Follow up of PPGLSphaeochromocytomas and paragangliomas Agenda follow


















- Slides: 38

Follow up of PPGLS(phaeochromocytomas and paragangliomas Agenda follow up importance recurrence rate recurrence risk factors follow up guideline(Biochemical, imaging , duration)

Ø Definition: Ø PPGL are rare neuroendocrine tumors that arise from neural crest-derived cells of the sympathetic and parasympathetic nervous systems. Ø Phaeochromocytoma and most thoraco-abdominopelvic paragangliom are of sympathetic origin. Ø Most parasympathetic paragangliomas are found in the head or neck and do not usually produce catecholamines

New tumoral events Ø New tumoral events are recurrences, defined as the reappearance of disease after complete tumor eradication, or new tumors. Ø Recurrences may be local (at the site of the primary tumor) or metastatic. Local recurrence may arise from microscopic cells of the primary tumour that escaped therapeutic intervention and later become detectable at the original site. However, persistence and recurrence are usually defined according to biochemical or imaging tests after the operation: if test results are positive, then the case shows persistence and if negative, recurrence Ø New tumours are phaeochromocytomas or paragangliomas that arise in the contralateral adrenal or in a previously unaffected paraganglion

Follow up importance Ø Following resection of the primary tumor, most patients are free of tumor , but tumor-free patients are at a risk of long-term new tumoral events. Ø Most of the recurrence are metastatic Ø The total duration of follow-up required remains unclear, however, as new events may occur decades after initial surgery Ø Consequently, long-term follow-up is recommended for patients who have undergone surgery for PH/PG

