FORCES BETWEEN PARTICLES NOBLE GAS CONFIGURATIONS PERIOD GROUP

FORCES BETWEEN PARTICLES NOBLE GAS CONFIGURATIONS PERIOD GROUP 1 2 He 2 10 Ne 3 18 Ar 4 36 Kr 5 54 Xe 6 86 Rn El Camino College Chemistry 21 A Dr. Dragan Marinkovic
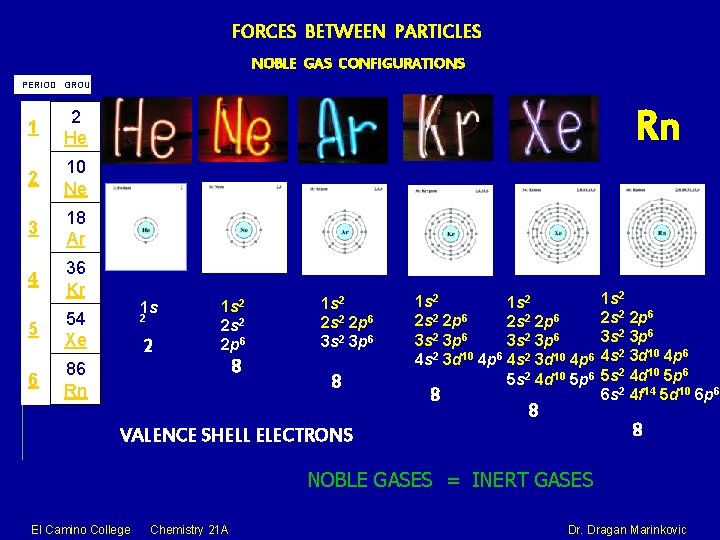
FORCES BETWEEN PARTICLES NOBLE GAS CONFIGURATIONS PERIOD GROUP 1 2 He 2 10 Ne 3 18 Ar 4 36 Kr 5 54 Xe 6 86 Rn Rn 1 s 2 2 s 2 2 p 6 8 1 s 2 2 p 6 3 s 2 3 p 6 8 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 8 8 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14 5 d 10 6 p 6 8 VALENCE SHELL ELECTRONS NOBLE GASES = INERT GASES El Camino College Chemistry 21 A Dr. Dragan Marinkovic
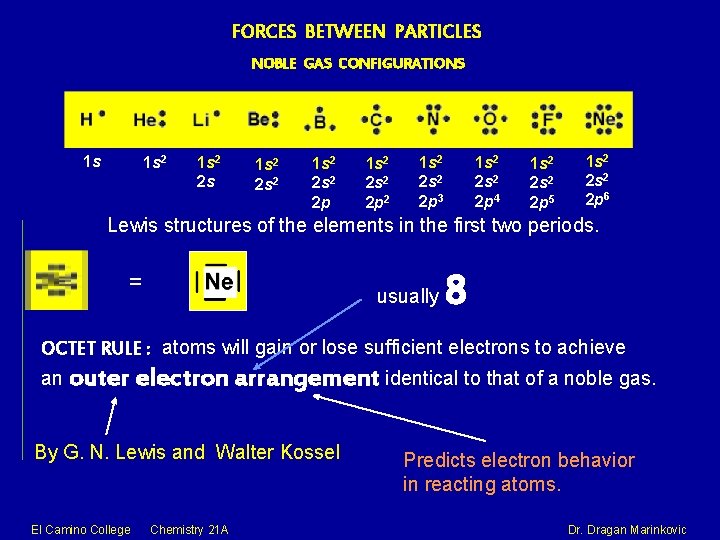
FORCES BETWEEN PARTICLES NOBLE GAS CONFIGURATIONS 1 s 1 s 2 2 s 2 2 p 1 s 2 2 p 2 1 s 2 2 p 3 1 s 2 2 p 4 1 s 2 2 p 5 1 s 2 2 p 6 Lewis structures of the elements in the first two periods. = usually 8 OCTET RULE : atoms will gain or lose sufficient electrons to achieve an outer electron arrangement identical to that of a noble gas. By G. N. Lewis and Walter Kossel El Camino College Chemistry 21 A Predicts electron behavior in reacting atoms. Dragan Marinkovic
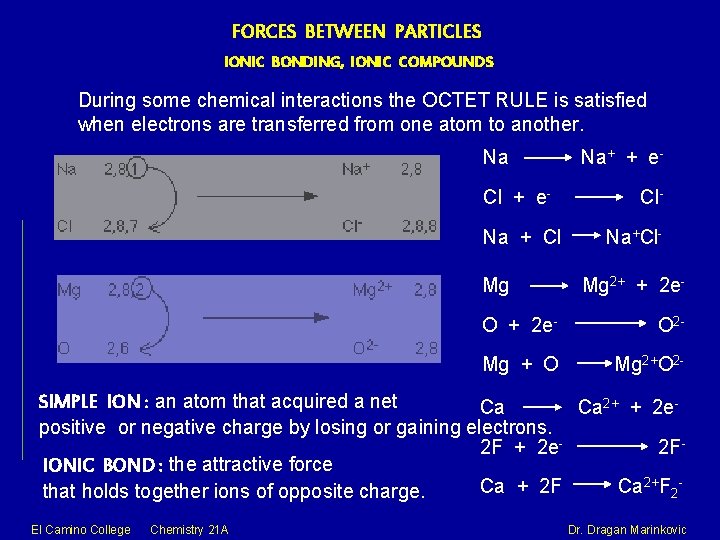
FORCES BETWEEN PARTICLES IONIC BONDING, IONIC COMPOUNDS During some chemical interactions the OCTET RULE is satisfied when electrons are transferred from one atom to another. Na Cl + e. Na + Cl Mg Na+ + e. Cl. Na+Cl. Mg 2+ + 2 e- O 2 - Mg + O Mg 2+O 2 - SIMPLE ION : an atom that acquired a net Ca Ca 2+ + 2 epositive or negative charge by losing or gaining electrons. 2 F + 2 e 2 FIONIC BOND : the attractive force Ca + 2 F Ca 2+F 2 that holds together ions of opposite charge. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
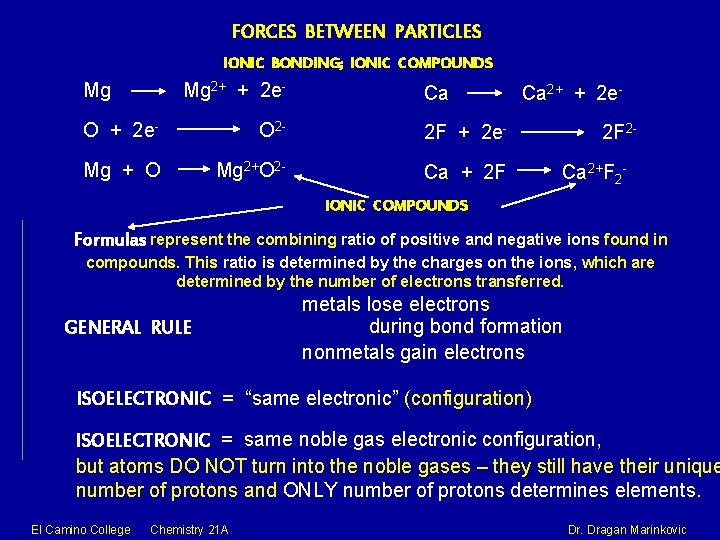
FORCES BETWEEN PARTICLES IONIC BONDING; IONIC COMPOUNDS Mg Mg 2+ + 2 e- Ca O + 2 e- O 2 - 2 F + 2 e- Mg + O Mg 2+O 2 - Ca + 2 F Ca 2+ + 2 e 2 F 2 Ca 2+F 2 - IONIC COMPOUNDS Formulas represent the combining ratio of positive and negative ions found in compounds. This ratio is determined by the charges on the ions, which are determined by the number of electrons transferred. GENERAL RULE metals lose electrons during bond formation nonmetals gain electrons ISOELECTRONIC = “same electronic” (configuration) ISOELECTRONIC = same noble gas electronic configuration, but atoms DO NOT turn into the noble gases – they still have their unique number of protons and ONLY number of protons determines elements. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
