Food Allergies Advances in Diagnosis and Management Greg


































- Slides: 55

Food Allergies: Advances in Diagnosis and Management Greg Black, MD USC SOM Department of Pediatrics Grand Rounds October 16, 2015 Carolina Allergy and Asthma Consultants, PA

Roadmap • • Food allergies: The Scope of the Problem Risk Factors and Theories on FA Development Conventional Methods of Diagnosis Component-Resolved Diagnostics LEAP Study and Prevention Advances in Immunotherapy Conclusions

Exclusions • • • Food Protein Induced Enterocolitis (acute and chronic FPIES) Allergic Proctocolitis Food Dependent Exercise Induced Anaphylaxis Red meat allergy (alpha-gal sensitivity) Oral Pollen Syndrome Celiac Disease Atopic Dermatitis Auriculotemporal Syndrome Eosinophilic Esophagitis and Gastrointestinal Disease Food Poisoning Irritable Bowel Syndrome/Mastocytic Enterocolitis Inflammatory Bowel Disease

Food Allergy (FA) • CDC estimates 8% of children and 4% of adults in US have a FA diagnosis. • CDC estimates 50% increase in pediatric FA 1997 -2011. • Gupta et al in 2012 reports economic burden of FA is $25 billion USD. • FA results in 300, 000 ambulatory care visits a year, and 200, 000 ED visits per year (half of ED visits are diagnosed as food allergen induced anaphylaxis). • FA is the leading cause of anaphylaxis outside of the hospital setting. • Co-diagnosis of asthma is a risk factor fatal food allergy.

Food Allergy (FA) Diagnosis • NIAID describes food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food. ” • Ninety percent of immediate hypersensitivity to foods in US due to milk, soy, egg, wheat, peanut, tree nut, fish, and shellfish. • Self reporting of food allergies by patients consistently is higher than true immediate hypersensitivity as documented by Oral Food Challenge (OFC). • Food allergy more common in children, and high cooccurrence of other atopic conditions. • Children with moderate to severe persistent atopic dermatitis at 35% increased risk to develop immediate hypersensitivity.

Fatal Food Allergy • AAAAI Statistics Page: 38. 7% of FA children have a history of severe reaction. • Xu et al in 2014 reported on 92 cases of fatal anaphylaxis from the Ontario, Canada Coroner’s office. • Forty (43%) due to food allergens. • Sixteen (17%) due to peanut. • RF for FA fatalities: asthma, peanut allergy, teenagers, delayed epinephrine administration. • Umasunthar et al reported on 10 studies in 2013 in a metaanalysis concerning reported on 240 cases of fatal food allergy at 1. 81 per million person years, assuming a FA prevalence of 3. 9%. • Incidence of fatal FA less than that of fatal accidents in Europe.


FA: Risk Factors • Family History • 7 fold increase in risk of Peanut allergy if primary relative is peanut allergic • Sex • M: F for peanut allergy in childhood 5: 1. • Ratio changes to 1: 1 with adulthood. • Ethnicity • Lieu et al documents risk of FA in non hispanic blacks increased over whites OR 3. 06 (95% CI 2. 1 -4. 3). NHANES 200506. • Genes • • STAT 6 IL 10 CD 14 TSLP

Food Allergy Diagnosis • History of Ingestion? • • Time to symptoms Foods ingested Means of preparation Location where food consumed • Symptoms? • • • Urticaria Angioedema Nausea, Vomiting Stridor Change in sensorium • Resolved/Recurring? • Time to resolution • Factors involved in resolution • Has patient returned to eating the food? • Confounders? • • • NSAIDS, Antibiotics Venom Exercise Latex Sexual activity


Theories on increase of FA Diagnoses • Hygiene Hypothesis • Lack of early infectious exposure leads to increased allergic sensitization • C-sections involved? • Early Probiotic administration may protect against eczema onset, but not allergic sensitization. • Dietary guidelines • Former AAP guidelines concerning families at risk (atopic history) was to delay introduction of typical allergenic foods, until recently. • No major role for dietary restrictions in pregnancy or lactation that would affect development of FA.

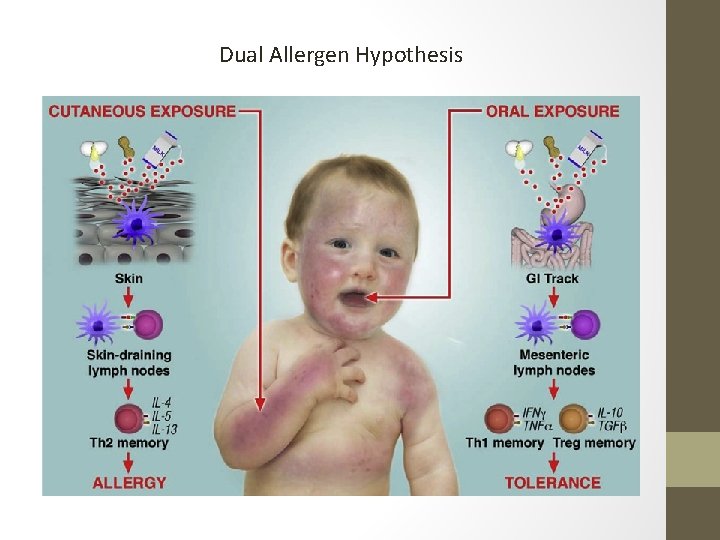
Dual Allergen Hypothesis

Insights into FA Inception • Bartnikas et al in 2013 epicutaneously sensitized BALB/c mice with ovalbumin (OVA) or orally dosed them with OVAcholeratoxin. • On oral challenge with OVA the epicutaneously sensitized mice had anaphylaxis, increased serum IL-4, and an expanded mast cell population in jejunum compared to orally dosed ova-CT mice that tolerated oral challenge. • Hough et al in 2013 determined that household peanut consumption was the most important variable in detecting peanut protein in the house dust of an infant’s crib and play area. • Peanut protein in house dust deemed biologically active as it stimulated basophils of peanut allergic patients in vitro.

