | 33.

IDM and HLA (F 2004 / F 2005) Revision Highlights • Donor/cord blood unit identification *not a complete list – Donor Identification to be auto-populated on form in key fields • Form 2004 – New question added to capture NAT testing for HBV – NAT testing for HIV-1 and HCV split – Removed questions for Anti-HTLV 1, syphyllis, CMV, WNV, toxoplasmosis • Form 2005 – Form will be collected for recipient and final donor only TRAINING & DEVELOPMENT | 3434.

Post-TED (F 2450) Revision Highlights*not a complete list • ‘Check all that apply’ capability: • Liver toxicity prophylaxis, post-HCT therapy, relapse or progression post-HCT therapy • GVHD – Added questions to capture organ staging since the date of the last report • Chimerism studies – Collecting chimerism data for beta thalassemia and sickle cell recipients TRAINING & DEVELOPMENT | 3535.

Post-TED (F 2450) Revision Highlights (cont. ) • Relapse or progression post-HCT – The intent of the F 2450 has always been to capture the first relapse only – Updated question text to say “was the date of the first clinical/hematologic relapse or progression previously reported” – Removed “decreased chimerism” from the intervention given for relapse question TRAINING & DEVELOPMENT | 36.

CRID Assignment Form (F 2804) Revision Highlights • Demographics – Added recipient ethnicity, race and race detail – Will be auto-populated on pre-TED – Race will be ‘check all that apply’ – Race detail list will automatically filter based on race selection TRAINING & DEVELOPMENT | 37.

Plasma Cell Disorder Revision Highlights • Revising the forms 2016/2116 and the PCD section of the 2402 • Capturing biclonal/triclonal scenarios • Capturing information on monoclonal gammopathy of renal significance (MRGS) and POEMS • Updated Amyloidosis sections • Fall release TRAINING & DEVELOPMENT | 38.

Contact form (F 2820) • Form will collect recipient contact details for use in: – CIBMTR observational research database: permission to contact for future CIBMTR research studies – BMT CTN studies for direct patient follow up – Additional future studies • Includes: – name – address – phone number – email TRAINING & DEVELOPMENT | 3939.

TRAINING & DEVELOPMENT | 40. 40

Questions TRAINING & DEVELOPMENT | 41.
 476 fighter group
476 fighter group Socorro isd schools
Socorro isd schools Passive voice revision
Passive voice revision Rachael blansett
Rachael blansett Rachael huntley
Rachael huntley Rachael blansett
Rachael blansett The soul of education
The soul of education Rachael edgerly
Rachael edgerly Rachael hale
Rachael hale Oh quel beau jour ou devant ta face lyrics
Oh quel beau jour ou devant ta face lyrics Rachael trotman
Rachael trotman Oh quel beau jour
Oh quel beau jour Rachael
Rachael Rachael corrie
Rachael corrie Rachael drazan
Rachael drazan Rachael scherer
Rachael scherer Gcse child development revision powerpoint
Gcse child development revision powerpoint Starbucks tangible and intangible resources
Starbucks tangible and intangible resources Revision verb form
Revision verb form Standard form revision
Standard form revision Rejoice dustin kensrue
Rejoice dustin kensrue Tiffany taylor georgia department of education
Tiffany taylor georgia department of education Tiffany tampa review
Tiffany tampa review Em tiffany
Em tiffany Em tiffany facts
Em tiffany facts Autumn landscape tiffany
Autumn landscape tiffany Nunca desayunaré en tiffany
Nunca desayunaré en tiffany Tiffany besse
Tiffany besse Tiffany wendler
Tiffany wendler Tiffany megnath
Tiffany megnath Tiffany mehdizadeh
Tiffany mehdizadeh Dr tiffany shaw
Dr tiffany shaw April dye
April dye Dr tiffany shaw
Dr tiffany shaw Tiffany neill
Tiffany neill Tiffany kesslar
Tiffany kesslar Em tiffany
Em tiffany What is the ffa emblem composed of
What is the ffa emblem composed of Bilderbuch von tomi ungerer
Bilderbuch von tomi ungerer Tiffany stephenson
Tiffany stephenson Ffa creed quiz
Ffa creed quiz Couchsurfing questions
Couchsurfing questions