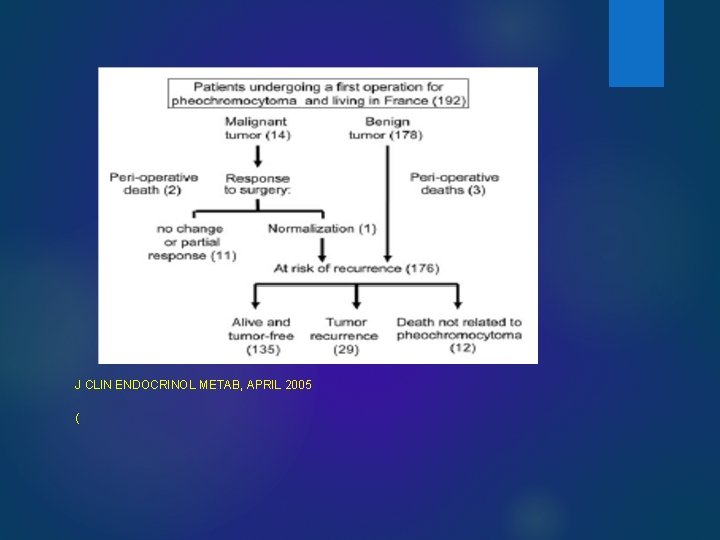
J CLIN ENDOCRINOL METAB, APRIL 2005 (

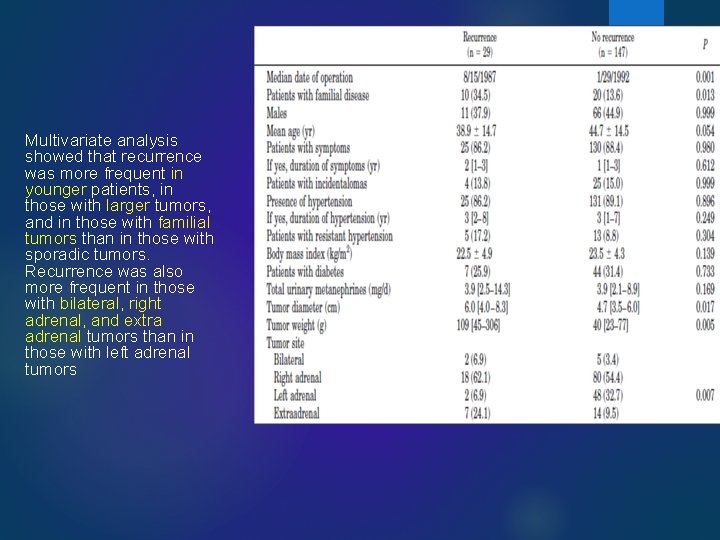
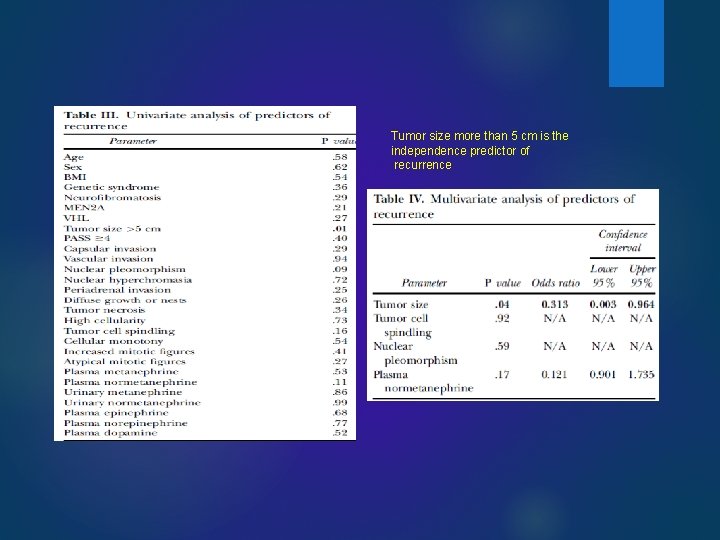
Multivariate analysis showed that recurrence was more frequent in younger patients, in those with larger tumors, and in those with familial tumors than in those with sporadic tumors. Recurrence was also more frequent in those with bilateral, right adrenal, and extra adrenal tumors than in those with left adrenal tumors

HORM METAB RES 2012 LONG-TERM POSTOPERATIVE FOLLOW-UP IN PATIENTS WITH APPARENTLY BENIGN PHEOCHROMOCYTOMA AND PARAGANGLIOMA

Ø more than 15 % of patients undergoing surgery for PH/PG develop new tumors or recurrences. A 15 % probability of new events implies a need for long-term follow-up. Ø most recurrences are metastatic Ø there are no robust prognostic indices of tumor recurrence in patients with PH/PG other than the higher probability of new events in patients with inherited tumors and probably, in patients with large tumors or in patients with primary tumors of extraadrenal location.


Follow up in this study Ø sporadic, single PH of 5 cm or less in diameter, ) follow-up, initially 1 year after surgery and then every other year for the rest of their lives. However this follow-up period can be reduced to yearly, if it is more simple and convenient for patients and physicians. Ø Patients with documented inherited diseases and those undergoing successful surgery for a PH of more than 5 cm in diameter, a PGL or multiple tumors should undergo clinical and biochemical follow-up 6 months after surgery and then every year for the rest of their lives.

Ø Earlier biochemical testing should be proposed if indicated by signs or symptoms of tumor recurrence, such as a rise in BP, weight loss, adrenergic symptoms, or compressive pain. Ø Patients with syndromic disease should also be offered screening tests for associated tumors. Ø nonfunctional thoraco-abdominal primary tumors, biochemical follow up is probably not helpful and should be replaced by or complemented with imaging tests, preferably magnetic resonance imaging of the abdomen and chest to prevent repeated radiation exposure. Ø We recommend the determination of plasma or urinary metanephrines (normetanephrine and metanephrine)for follow up

Assessment of recurrences Ø Patients testing positive for metanephrines should undergo imaging tests for tumor diagnosis or staging. The combinationof thoraco-abdomino-pelvic imaging by CT-scan or magnetic resonance imaging plus 18 F-fl uorodeoxyglucose positron emission tomography is probably the best option for distinguishing new tumors from metastases and for tumor staging. Ø In cases of metastasis, 123 I-MIBG scintigraphy should also be performed to determine whether the patient should be given palliative 131 I-MIBG treatment.

Surgury; 2014 Predictors of recurrence in pheochromocytoma Ø In this retrospective study(2000 -2013) we reviewed all patients who underwent adrenalectomy for pheochromocytoma during a 14 -year period at a single institution(135 patients). Ø With a median follow up of 85 months (range 8– 172 months), 8 patients (6%) developed recurrent disease. Recurrence was diagnosed by increased plasma or urine metanephrines and positive cross sectional imaging (such as computed tomography, magnetic resonance imaging, or metaiodobenzylguanidine study) in 6 patients, and by positive imaging and normal biochemical levels in 2 patients Ø The optimal protocol, in our opinion, is measurement of metanephrines at 1, 6, and 12 months postoperatively and then annually thereafter. Because up to 25% of our patients with recurrence had normal laboratory results with recurrence of disease, we also recommend annual cross-sectional imaging, preferably computed tomography, to look for recurrence.