Insights into FA Inception • Noti et al in 2014 sensitized mice via atopic dermatitis skin lesions to OVA and peanut allergen followed by intragastric allergen challenge, with immunologic changes measured thereafter. • Sensitization via this method was associated with expansion of TSLP-elicited basophils in the skin that promote allergen specific Th 2 cytokine responses, increased allergen specific serum Ig. E levels, and mast cell accumulation in the intestine, promoting the development of food allergy. • Basophil depletion or disruption of TSLP response decreased susceptibility of food allergic response. • Cell transfer of TSLP-elicited basophils into intact skin promoted disease!

Methods of FA Screening • Skin prick testing • Inexpensive • Outpatient setting • Immediate results (1520 minutes) • May use extract or “prick to prick” method with fresh allergen. • Intradermal testing to food allergens prone to false positivity and anaphylaxis.


Skin Prick Testing (SPT) for FA • Skin prick testing for FA has a sensitivity of 85% and a specificity of 74% (Sampson H et al JACI 1984). • Judicious and limited skin prick testing should be done within the context of a given anaphylactic or allergic event. • Proves sensitization, not clinical allergy. • Based on wheal diameter of positive prick tests, threshold values to determine if OFC’s are necessary have been set: • • Milk 8 mm Egg 7 mm Peanut 8 mm Sesame 8 mm

Serology (s. Ig. E) Testing for FA • Fluoroscence-labeled antibody assays to detect the presence of circulating Ig. E to a suspected food allergen. • Also only proves sensitizations, not clinical allergy. • RAST, Immunocap, Immulite 2000, etc. • Non specific testing to multiple allergens should be avoided. • Can be useful if history is suggestive of anaphylaxis and spt is negative or if patient cannot do without antihistamine, severe skin disease, dermatographism, etc. • Published cutoff values used to avoid OFC: • • Egg 7 k. UA/L Peanut 14 k. UA/L Milk 15 k. UA/L Sesame 7 k. UA/L

Limitations • Skin Prick Test • PPV based on population and food • NPV relatively high but negative result does not rule out allergy • Food allergy extract not standardized • Wheal size to establish OFC reactivity not determined for most allergens • Cannot skin test patients with active dermatitis • s. Ig. E Test • Often leads to unnecessary elimination diet • Total Ig. E level and nonspecific cross reactive binding • Fleischer et al in 2011 shows large majority of pediatric patients successfully reintroduced foods avoided based on s. Ig. E values only.


FA Diagnosis: Food Challenge • Gold standard of FA diagnosis is Double Blind Placebo Controlled Food Challenge (DBPCFC). • Cumbersome, lengthy, and usually only performed in academic centers. • OFC simple and easier to administer in outpatient setting. • Lieberman et al in 2011 evaluated 701 OFC’s for 521 patients. • 18. 8% elicited reaction, 1. 7% required epinephrine. • OFC’s should start with 0. 1% - 1 % of total challenge food. • OFC’s goal challenge dose should be 8 -10 gm dry food, 16 -20 gm meat or fish, 100 ml wet food; dosing interval should be every 15 minutes.

Unproven Diagnostic Methods • Serum Ig. G level to potential food allergens • Applied Kinesiology • Hair analysis • Cytotoxicity • Electrodermal testing • Rescue dogs

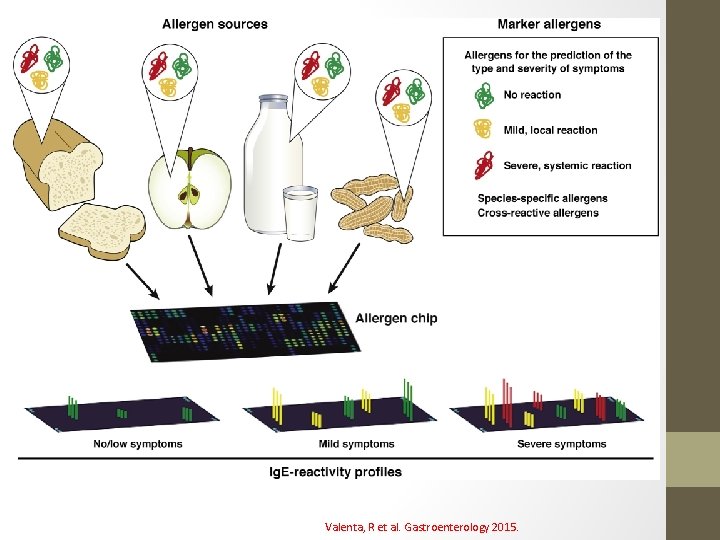
Component Resolved Diagnostics (CRD) • Diagnosis of immediate hypersensitivity to FA historically based on skin testing and s. Ig. E, but this approach has disadvantages that may affect the patient’s management. • CRD uses purified allergen proteins from natural sources or recombinant expression of complementary DNA. Food Components Peanut Ara h 1, 2, 3, 6, 8, 9 Milk Bos d 4, 5, 6, 8, 12 Egg Gal d 1, 2, 3, 4, 5

Valenta, R et al. Gastroenterology 2015.