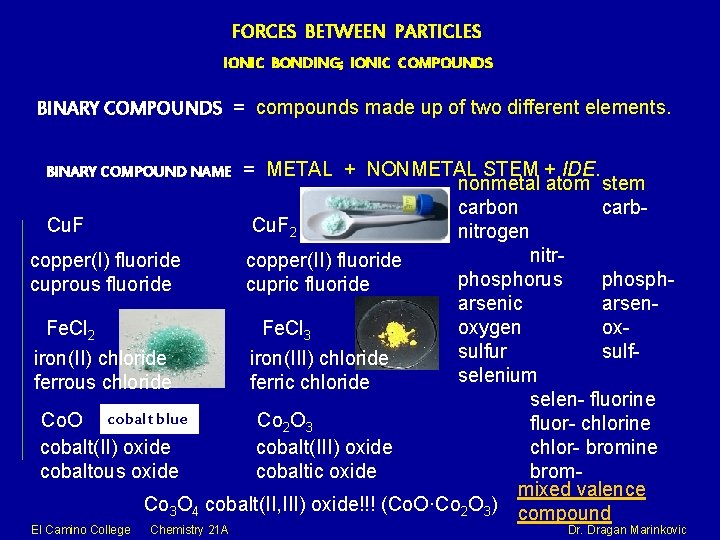
FORCES BETWEEN PARTICLES IONIC BONDING; IONIC COMPOUNDS BINARY COMPOUNDS = compounds made up of two different elements. = METAL + NONMETAL STEM + IDE. nonmetal atom stem carbon carb. Cu. F 2 nitrogen nitrcopper(I) fluoride copper(II) fluoride phosphorus phosphcuprous fluoride cupric fluoride arsenic arsenoxygen ox. Fe. Cl 2 Fe. Cl 3 sulfur sulfiron(II) chloride iron(III) chloride selenium ferrous chloride ferric chloride selen- fluorine Co. O cobalt blue Co 2 O 3 fluor- chlorine chlor- bromine cobalt(II) oxide cobalt(III) oxide bromcobaltous oxide cobaltic oxide mixed valence Co 3 O 4 cobalt(II, III) oxide!!! (Co. O·Co 2 O 3) compound BINARY COMPOUND NAME El Camino College Chemistry 21 A Dr. Dragan Marinkovic

FORCES BETWEEN PARTICLES IONIC BONDING MOLECULES are the smallest particle of pure substance that has properties of that substance and is capable of stable independent existence. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
FORCES BETWEEN PARTICLES IONIC BONDING MOLECULES are the smallest particle of pure substance that has properties of that substance and is capable of stable independent existence. Compound formula is a symbol for the molecule of a compound, consisting of the symbols of the atoms found in the molecule. Some compound formulas are used to represent a single molecule. Molecular formulas represent the precise number of atoms of each element found in a molecule. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
FORCES BETWEEN PARTICLES IONIC BONDING MOLECULES are the smallest particle of pure substance that has properties of that substance and is capable of stable independent existence. Compound formula is a symbol for the molecule of a compound, consisting of the symbols of the atoms found in the molecule. Some compound formulas are used to represent a single molecule. Molecular formulas represent the precise number of atoms of each element found in a molecule. Formulas of ionic compounds represent only a simplest combining ratio of the ions in the compounds. A stable form of an ionic compound is not a molecule, but a CRYSTAL in which many ions of opposite charge occupy LATTICE SITES in a rigid three dimensional arrangement called a CRYSTAL LATTICE. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
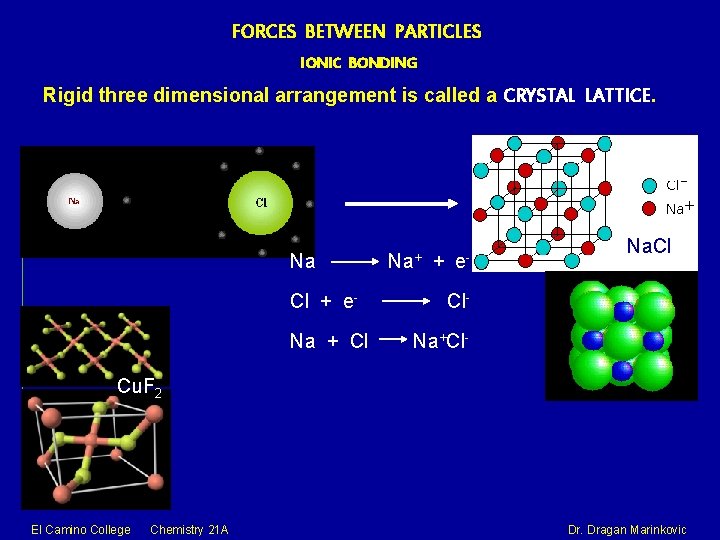
FORCES BETWEEN PARTICLES IONIC BONDING Rigid three dimensional arrangement is called a CRYSTAL LATTICE. Na Cl + e. Na + Cl Na+ + e- Na. Cl Cl. Na+Cl- Cu. F 2 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
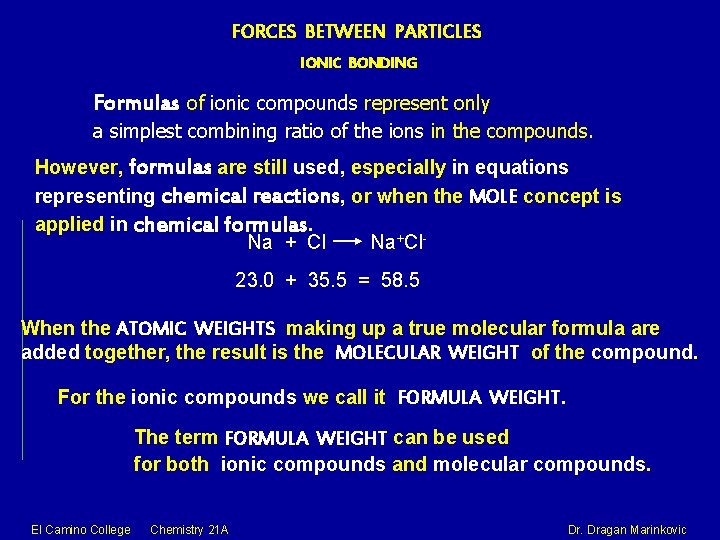
FORCES BETWEEN PARTICLES IONIC BONDING Formulas of ionic compounds represent only a simplest combining ratio of the ions in the compounds. However, formulas are still used, especially in equations representing chemical reactions, or when the MOLE concept is applied in chemical formulas. Na + Cl Na+Cl 23. 0 + 35. 5 = 58. 5 When the ATOMIC WEIGHTS making up a true molecular formula are added together, the result is the MOLECULAR WEIGHT of the compound. For the ionic compounds we call it FORMULA WEIGHT. The term FORMULA WEIGHT can be used for both ionic compounds and molecular compounds. El Camino College Chemistry 21 A Dr. Dragan Marinkovic

FORCES BETWEEN PARTICLES COVALENT BONDING A chemical bond is a strong attraction between two or more atoms. Bonds hold atoms in molecules and crystals together. There are many types of chemical bonds, but all involve electrons which are either shared or transferred between the bonded atoms. El Camino College Chemistry 21 A Dr. Dragan Marinkovic

FORCES BETWEEN PARTICLES COVALENT BONDING A chemical bond is a strong attraction between two or more atoms. Bonds hold atoms in molecules and crystals together. There are many types of chemical bonds, but all involve electrons which are either shared or transferred between the bonded atoms. It is known that the stable form of gases, like oxygen, nitrogen and chlo are diatomic molecules O 2, N 2 and Cl 2. Obviously, such molecular bonds be formed by electron transfer from one atom to another like in ionic mo G. W. Lewis proposed that in these molecules VALENCE SHELL ELECTRONS ARE SHARED in order to satisfy the OCTET RULE for EACH of the ATO Such El Camino College Chemistry 21 A BONDS are called COVALENT BONDS. Dragan Marinkovic