Tumor size more than 5 cm is the independence predictor of recurrence

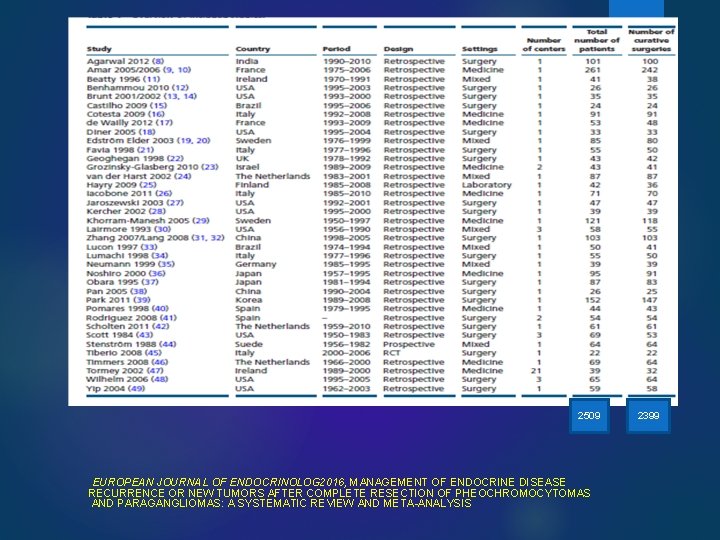
2509 EUROPEAN JOURNAL OF ENDOCRINOLOG 2016, MANAGEMENT OF ENDOCRINE DISEASE RECURRENCE OR NEW TUMORS AFTER COMPLETE RESECTION OF PHEOCHROMOCYTOMAS AND PARAGANGLIOMAS: A SYSTEMATIC REVIEW AND META-ANALYSIS 2399

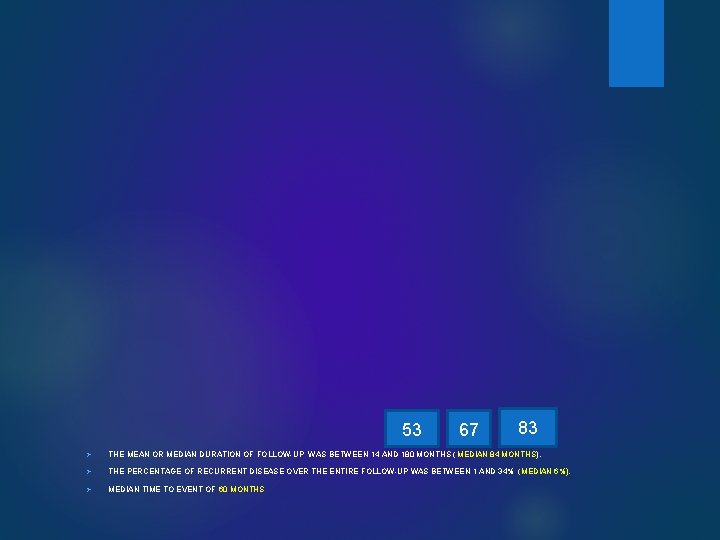
53 67 83 Ø THE MEAN OR MEDIAN DURATION OF FOLLOW-UP WAS BETWEEN 14 AND 180 MONTHS ( MEDIAN 84 MONTHS). Ø THE PERCENTAGE OF RECURRENT DISEASE OVER THE ENTIRE FOLLOW-UP WAS BETWEEN 1 AND 34% (MEDIAN 6%). Ø MEDIAN TIME TO EVENT OF 60 MONTHS

ASSUMING A STEADY INCIDENCE OVER TIME, THIS CONVERTS INTO A 5 -YEAR CUMULATIVE INCIDENCE OF 4. 7% (95% CI: 3. 4, 6. 1; ), DISTRIBUTED AS FOLLOWS: NEW TUMORS 22%, LOCAL RECURRENCES 23%, AND METASTATIC RECURRENCES 55%.