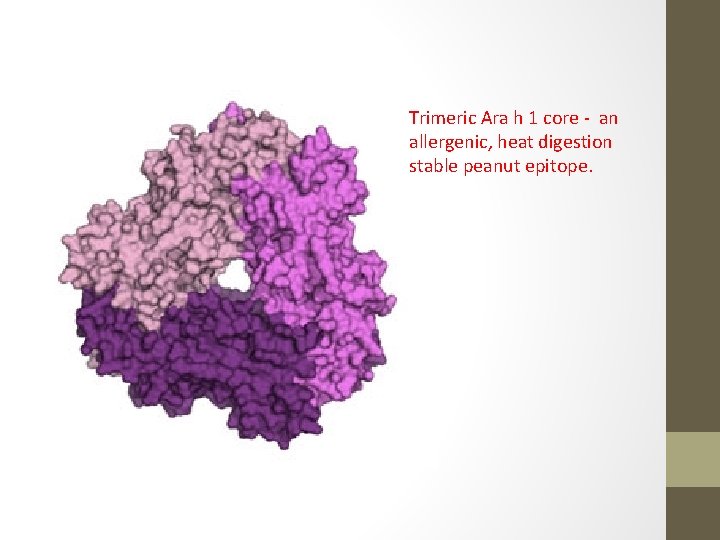
Trimeric Ara h 1 core - an allergenic, heat digestion stable peanut epitope.

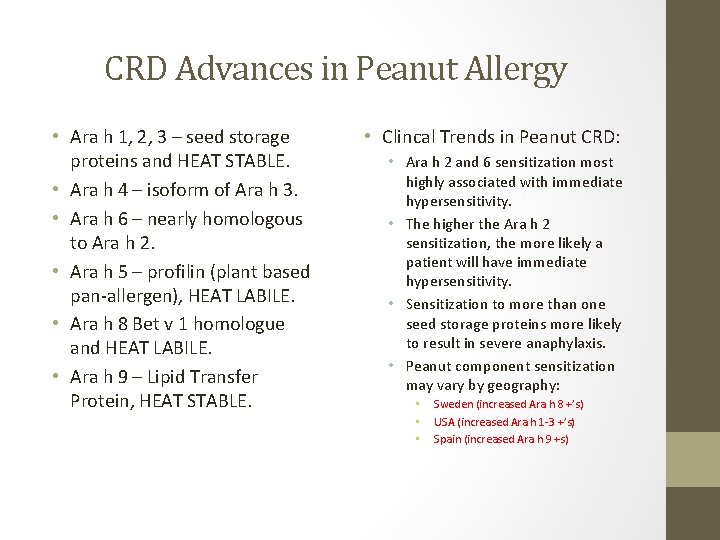
CRD Advances in Peanut Allergy • Ara h 1, 2, 3 – seed storage proteins and HEAT STABLE. • Ara h 4 – isoform of Ara h 3. • Ara h 6 – nearly homologous to Ara h 2. • Ara h 5 – profilin (plant based pan-allergen), HEAT LABILE. • Ara h 8 Bet v 1 homologue and HEAT LABILE. • Ara h 9 – Lipid Transfer Protein, HEAT STABLE. • Clincal Trends in Peanut CRD: • Ara h 2 and 6 sensitization most highly associated with immediate hypersensitivity. • The higher the Ara h 2 sensitization, the more likely a patient will have immediate hypersensitivity. • Sensitization to more than one seed storage proteins more likely to result in severe anaphylaxis. • Peanut component sensitization may vary by geography: • • • Sweden (increased Ara h 8 +’s) USA (increased Ara h 1 -3 +’s) Spain (increased Ara h 9 +s)

CRD Advances in Peanut Allergy • Johannsen et al in 2011 in Clin Exp Allergy found that Peanut SPT < 7 mm and s. Ig. E < 2 k. Ua/L was associated with 95% OFC tolerance. • Nicolaou et al in 2010 showed that in a birth cohort of 933 children 11. 8% were sensitized to peanut at 8 years of age. OFC’s were performed on children with unconvincing reactions and s. Ig. E < 15 k. Ua/L and spt < 8 mm, finding a prevalence of immediate HS of 22. 4% among sensitized patients. • Nicolaou et al in 2010 also found that peanut sensitized children that were tolerant often were concomitantly sensitized to grass pollen and birch pollen. • Dang et al in 2012 found in 200 Australian children that peanut skin prick test followed by Ara h 2 measurement limited the need for OFC when compared to a combination of other tests.