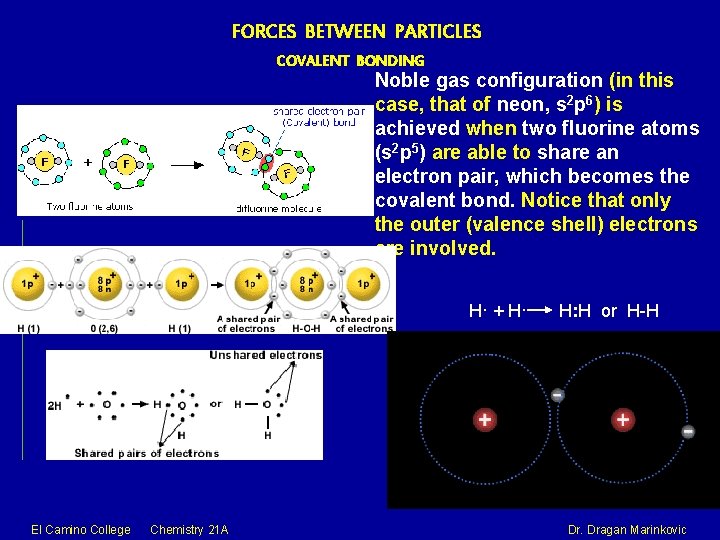
FORCES BETWEEN PARTICLES COVALENT BONDING Noble gas configuration (in this case, that of neon, s 2 p 6) is achieved when two fluorine atoms (s 2 p 5) are able to share an electron pair, which becomes the covalent bond. Notice that only the outer (valence shell) electrons are involved. H· + H· El Camino College Chemistry 21 A H: H or H-H Dr. Dragan Marinkovic
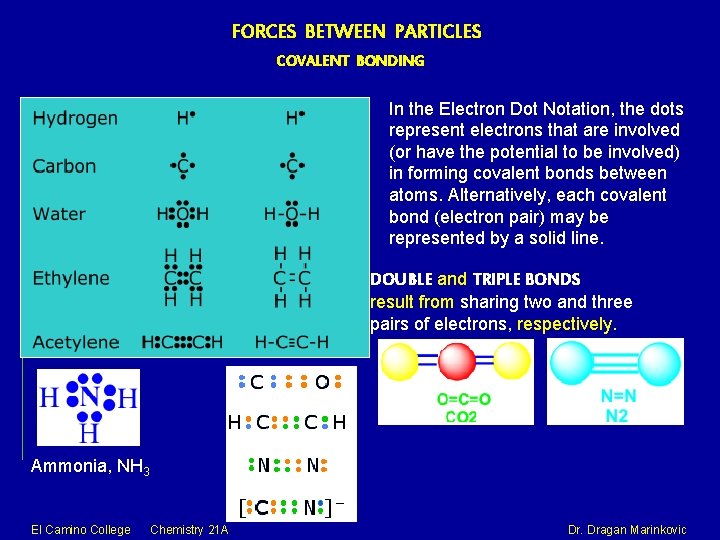
FORCES BETWEEN PARTICLES COVALENT BONDING In the Electron Dot Notation, the dots represent electrons that are involved (or have the potential to be involved) in forming covalent bonds between atoms. Alternatively, each covalent bond (electron pair) may be represented by a solid line. DOUBLE and TRIPLE BONDS result from sharing two and three pairs of electrons, respectively. Ammonia, NH 3 El Camino College Chemistry 21 A Dr. Dragan Marinkovic
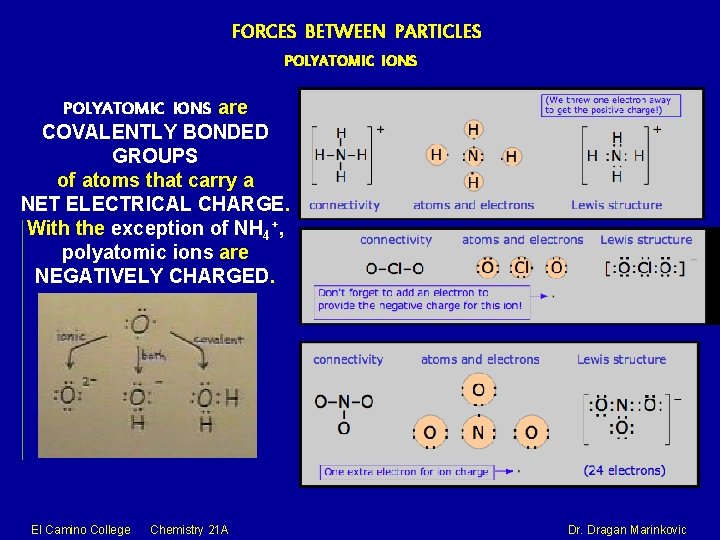
FORCES BETWEEN PARTICLES POLYATOMIC IONS are COVALENTLY BONDED GROUPS of atoms that carry a NET ELECTRICAL CHARGE. With the exception of NH 4+, polyatomic ions are NEGATIVELY CHARGED. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
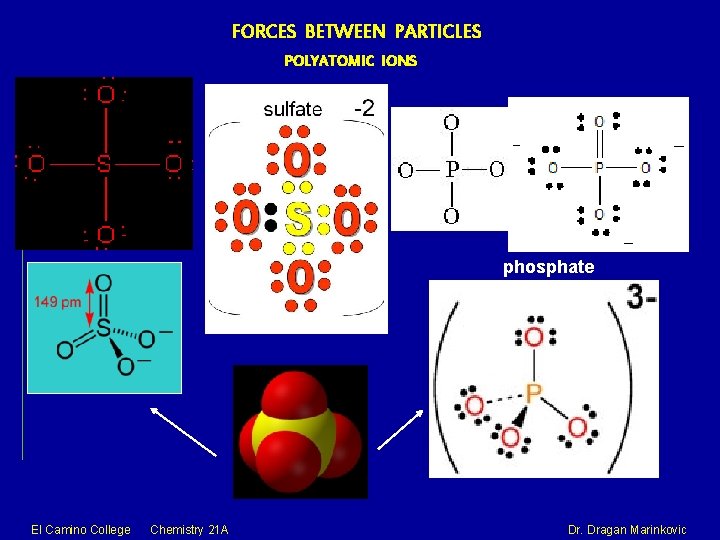
FORCES BETWEEN PARTICLES POLYATOMIC IONS phosphate El Camino College Chemistry 21 A Dr. Dragan Marinkovic
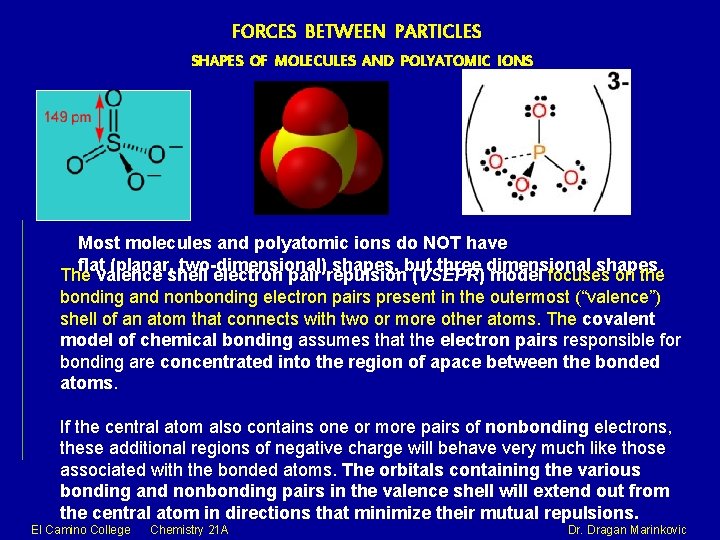
FORCES BETWEEN PARTICLES SHAPES OF MOLECULES AND POLYATOMIC IONS Most molecules and polyatomic ions do NOT have flat (planar, two-dimensional) shapes, but three dimensional shapes. The valence shell electron pair repulsion (VSEPR) model focuses on the bonding and nonbonding electron pairs present in the outermost (“valence”) shell of an atom that connects with two or more other atoms. The covalent model of chemical bonding assumes that the electron pairs responsible for bonding are concentrated into the region of apace between the bonded atoms. If the central atom also contains one or more pairs of nonbonding electrons, these additional regions of negative charge will behave very much like those associated with the bonded atoms. The orbitals containing the various bonding and nonbonding pairs in the valence shell will extend out from the central atom in directions that minimize their mutual repulsions. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