Ø However, the risk of recurrence remains nonnegligible, approximately 5% per 5 years of followup, and late events are possible, up to 15 years after surgery in the included studies Ø Paragangliomas and familial disease are the two main independent risk factors of recurrent disease identified by several studies and by meta-regression analyses across studies. Ø The association between the size of the primary tumor and the risk of recurrence after complete removal was weaker.

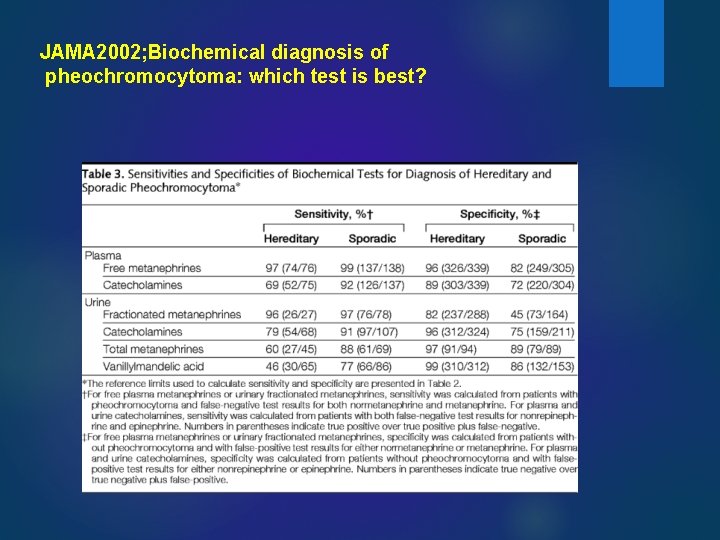
JAMA 2002; Biochemical diagnosis of pheochromocytoma: which test is best?

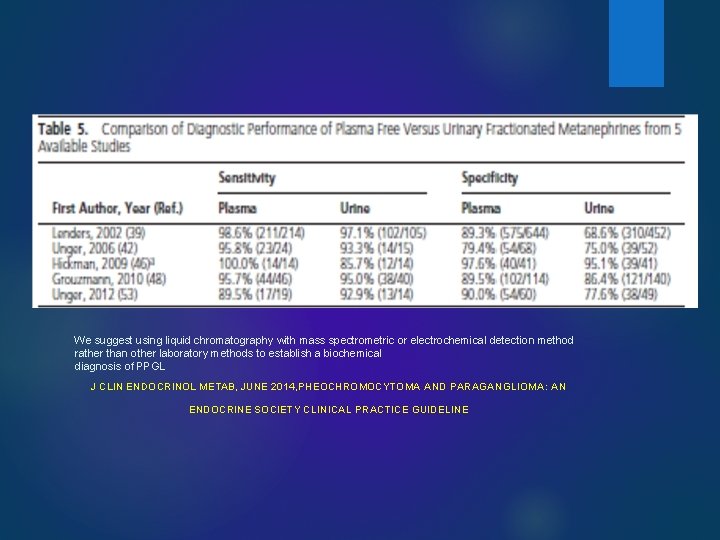
We suggest using liquid chromatography with mass spectrometric or electrochemical detection method rather than other laboratory methods to establish a biochemical diagnosis of PPGL J CLIN ENDOCRINOL METAB, JUNE 2014, PHEOCHROMOCYTOMA AND PARAGANGLIOMA: AN ENDOCRINE SOCIETY CLINICAL PRACTICE GUIDELINE

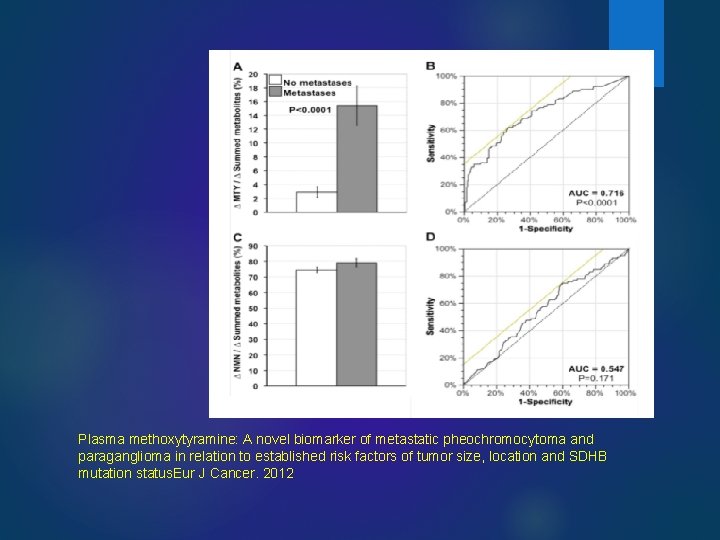
Plasma methoxytyramine: A novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumor size, location and SDHB mutation status. Eur J Cancer. 2012