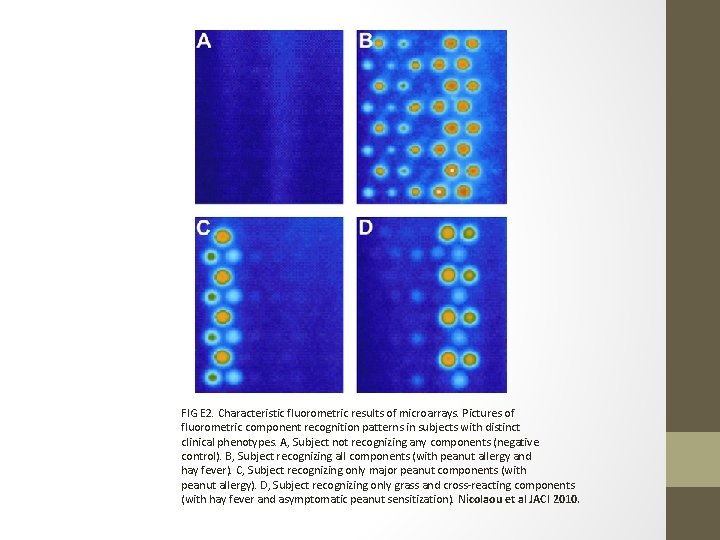
FIG E 2. Characteristic fluorometric results of microarrays. Pictures of fluorometric component recognition patterns in subjects with distinct clinical phenotypes. A, Subject not recognizing any components (negative control). B, Subject recognizing all components (with peanut allergy and hay fever). C, Subject recognizing only major peanut components (with peanut allergy). D, Subject recognizing only grass and cross-reacting components (with hay fever and asymptomatic peanut sensitization). Nicolaou et al JACI 2010.

Ara h 8 = Bet v 1 homologue • Extensive Ig. E cross-sensitization exists between peanut allergens and botanical allergens such as birch, alder, and grass allergens. • Asarnoj et al in 2010 described a group of peanut sensitized children that were sensitized to Ara h 8 only on Peanut CRD. Seventeen percent of these patients described mild symptoms only with peanut ingestion. • Asarnoj et al in 2012 examined 144 children with lone Ara h 8 sensitization: • 82 eating peanuts regularly at time of recruitment. • 62 underwent OFC to peanut • 1 experienced anaphylaxis (DEVELOPED ARA H 6 SENSITIZATION IN INTERIM FROM RECRUITMENT TO CHALLENGE).

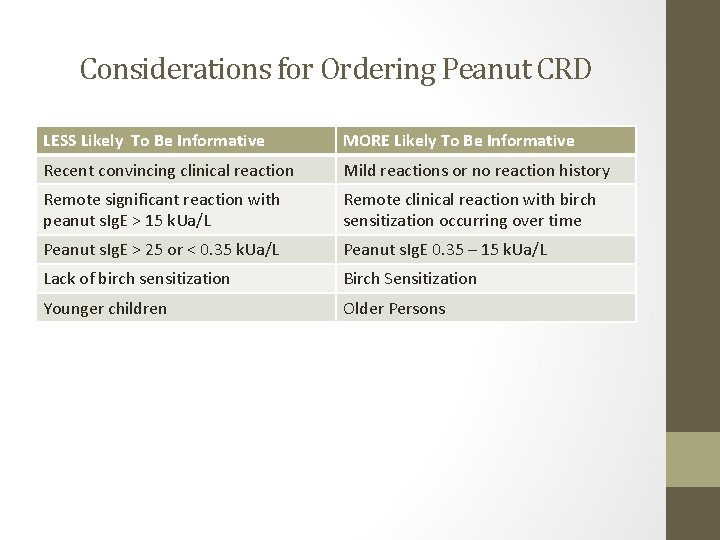
Considerations for Ordering Peanut CRD LESS Likely To Be Informative MORE Likely To Be Informative Recent convincing clinical reaction Mild reactions or no reaction history Remote significant reaction with peanut s. Ig. E > 15 k. Ua/L Remote clinical reaction with birch sensitization occurring over time Peanut s. Ig. E > 25 or < 0. 35 k. Ua/L Peanut s. Ig. E 0. 35 – 15 k. Ua/L Lack of birch sensitization Birch Sensitization Younger children Older Persons

CRD Advances in Egg Allergy • Egg allergy affects up to 2% of US children. • Previous estimates that egg allergy is outgrown by a majority of patients at 5 years of age is now considered wrong: • Savage et al 2007 in a chart review estimated 32% of patients still allergic at 16 years. • Sicherer et al 2014 used birth cohort to document that only half of infants diagnosed with egg allergy were tolerant at age 6 years. • Gal d 1 (ovomucoid) • HEAT STABLE • • Gal d 2 (ovalbumin) Gal d 3 (conalbumin) Gal d 4 (lysozyme) Gal d 5 (albumin)

CRD Advances in Egg Allergy • Alessandri et al in 2012 examined 68 Italian children for suspected egg allergy using CRD and egg-OFC. • 44/47 Gal d 1 (ovomucoid) negative tolerated boiled egg. • 20/21 Gal d 1 positive patients reacted to raw egg. • Lemon-Mule et al in 2008 challenged 117 children to heatedegg OFC and measured egg-CRD and skin test data in the reactive and tolerant groups. • 64/117 heated egg tolerant and placed on heated egg diet. • Heated egg diet was associated with the following over 1 year: • Decreased skin prick test wheal to egg white • Increased Ig. G 4 levels to ovalbumin and ovomucoid • No control group for heated egg diet; followed x 12 months only.