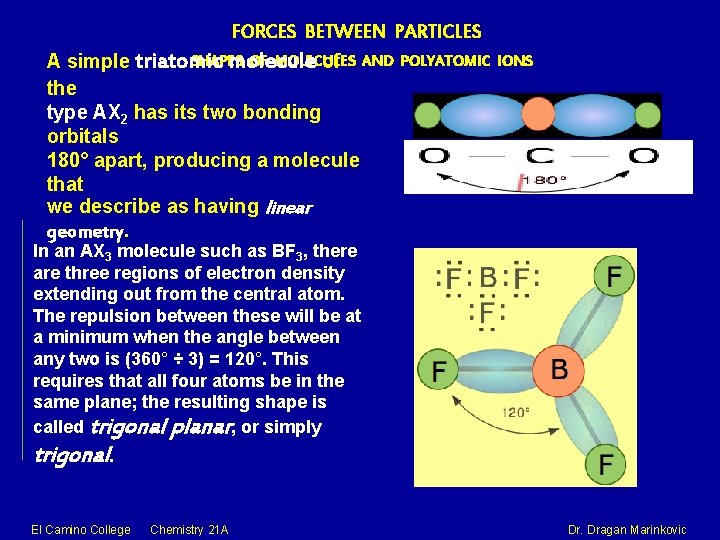
FORCES BETWEEN PARTICLES SHAPES OF MOLECULES A simple triatomic molecule of AND POLYATOMIC the type AX 2 has its two bonding orbitals 180° apart, producing a molecule that we describe as having linear geometry. IONS In an AX 3 molecule such as BF 3, there are three regions of electron density extending out from the central atom. The repulsion between these will be at a minimum when the angle between any two is (360° ÷ 3) = 120°. This requires that all four atoms be in the same plane; the resulting shape is called trigonal planar, or simply trigonal. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
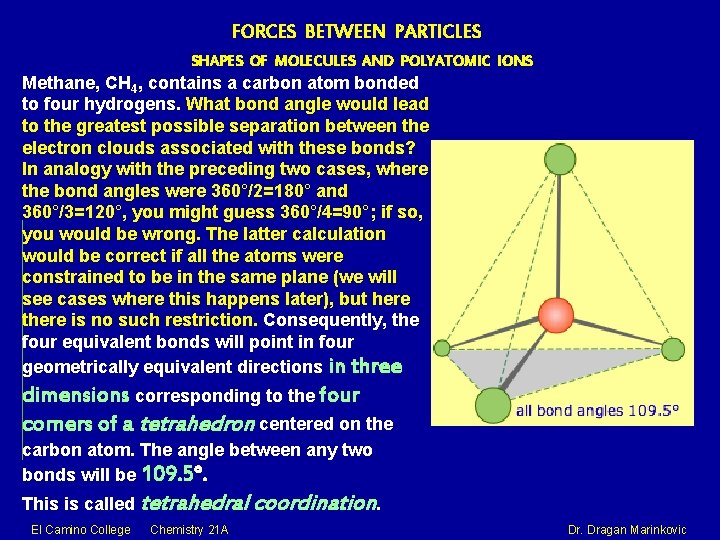
FORCES BETWEEN PARTICLES SHAPES OF MOLECULES AND POLYATOMIC IONS Methane, CH 4, contains a carbon atom bonded to four hydrogens. What bond angle would lead to the greatest possible separation between the electron clouds associated with these bonds? In analogy with the preceding two cases, where the bond angles were 360°/2=180° and 360°/3=120°, you might guess 360°/4=90°; if so, you would be wrong. The latter calculation would be correct if all the atoms were constrained to be in the same plane (we will see cases where this happens later), but here there is no such restriction. Consequently, the four equivalent bonds will point in four geometrically equivalent directions in three dimensions corresponding to the four corners of a tetrahedron centered on the carbon atom. The angle between any two bonds will be 109. 5°. This is called tetrahedral coordination. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
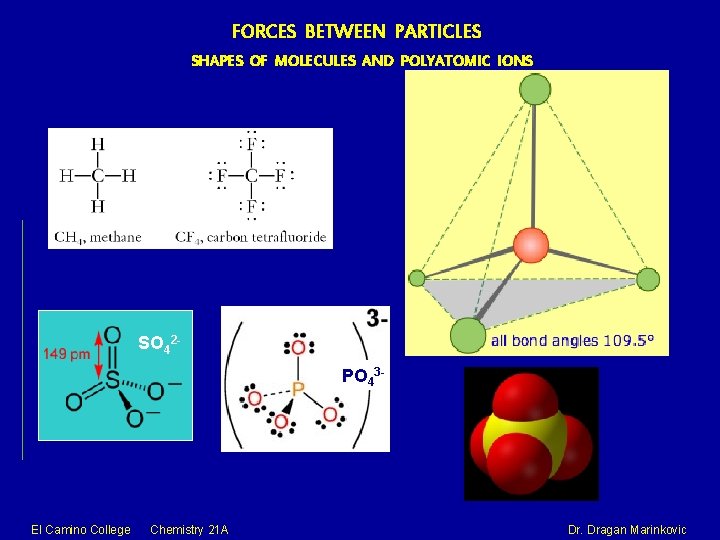
FORCES BETWEEN PARTICLES SHAPES OF MOLECULES AND POLYATOMIC IONS SO 42 PO 43 - El Camino College Chemistry 21 A Dr. Dragan Marinkovic
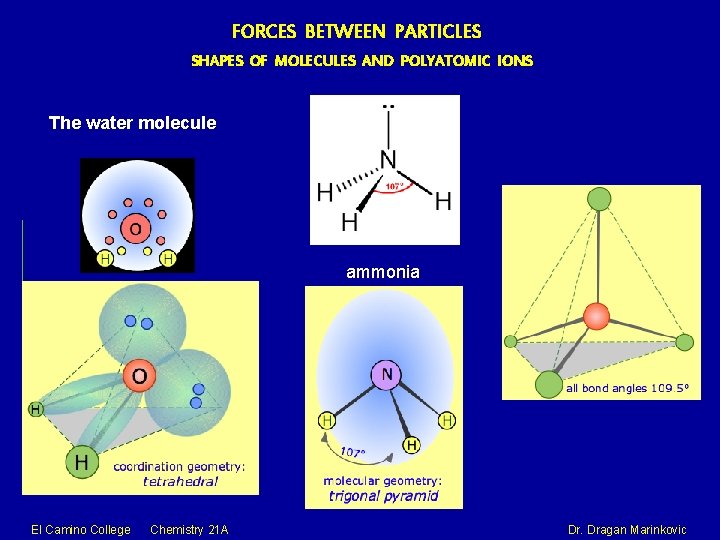
FORCES BETWEEN PARTICLES SHAPES OF MOLECULES AND POLYATOMIC IONS The water molecule ammonia El Camino College Chemistry 21 A Dr. Dragan Marinkovic