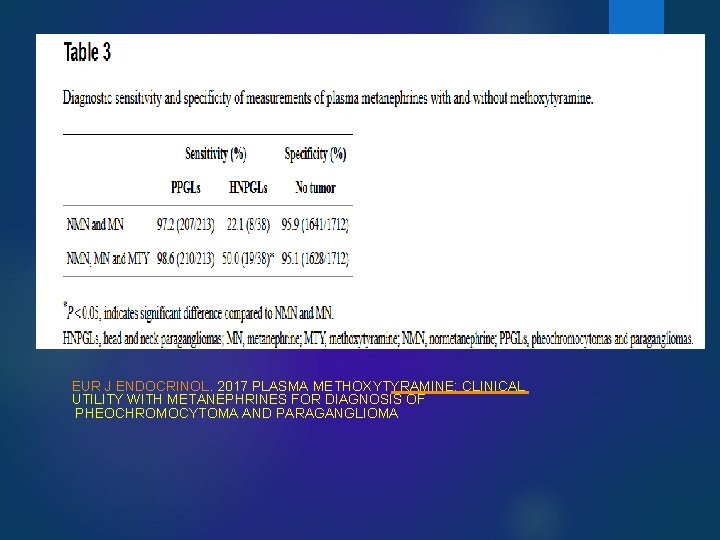
EUR J ENDOCRINOL. 2017 PLASMA METHOXYTYRAMINE: CLINICAL UTILITY WITH METANEPHRINES FOR DIAGNOSIS OF PHEOCHROMOCYTOMA AND PARAGANGLIOMA

Comparison of metaiodobenzylguanidine scintigraphy with positron emission tomography in the diagnostic work-up of pheochromocytoma and paraganglioma: a systematic review THE QUARTERLY JOURNAL OF NUCLEAR MEDICINE AND MOLECULAR IMAGIN 2013 Twenty-eight studies comprising 852 patients who underwent both MIBG scintigraphy and PET or PET/CT with different radiopharmaceuticals were included and discussed. Three studies evaluated carbon-11 hydroxyephedrin as PET radiopharmaceutical, 9 studies fluorin 18 dopamin, 8 studies with fluorin 18 dihydroxyphenylalanine, 12 studies fluorin 18 fluorodeoxyglocose, 5 gallium 68 somatostatin analogue Despite the heterogeneity of the studies included in the analysis, it can be concluded that the diagnostic performance of PET with various agents is clearly superior to that of MIBG scintigraphy in patients with Pheo/PGL, mainly for familial, extra- adrenal and metastatic diseases;

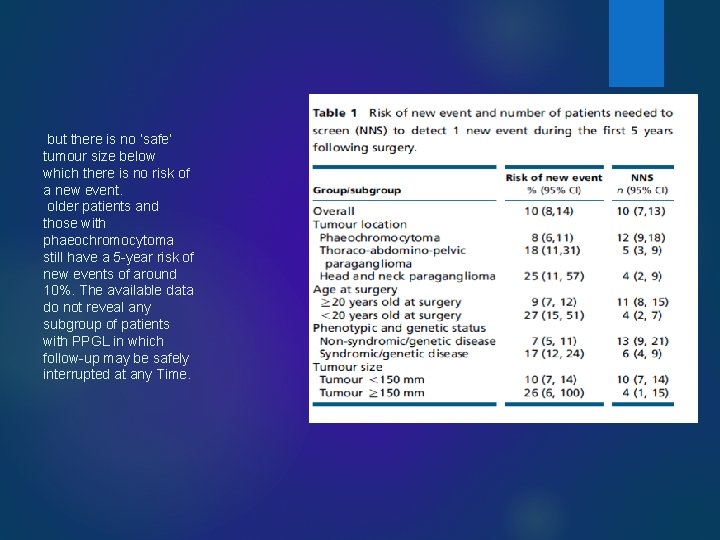
European Journal of Endocrinology; 2016 European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma We performed a systematic review of the literature and analysed the European Network for the Study of Adrenal Tumours (ENS@T) database Ens@t: Median follow-up was 54 months. The risk of new events in the whole population was 10% (95% CI 8, 14) over the first 5 years of follow-up (new tumours 42%, local recurrences 13% and metastatic recurrences 45%). .

but there is no ’safe’ tumour size below which there is no risk of a new event. older patients and those with phaeochromocytoma still have a 5 -year risk of new events of around 10%. The available data do not reveal any subgroup of patients with PPGL in which follow-up may be safely interrupted at any Time.