Baked Egg As a Form of Immunotherapy? • Leonard et al in 2012 divided regular egg allergic children based on their reactivity to baked egg or not. Control group avoided egg completely. • Of the Intention to Treat Group (79 children), 53% of these patients achieved REGULAR EGG TOLERANCE, by incorporating daily baked egg ingestion at 37. 8 months. • Patients on baked egg diet saw an decrease in size of skin test, and an increase in egg white specific Ig. G 4 levels.

CRD Advances in Milk Allergy • D’Urbano et al in 2010 performed skin prick tests, s. Ig. E, CRD, and OFC to 58 milk allergic children. • Ig. E reactivity to Bos d 8 much higher than other milk components. • + CRD to Bos d 8 outperformed s. Ig. E when using a clinical decision point of > 0. 6 ISU. • PPV 96% • NPV 78% • Bos d 4 (alactalbumin) • Bos d 5 (Blactoglobulin) • Bos d 7 (bovine Ig. G) • Bos d 8 (casein) • HEAT STABLE • Lactoferrin

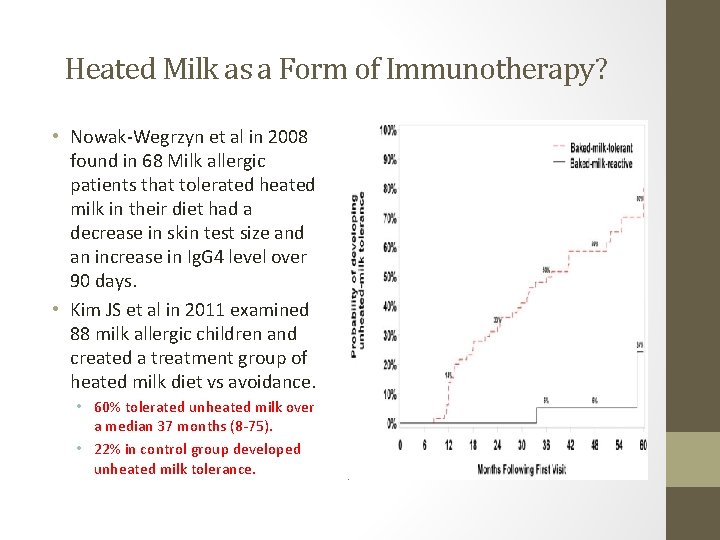
Heated Milk as a Form of Immunotherapy? • Nowak-Wegrzyn et al in 2008 found in 68 Milk allergic patients that tolerated heated milk in their diet had a decrease in skin test size and an increase in Ig. G 4 level over 90 days. • Kim JS et al in 2011 examined 88 milk allergic children and created a treatment group of heated milk diet vs avoidance. • 60% tolerated unheated milk over a median 37 months (8 -75). • 22% in control group developed unheated milk tolerance.

Du Toit et al. JACI. 2008: 122; 984 -91.

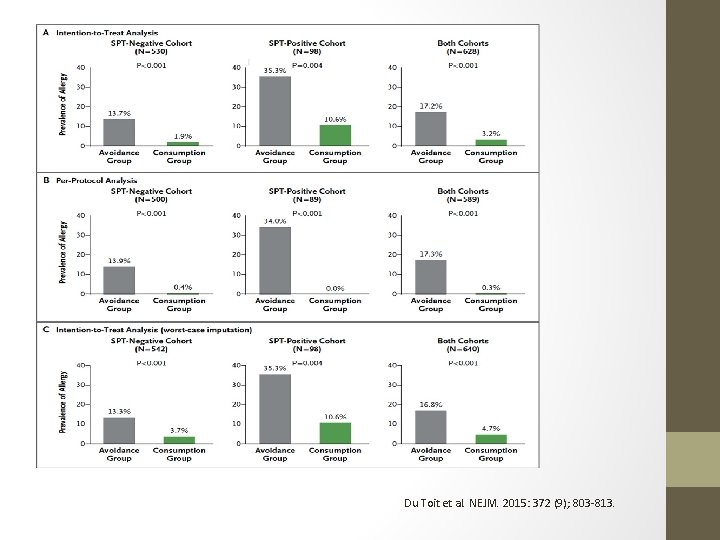
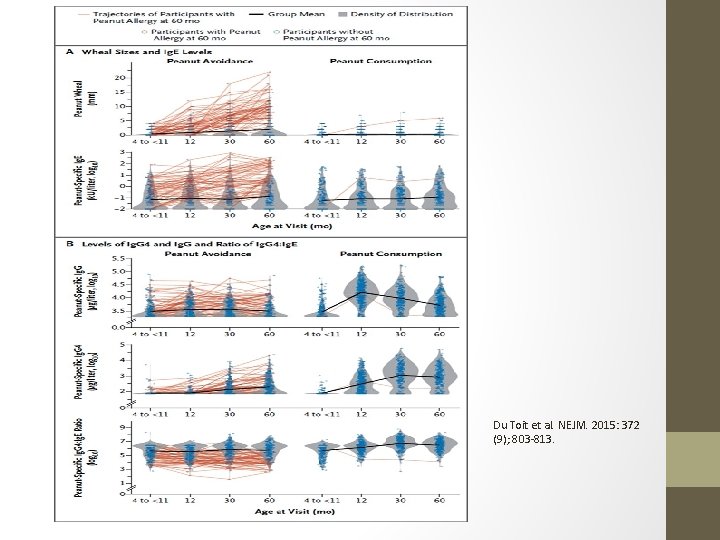
LEAP: Learning Early About Peanut Allergy • Du Toit et al reported this year in the NEJM a randomized trial for infants at risk for the development of peanut allergy. • 640 infants (4 -11 months) with egg allergy and eczema were skin tested for peanut. Skin test wheal of 1 -4 mm was considered positive. • Skin test results stratified patients into skin test negative and skin test positive groups. • These groups were then randomly assigned into a consumption cohort or an avoidance cohort. Both groups followed regularly with visits, skin testing, s. Ig. E to peanut, Ig. G 4 to peanut, up to 60 months. • Open food challenge or DBPCFC at end of 60 months assessed rates of peanut allergy development.