FORCES BETWEEN PARTICLES THE POLARITY OF COVALENT MOLECULES Cl 2 (Cl-Cl) El Camino College Chemistry 21 A Dr. Dragan Marinkovic
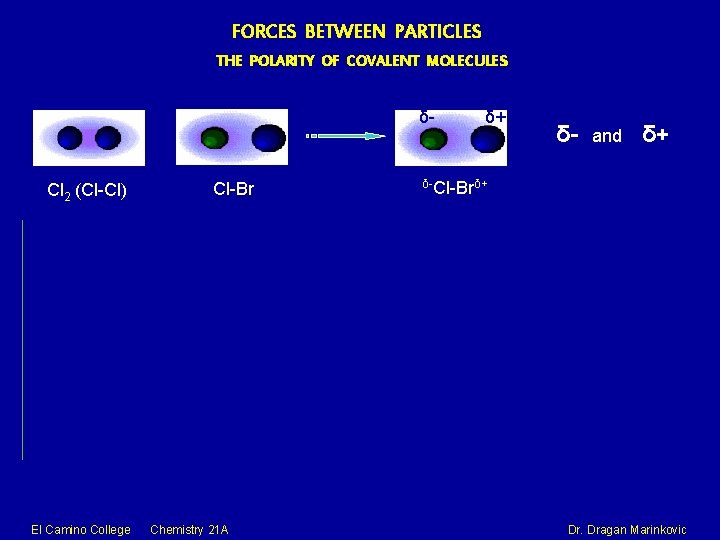
FORCES BETWEEN PARTICLES THE POLARITY OF COVALENT MOLECULES δ- Cl 2 (Cl-Cl) El Camino College Cl-Br Chemistry 21 A δ+ δ- and δ+ δ-Cl-Brδ+ Dr. Dragan Marinkovic
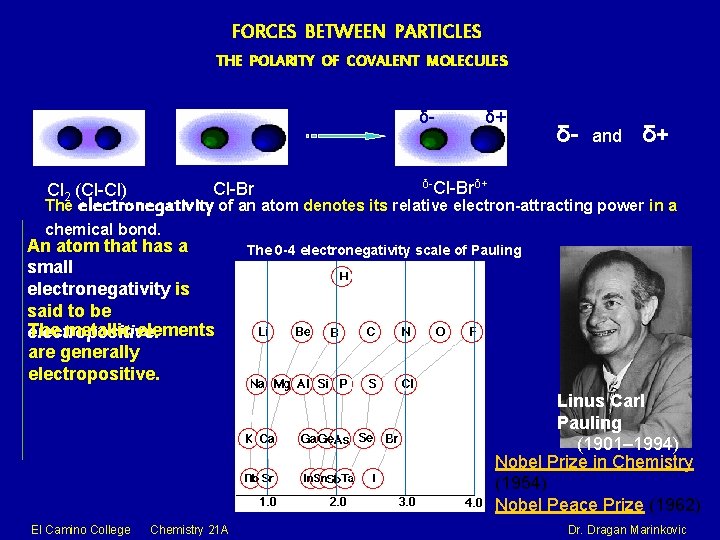
FORCES BETWEEN PARTICLES THE POLARITY OF COVALENT MOLECULES δ- Cl-Br Cl 2 (Cl-Cl) δ+ δ- and δ+ δ-Cl-Brδ+ The electronegativity of an atom denotes its relative electron-attracting power in a chemical bond. An atom that has a small electronegativity is said to be The metallic elements electropositive. are generally electropositive. The 0 -4 electronegativity scale of Pauling Linus Carl Pauling (1901– 1994) Nobel Prize in Chemistry (1954) Nobel Peace Prize (1962) El Camino College Chemistry 21 A Dr. Dragan Marinkovic
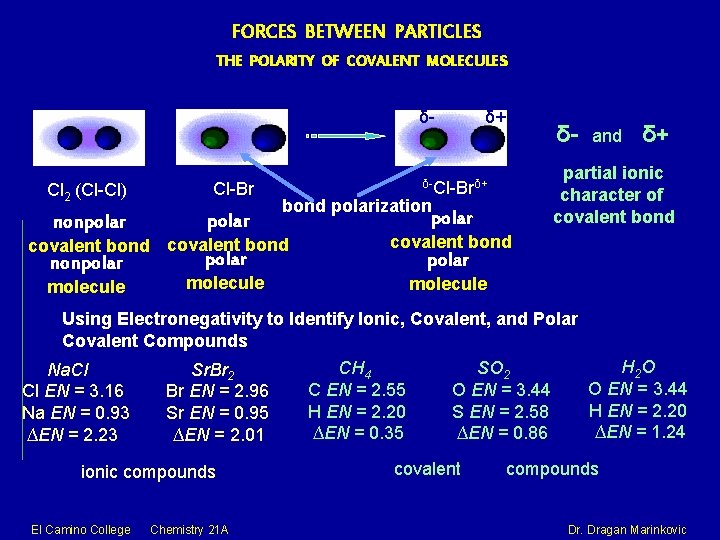
FORCES BETWEEN PARTICLES THE POLARITY OF COVALENT MOLECULES δ- Cl 2 (Cl-Cl) Cl-Br nonpolar molecule polar δ- and δ+ partial ionic character of covalent bond δ-Cl-Brδ+ bond polarization covalent bond nonpolar δ+ polar covalent bond polar molecule Using Electronegativity to Identify Ionic, Covalent, and Polar Covalent Compounds Na. Cl Cl EN = 3. 16 Na EN = 0. 93 ∆EN = 2. 23 Sr. Br 2 Br EN = 2. 96 Sr EN = 0. 95 ∆EN = 2. 01 ionic compounds El Camino College Chemistry 21 A CH 4 C EN = 2. 55 H EN = 2. 20 ∆EN = 0. 35 SO 2 O EN = 3. 44 S EN = 2. 58 ∆EN = 0. 86 covalent H 2 O O EN = 3. 44 H EN = 2. 20 ∆EN = 1. 24 compounds Dr. Dragan Marinkovic
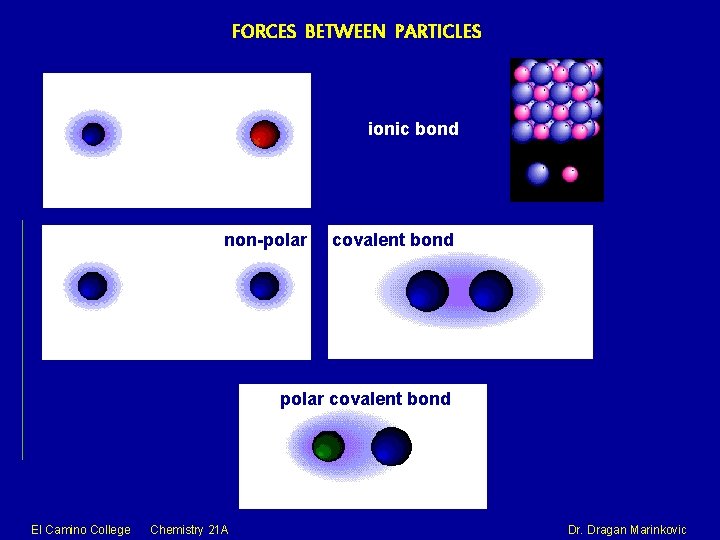
FORCES BETWEEN PARTICLES ionic bond non-polar covalent bond El Camino College Chemistry 21 A Dr. Dragan Marinkovic
FORCES BETWEEN PARTICLES OTHER INTERPARTICLE FORCES Ionic and covalent bonding explains certain properties of substances. Some experimental observations can be explained only by proposing other types of forces between particles. Ionic compounds in solid state form crystal lattice. Actually, most pure substances (molecules or atoms) form crystal lattices in solid state. When heated, solids melt and forces holding particles in dense organized form weaken and particles move about more freely – in liquid state. More heating overcomes attractive interparticle forces even more turning the liquid into a gas or vapor (the liquid boils). Interparticle forces are minimal and particles move about freely – gas (vapor) state. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