Diagnosis of malignancy Ø we recommend defining malignancy in PPGL as the presence of metastasis in lymph nodes or other distant sites. Ø Rationale. No single clinical, biochemical or histological feature can distinguish malignant from benign PPGL. Ø The multi-parameter Phaeochromocytoma of the Adrenal Gland Scaled Score is not reliable and the reproducibility of the recent Grading system for Adrenal Phaeochromocytoma and Paraganglioma requires further validation. Ø A metastasis is therefore defined as the presence of chromaffin tissue in a location where normally no chromaffin tissue is expected Ø Remark 1. The proposed definition of malignancy relies on overt metastatic spread: it is specific but not sensitive Ø Remark 2. This definition applies to metastatic primary tumours and to metastatic recurrences.

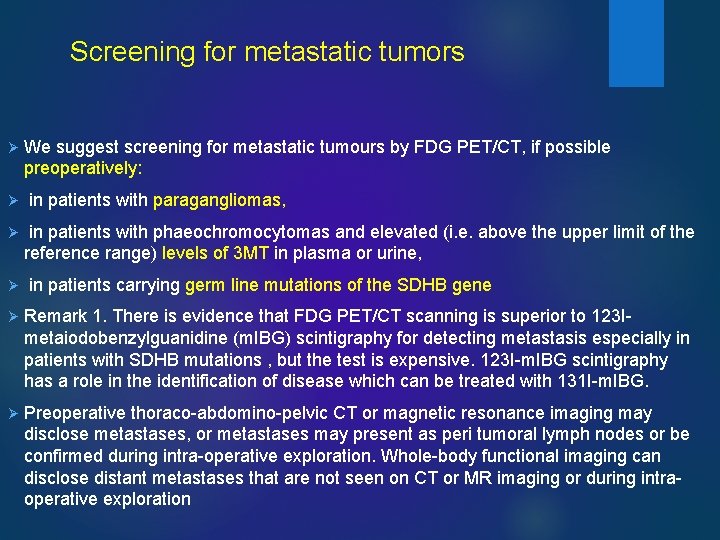
Screening for metastatic tumors Ø Ø We suggest screening for metastatic tumours by FDG PET/CT, if possible preoperatively: in patients with paragangliomas, in patients with phaeochromocytomas and elevated (i. e. above the upper limit of the reference range) levels of 3 MT in plasma or urine, in patients carrying germ line mutations of the SDHB gene Ø Remark 1. There is evidence that FDG PET/CT scanning is superior to 123 Imetaiodobenzylguanidine (m. IBG) scintigraphy for detecting metastasis especially in patients with SDHB mutations , but the test is expensive. 123 I-m. IBG scintigraphy has a role in the identification of disease which can be treated with 131 I-m. IBG. Ø Preoperative thoraco-abdomino-pelvic CT or magnetic resonance imaging may disclose metastases, or metastases may present as peri tumoral lymph nodes or be confirmed during intra-operative exploration. Whole-body functional imaging can disclose distant metastases that are not seen on CT or MR imaging or during intraoperative exploration