Du Toit et al. NEJM. 2015: 372 (9); 803 -813.

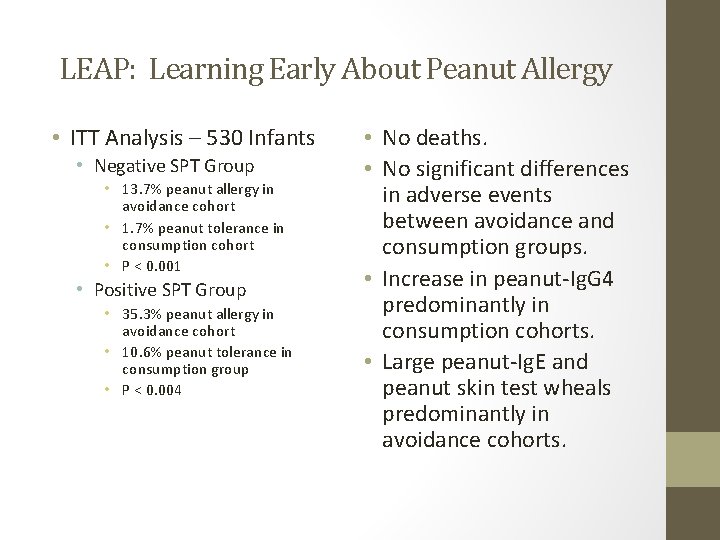
LEAP: Learning Early About Peanut Allergy • ITT Analysis – 530 Infants • Negative SPT Group • 13. 7% peanut allergy in avoidance cohort • 1. 7% peanut tolerance in consumption cohort • P < 0. 001 • Positive SPT Group • 35. 3% peanut allergy in avoidance cohort • 10. 6% peanut tolerance in consumption group • P < 0. 004 • No deaths. • No significant differences in adverse events between avoidance and consumption groups. • Increase in peanut-Ig. G 4 predominantly in consumption cohorts. • Large peanut-Ig. E and peanut skin test wheals predominantly in avoidance cohorts.

Du Toit et al. NEJM. 2015: 372 (9); 803 -813.

LEAP: Learning Early About Peanut Allergy • Pro’s: LEAP study documented an intervention that was “safe, tolerated, and highly efficacious. ” • 92% adherence rate in both intervention cohorts. • Patients with negative SPT at entry who consumed peanut had 86% risk reduction, compared to patients with positive SPT at entry who consumed peanut with a 70% risk reduction. • Con’s: • Lack of a placebo group. • 76 infants in the original recruitment, prior to randomization had SPT wheal > 4 mm. • Of 319 children randomly assigned to peanut consumption group, 7 had positive results of entry food challenge and 9 terminated their consumption based on development of allergic symptoms.

Response to LEAP Study • Consensus Communication on Early Peanut Introduction and the Prevention of Peanut Allergy in High Risk Infants. Pediatrics. August 2015: 136; 600. • Doctors “should recommend” peanut introduction in high risk infants in countries where peanut allergy is prevalent. • Infants with eczema and egg allergy under 6 months of age should have allergy referral to determine peanut status and whether they should be challenged using LEAP Protocol. • More formal NIAID/EAACI food introduction guidelines to follow. • Editorial Response JACI: In Practice. 2015: 3(5); 649 -51. • Protocol suggested in Consensus non-specific and open ended. • Leaves up to primary care doctors to define “high risk. ” • Did not discuss implications of adherence with target dose. • Did not address implications of widespread testing and financial burden of this practice.

Management of FA • Avoidance! • There is no cure. • Education of patient, family, friends, school, social contacts etc. • Training of patient and caregivers on correct Epi Pen or Auvi Q technique. • Re-assessment of immunologic reactivity. • Tolerated an accidental ingestion after years of consisted avoidance? • Twenty percent likelihood of outgrowing peanut allergy.

Immunotherapy (IT) for FA • Need is clear as prevalence of FA worldwide is growing. • Avoidance is a imperfect management. • Accidental ingestion is common (20%). • Gupta et al in 2011 reports 38. 7% of children diagnosed with FA have experienced anaphylaxis that includes hypotension and/or dyspnea. • Terms for defining goals of Immunotherapy FA Trials. • Desensitization: tolerating escalating doses of food allergen during a trial. • Sustained unresponsiveness: tolerated a controlled food challenge 2 -6 weeks after desensitization was halted and food allergen was avoided. • Immunological Tolerance: “Growing Out of It. ”

Forms of IT for FA • OIT: Oral Immunotherapy • SLIT: Sublingual immunotherapy • Recombinant Protein Therapy • Epicutaneous Immunotherapy • OIT trials are most numerous and better characterized compared to other forms. • More patients reach active desensitization but with a higher rate of adverse events.