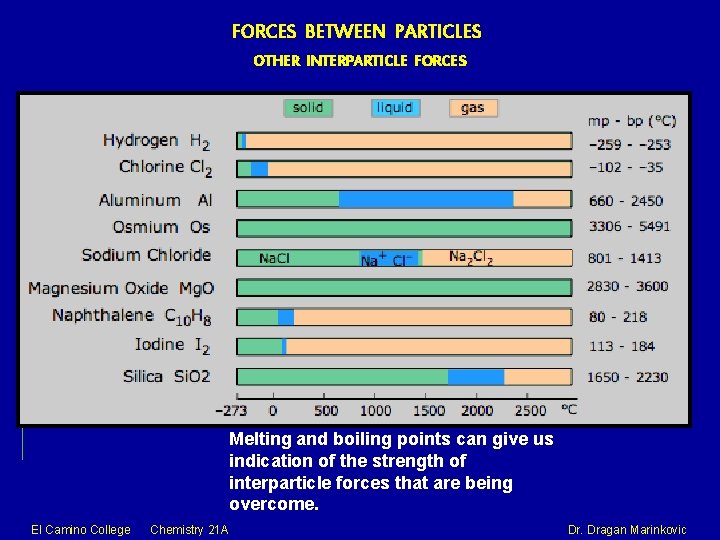
FORCES BETWEEN PARTICLES OTHER INTERPARTICLE FORCES Melting and boiling points can give us indication of the strength of interparticle forces that are being overcome. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
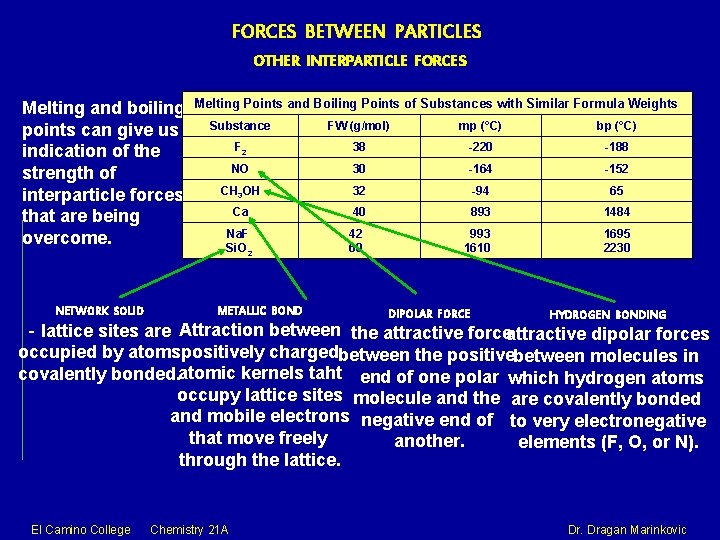
FORCES BETWEEN PARTICLES OTHER INTERPARTICLE FORCES Melting and boiling points can give us indication of the strength of interparticle forces that are being overcome. NETWORK SOLID Melting Points and Boiling Points of Substances with Similar Formula Weights Substance FW (g/mol) mp (°C) bp (°C) F 2 38 -220 -188 NO 30 -164 -152 CH 3 OH 32 -94 65 Ca 40 893 1484 Na. F Si. O 2 42 60 993 1610 1695 2230 METALLIC BOND DIPOLAR FORCE HYDROGEN BONDING - lattice sites are Attraction between the attractive forceattractive dipolar forces occupied by atomspositively chargedbetween the positivebetween molecules in covalently bonded. atomic kernels taht end of one polar which hydrogen atoms occupy lattice sites molecule and the are covalently bonded and mobile electrons negative end of to very electronegative that move freely another. elements (F, O, or N). through the lattice. El Camino College Chemistry 21 A Dr. Dragan Marinkovic
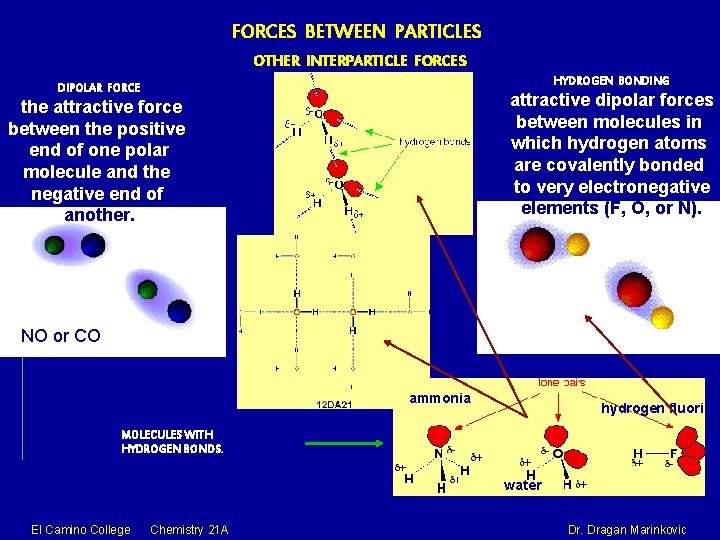
FORCES BETWEEN PARTICLES OTHER INTERPARTICLE FORCES HYDROGEN BONDING DIPOLAR FORCE attractive dipolar forces between molecules in which hydrogen atoms are covalently bonded to very electronegative elements (F, O, or N). the attractive force between the positive end of one polar molecule and the negative end of another. NO or CO ammonia hydrogen fluoride MOLECULES WITH HYDROGEN BONDS. water El Camino College Chemistry 21 A Dr. Dragan Marinkovic
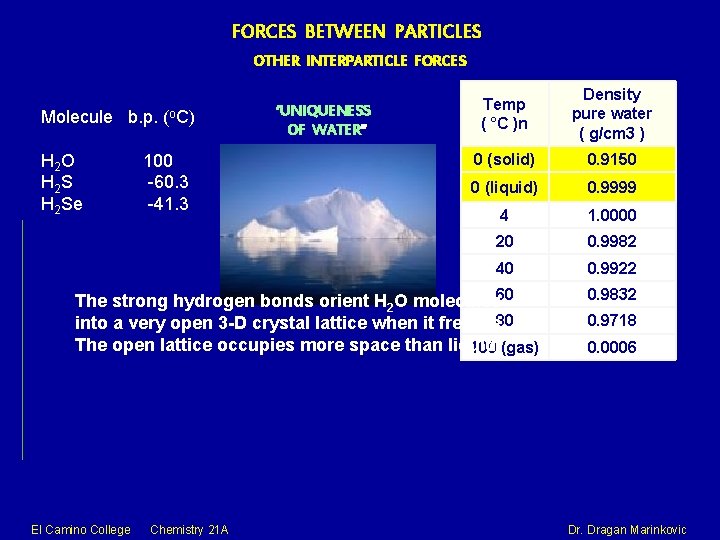