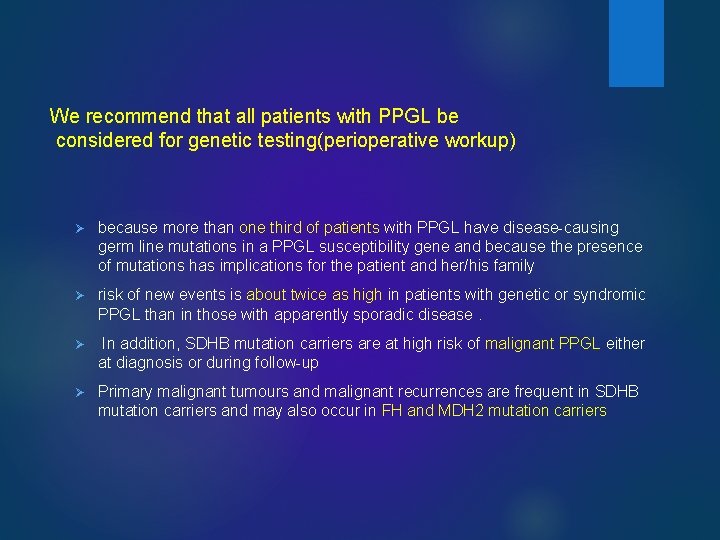
We recommend that all patients with PPGL be considered for genetic testing(perioperative workup) Ø because more than one third of patients with PPGL have disease-causing germ line mutations in a PPGL susceptibility gene and because the presence of mutations has implications for the patient and her/his family Ø risk of new events is about twice as high in patients with genetic or syndromic PPGL than in those with apparently sporadic disease. Ø In addition, SDHB mutation carriers are at high risk of malignant PPGL either at diagnosis or during follow-up Ø Primary malignant tumours and malignant recurrences are frequent in SDHB mutation carriers and may also occur in FH and MDH 2 mutation carriers

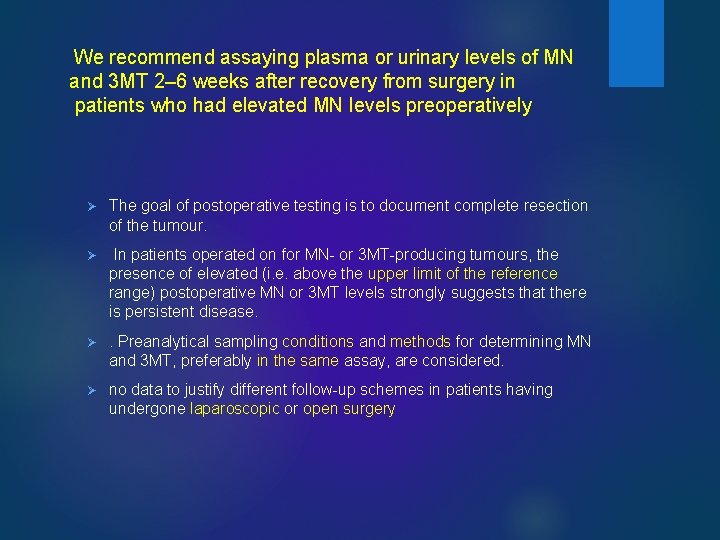
We recommend assaying plasma or urinary levels of MN and 3 MT 2– 6 weeks after recovery from surgery in patients who had elevated MN levels preoperatively Ø The goal of postoperative testing is to document complete resection of the tumour. Ø In patients operated on for MN- or 3 MT-producing tumours, the presence of elevated (i. e. above the upper limit of the reference range) postoperative MN or 3 MT levels strongly suggests that there is persistent disease. Ø . Preanalytical sampling conditions and methods for determining MN and 3 MT, preferably in the same assay, are considered. Ø no data to justify different follow-up schemes in patients having undergone laparoscopic or open surgery

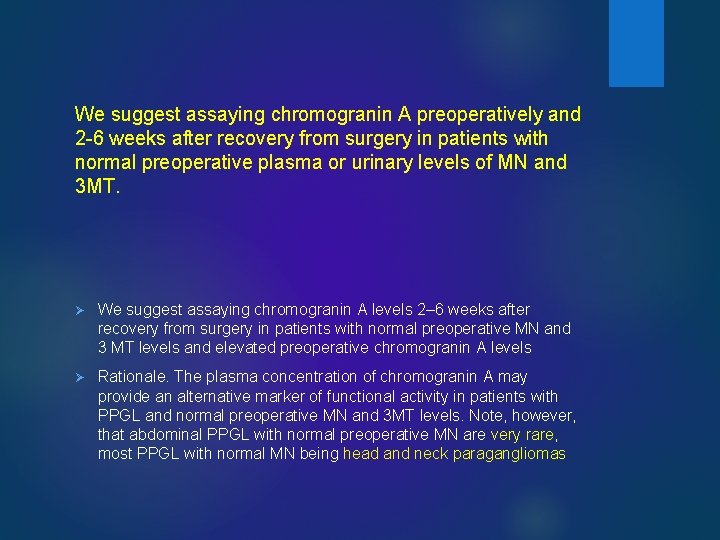
We suggest assaying chromogranin A preoperatively and 2 -6 weeks after recovery from surgery in patients with normal preoperative plasma or urinary levels of MN and 3 MT. Ø We suggest assaying chromogranin A levels 2– 6 weeks after recovery from surgery in patients with normal preoperative MN and 3 MT levels and elevated preoperative chromogranin A levels Ø Rationale. The plasma concentration of chromogranin A may provide an alternative marker of functional activity in patients with PPGL and normal preoperative MN and 3 MT levels. Note, however, that abdominal PPGL with normal preoperative MN are very rare, most PPGL with normal MN being head and neck paragangliomas