OIT Schema • Initial Dose Escalation • Build-up phase • Maintenance Phase • Avoidance Phase • Assessment of response by food challenge

Peanut OIT • Anagnostou et al in 2014 released results of STOP II trial for peanut allergic children undergoing OIT. 39/49 patients in the treatment completed 6 months of maintenance 800 mg peanut dosing; 24/39 passed a 1400 mg oral challenge. • Study did not assess for sustained unresponsiveness. • Syed et al in 2014 compared antigen induced Treg characteristics of 23 patients on peanut OIT vs peanut allergic controls. • After 2 years of maintenance peanut OIT, therapy was stopped and 35% were peanut tolerant at 3 months, and 13% at 6 months. • Vickery et al in 2014 documented 24/39 patients that completed 4 years of peanut OIT • 50% passed peanut OFC at 1 month after peanut abstinence.

OIT for Other Food Allergens • Escudero et al in 2015 completed a RCT for egg allergic children to examine effectiveness of egg OIT over 90 days. • 11/30 of egg-OIT (37%) tolerated egg OFC 1 month after discontinuing egg OIT; for this group, they were still consuming all forms of egg ad lib with 36 months of documented follow up. • Burks et al in 2012 randomized 40/55 children into an Egg OIT group with 22 month treatment phase. • 11/40 (28%) passed egg OFC two months after egg OIT discontinuation; these children had documented sustained unresponsiveness at 30 and 36 months of follow up. • Meglio et al published follow up study of 21 milk allergic children 4 years after they were desensitized. • 15/21 (71%) achieved desensitization over 6 months in 2004 • 14/21 (66%) had sustained unresponsiveness at 4 year follow up

Novel Approaches • • • Co-administer Peanut OIT with probiotic OIT for multiple foods (Stanford Program) Omalizumab usage for rush oral desensitization DARPins Epicutaneous patch therapy Rectal delivered peanut vaccine of Ara h 1/2/3 with E. coli as adjuvant

Take Home Points • s. Ig. E testing for random foods indicates sensitization only and may lead to unnecessary anxiety and elimination. • CRD can improve FA diagnosis and management. • Ara h 1, 2, 3, 9 + for peanut associated with immediate HS • Gal d 1 or Ovomucoid + associated with immediate HS • Bos d 8 or Casein + associated with immediate HS • Children with confirmed unheated milk and/or regular egg allergy are likely to gain tolerance more quickly if they tolerate heated milk and/or baked egg, and eat foods containing these regularly. • Early peanut introduction for selected high risk patients likely can protect against development of peanut allergy. • OIT for FA is NOT READY FOR CLINICAL USE.

It’s a Long Way to the Top if You Want to Rock ‘N’ Roll!!!

References • Sicherer et al. Advances in diagnosing peanut allergy. JACI In Practice. 2013: 1 (1); 1 -13. • O’Keefe et al. Diagnosis and management of food allergies: new and emerging options: a systematic review. Journal of Asthma and Allergy. 2014: 7; 141 -164. • Lack, G. Update on risk factors for food allergy. JACI. 2012: 129 (5); 1187 -97. • Kattan, JD et al. Allergen component testing for food allergy: ready for prime time? Curr Allergy asthma Rep. 2013: 13; 58 -63. • Chhiba, KD et al. New development in immunotherapies for food allergy. Immunotherapy. 2015: 7(8); 913 -22. • Fleischer et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high risk infants. Pediatrics. 2015: 136; 600 -04. • Bartnikas, LM et al. Epicutaneous sensitization result in Ig. E-dependent intestinal mast cell expansion and food-induced anaphylaxis. JACI. 2013; 131 (2); 451 -60. • Noti et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. JACI. 2014: 133 (5); 1390 -99. • Du Toit, G et al. Randomized Trial of Peanut Consumption in Infants arisk for peanut allergy. NEJM. 2015: 372 (9); 803 -13. • Valenta, R et al. Food allergies: the basics. Gastroenterology. 2015: 148; 11201131.

References • Asarnoj, A et al. Peanut component Ara h 8 sensitization and tolerance to peanut. JACI. 2012; 130(2): 468 -472. • Nicolaou, N et al. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component resolved diagnostics. JACI. 2010; 125(1); 191 -7. • Caubet, J et al. Significance of ovomucoid and ovalumin specific Ig. E/Ig. G 4 ratios in egg allergy. JACI. 2012; 129(3): 739 -747. • Umasunthar, T et al. Incidence of fatal food anaphylaxis in people with food allergy. Clin Exp Allergy. 2013; 43: 1333 -41. • Xu et al. Anaphylaxis related deaths in Ontario: a retrospective review of cases from 19862011. Allergy, Asthma, and Clin Imm. 2014; 10(38): 1 -8. • Leonard, SA et al. Dietary baked egg accelerates resolution of egg allergy in children. JACI. 2012; 130(2): 473 -480. • Lemon-Mule, H et al. Immunologic changes in children with egg allergy ingesting extensively heated egg. JACI. 2008; 122 (5): 977 -83. • D’Urbano LE et al. Performance of component based micro-array in the diagnosis of cow’s milk and hen’s egg allergy. Clin Exp Allergy. 2010; 40: 1561 -1570. • Escudero C et al. Early Sustained Unresponsiveness after short course egg OIT: a RCT study in egg allergic children. Clin Exp Allergy. Ahead of publication. • Alessandri C et al. Ovomucoid specific Ig. E detected by microarray predicted tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2011; 42: 441 -450.