FORCES BETWEEN PARTICLES OTHER INTERPARTICLE FORCES Molecule b. p. (o. C) H 2 O H 2 S H 2 Se 100 -60. 3 -41. 3 ‘UNIQUENESS OF WATER” Temp ( °C )n Density pure water ( g/cm 3 ) 0 (solid) 0. 9150 0 (liquid) 0. 9999 4 1. 0000 20 0. 9982 40 0. 9922 The strong hydrogen bonds orient H 2 O molecules 60 80 into a very open 3 -D crystal lattice when it freezes. The open lattice occupies more space than liquid. 100 (gas) El Camino College Chemistry 21 A 0. 9832 0. 9718 0. 0006 Dr. Dragan Marinkovic
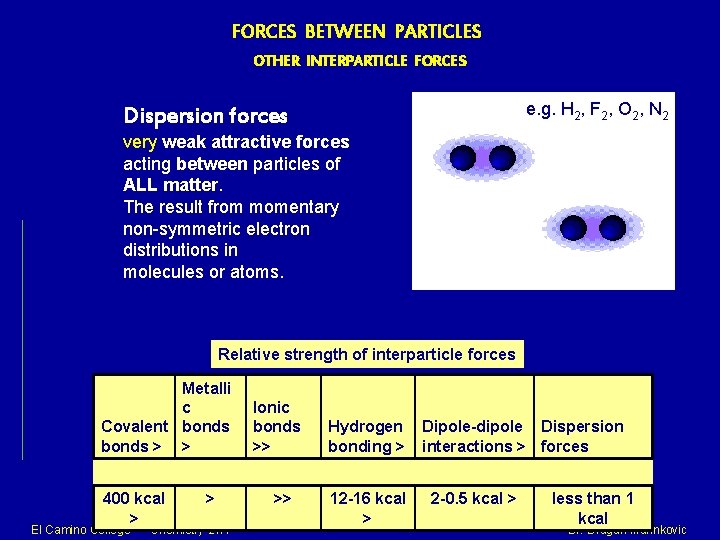
FORCES BETWEEN PARTICLES OTHER INTERPARTICLE FORCES e. g. H 2, F 2, O 2, N 2 Dispersion forces very weak attractive forces acting between particles of ALL matter. The result from momentary non-symmetric electron distributions in molecules or atoms. Relative strength of interparticle forces Metalli c Covalent bonds > > 400 kcal > El Camino College > Chemistry 21 A Ionic bonds >> >> Hydrogen bonding > 12 -16 kcal > Dipole-dipole Dispersion interactions > forces 2 -0. 5 kcal > less than 1 kcal Dr. Dragan Marinkovic
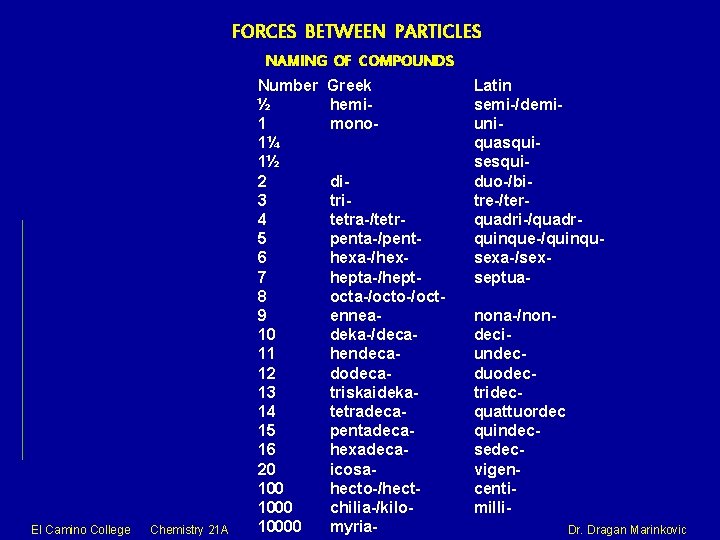
FORCES BETWEEN PARTICLES NAMING OF COMPOUNDS El Camino College Chemistry 21 A Number ½ 1 1¼ 1½ 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 20 10000 Greek hemimonoditritetra-/tetrpenta-/penthexa-/hexhepta-/heptocta-/octo-/octenneadeka-/decahendecadodecatriskaidekatetradecapentadecahexadecaicosahecto-/hectchilia-/kilomyria- Latin semi-/demiuniquasquisesquiduo-/bitre-/terquadri-/quadrquinque-/quinqusexa-/sexseptuanona-/nondeciundecduodectridecquattuordec quindecsedecvigencentimilli. Dragan Marinkovic

FORCES BETWEEN PARTICLES NAMING OF COMPOUNDS Number Greek Latin ½ 1 1¼ 1½ 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 20 10000 semi-/demiuniquasquisesquiduo-/bitre-/terquadri-/quadrquinque-/quinqusexa-/sexseptua- hemimonoditritetra-/tetrpenta-/penthexa-/hexhepta-/heptocta-/octo-/octenneadeka-/decahendecadodecatriskaidekatetradecapentadecahexadecaicosahecto-/hectchilia-/kilomyria- El Camino College Chemistry 21 A nona-/nondeciundecduodectridecquattuordec quindecsedecvigencentimilli- Examples: Cr. O 3 chromium trioxide Na. OH sodium hydroxide KMn. O 4 potassium permanganate Os. O 4 osmium tetroxide SF 6 sulfur hexafluoride (NH 4)2 Cr. O 4 (di)ammonium chromate Li 3 PO 4 trilithium phosphate PCl 5 phosphorus pentachloride Dr. Dragan Marinkovic

FORCES BETWEEN PARTICLES El Camino College Chemistry 21 A Dr. Dragan Marinkovic
- Slides: 36