Imaging tests Ø We recommend performing an imaging test 3 months after presumably complete surgery in patients operated on for PPGL who had elevated MN or 3 MT postoperatively, in patients in whom MN and 3 MT were normal preoperatively and in patients in whom MN and 3 MT were not measured preoperatively Ø Rationale: Persistently elevated concentrations of MN and/or 3 MT postoperatively suggest incomplete tumour resection, and imaging can be used for assessing persistent residual tumour mass. Ø Patients operated on for a biochemically inactive tumour can subsequently develop new biochemically active tumours, and this is particularly true of patients with hereditary disease

Duration of follow-up We suggest follow-up for at least 10 years in all patients operated on for a PPGL to monitor local or metastatic recurrences or new tumours. High-risk patients (young patients and those with a genetic disease, a large tumour and/or a paraganglioma) should be offered lifelong annual follow-up.

Monitoring methods Ø We recommend assaying plasma or urinary MN and 3 MT every year to screen for local or metastatic recurrence or new tumours. Ø Rationale. There is evidence that determination of plasma free or urinary fractionated MN is more sensitive than assaying catecholamine and vanillylmandelic acid for detection of PPGL

Monitoring methods Ø There is no evidence that MN and 3 MT determinations allow earlier detection of recurrence or new tumours than imaging tests, but MN determination is widely available, harmless and cheap, and pre-analytical conditions are relatively simple. Ø We suggest assaying plasma chromogranin A every year to screen in patients operated on for MN- and 3 MTnegative and chromogranin Apositive PPGL. Ø SDHB mutation carriers with PPGL may exhibit normal MN and elevated chromogranin A levels in plasma

Is adrenergic symptoms important in follow up? Ø Determining MN and 3 MT only in patients who present with hypertension or adrenergic symptoms during follow-up is not sound because these signs and symptoms have poor sensitivity and specificity for predicting recurrence or new tumours

Imaging test for long term follow up Ø We suggest performing imaging tests every 1– 2 years in patients with biochemically inactive PPGL to screen for local or metastatic recurrences or new tumors Ø Imaging tests are the only option in cases where there are no reliable biochemical markers Ø It is not known which imaging technique is best. To minimise radiation exposure, one option is to perform thoracoabdominopelvic MRi. Ø This applies mostly to patients with head and neck paraganglioma

Specific conditions Ø Pregnancy. Although pregnancy and delivery can trigger acute cardiovascular events in patients with PPGL , it is not known whether pregnancy alters tumour growth or the metabolism of catecholamines in these conditions. Tests intended to detect new events should be proposed to women who have been operated on for a PPGL and who plan to become pregnant. The above recommendations for long-term follow-up apply to patients of childbearing age. Ø Elderly and frail patients. Considering the absolute risks, screening for new events does not appear necessary for patients who have been operated on for PPGL and have a reduced life expectancy.

plasma or urinary levels of MN and 3 MT 2– 6 weeks after recovery from surgery chromogranin A preoperatively and 2 -6 weeks after recovery from surgery in patients with normal preoperative plasma or urinary levels of MN and 3 MT. We recommend performing an imaging test 3 months after presumably complete surgery in patients operated on for PPGL who had elevated MN or 3 MT postoperatively, in patients in whom MN and 3 MT were normal preoperatively and in patients in whom MN and 3 MT were not measured preoperatively We suggest follow-up for at least 10 years in all patients High-risk patients (young patients and those with a genetic disease, a large tumour and/or a paraganglioma) should be offered lifelong annual follow-up We recommend assaying plasma or urinary MN and 3 MT every year to screen for local or metastatic recurrence or new tumours We suggest performing imaging tests every 1– 2 years in patients with biochemically inactive PPGL to screen