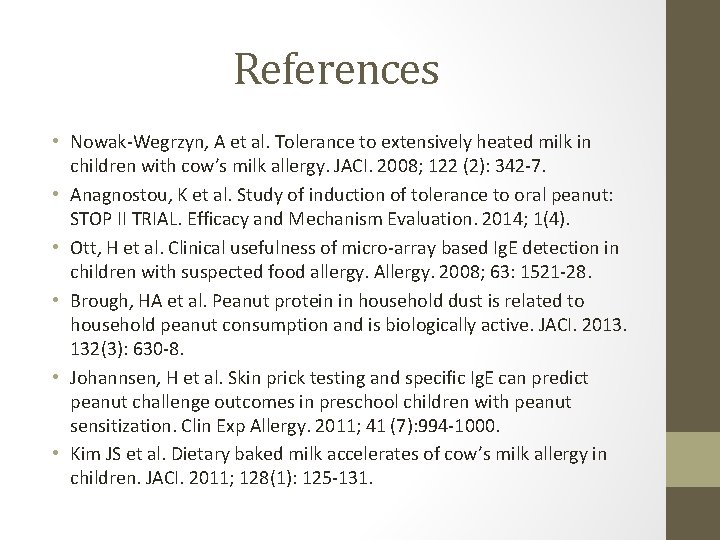
References • Nowak-Wegrzyn, A et al. Tolerance to extensively heated milk in children with cow’s milk allergy. JACI. 2008; 122 (2): 342 -7. • Anagnostou, K et al. Study of induction of tolerance to oral peanut: STOP II TRIAL. Efficacy and Mechanism Evaluation. 2014; 1(4). • Ott, H et al. Clinical usefulness of micro-array based Ig. E detection in children with suspected food allergy. Allergy. 2008; 63: 1521 -28. • Brough, HA et al. Peanut protein in household dust is related to household peanut consumption and is biologically active. JACI. 2013. 132(3): 630 -8. • Johannsen, H et al. Skin prick testing and specific Ig. E can predict peanut challenge outcomes in preschool children with peanut sensitization. Clin Exp Allergy. 2011; 41 (7): 994 -1000. • Kim JS et al. Dietary baked milk accelerates of cow’s milk allergy in children. JACI. 2011; 128(1): 125 -131.

Questions?
 Allergies and arthritis
Allergies and arthritis Arthritis and food allergies
Arthritis and food allergies Actual diagnosis
Actual diagnosis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Second phase of nursing process
Second phase of nursing process Types of nursing diagnoses
Types of nursing diagnoses Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Payroll in tally
Payroll in tally Short term loans and advances
Short term loans and advances Lurbinectedin posologie
Lurbinectedin posologie Allergic rhinitis icd 10
Allergic rhinitis icd 10 Beta glucan allergies
Beta glucan allergies Allergies croisées tableau
Allergies croisées tableau Chapter 17 section 2 the axis advances
Chapter 17 section 2 the axis advances Advances in technology during wwii
Advances in technology during wwii Lesson 9.1 intellectual advances in the first year
Lesson 9.1 intellectual advances in the first year Advances in real time rendering
Advances in real time rendering Coherent scattering
Coherent scattering Opto-electronic advances
Opto-electronic advances Recent advances in ceramics
Recent advances in ceramics Dcco
Dcco Advances in memory technology
Advances in memory technology Unit 2 food food food
Unit 2 food food food Sequence of food chain
Sequence of food chain Type 1 diabetes in adults diagnosis and management
Type 1 diabetes in adults diagnosis and management Greg and the ballistic missile
Greg and the ballistic missile Food web and food chain difference
Food web and food chain difference Create a food chain
Create a food chain How does the food chain go
How does the food chain go Levels of nourishment in a food chain
Levels of nourishment in a food chain Food chain and food web
Food chain and food web Food chains, food webs and ecological pyramids
Food chains, food webs and ecological pyramids Food webs and energy pyramids
Food webs and energy pyramids Food webs and energy pyramids answer key
Food webs and energy pyramids answer key Chaparral food chain
Chaparral food chain Role play on healthy food and junk food
Role play on healthy food and junk food Of junk food
Of junk food Desert food chain
Desert food chain Name junk food
Name junk food Introduction on fast food
Introduction on fast food Gregory kesden
Gregory kesden Schaffner esd gun
Schaffner esd gun Gregory reznik
Gregory reznik Where are liver flukes found
Where are liver flukes found Greg camm
Greg camm Greg gordon md
Greg gordon md Il clown killer
Il clown killer Greg provenzano
Greg provenzano Kentucky physicians health foundation
Kentucky physicians health foundation Greg tielke
Greg tielke Greg ashman
Greg ashman Greg dudkin ppl
Greg dudkin ppl Gstitt
Gstitt Greg stitt
Greg stitt Onderwereld opstel vrae
Onderwereld opstel vrae Greg kuperberg
Greg kuperberg














































































