Focus on Genetics and Gastrointestinal Cancers Jamin Morrison




















- Slides: 50


Focus on Genetics and Gastrointestinal Cancers Jamin Morrison, MD Hematology / Medical Oncology

Overview • • Cancer “genetics” in the news Gastrointestinal cancer genetic syndromes Lynch Syndrome & recommendations Translating cancer genetics to the bedside

https: //www. nytimes. com/2017/02/28/well/live/colon-andrectal-cancers-rising-in-youngpeople. html? action=click&content. Collection=Well&module=R elated. Coverage®ion=Marginalia&pgtype=article

https: //www. cnn. com/2017/02/28/health/colon-cancerrectal-cancer-risk-young-people-study/index. html

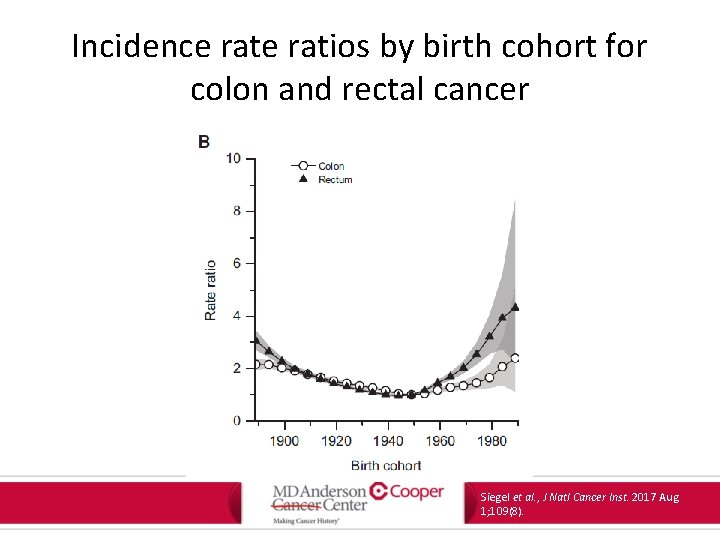
Incidence ratios by birth cohort for colon and rectal cancer Siegel et al. , J Natl Cancer Inst. 2017 Aug 1; 109(8).

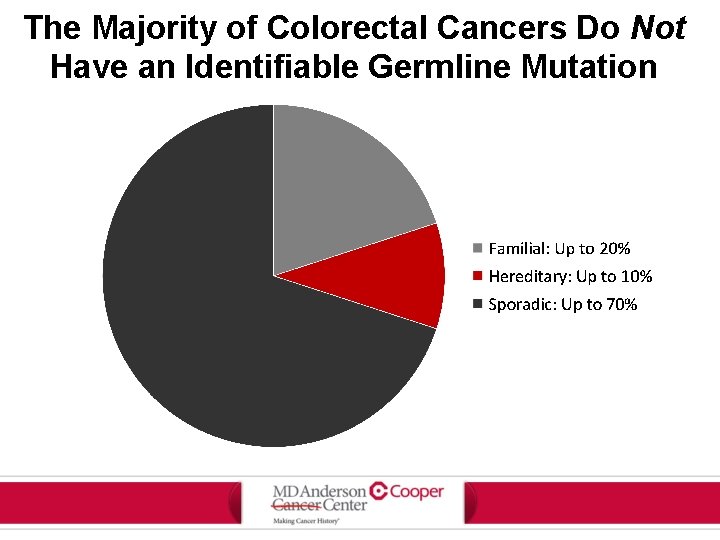
The Majority of Colorectal Cancers Do Not Have an Identifiable Germline Mutation

GI Cancer syndromes • • Cowden syndrome MUTYH-associated polyposis Hereditary pancreatic cancer Peutz-Jeghers syndrome Familial adenomatous polyposis (FAP) Serrated polyopsis syndrome Hereditary gastric cancer Lynch syndrome

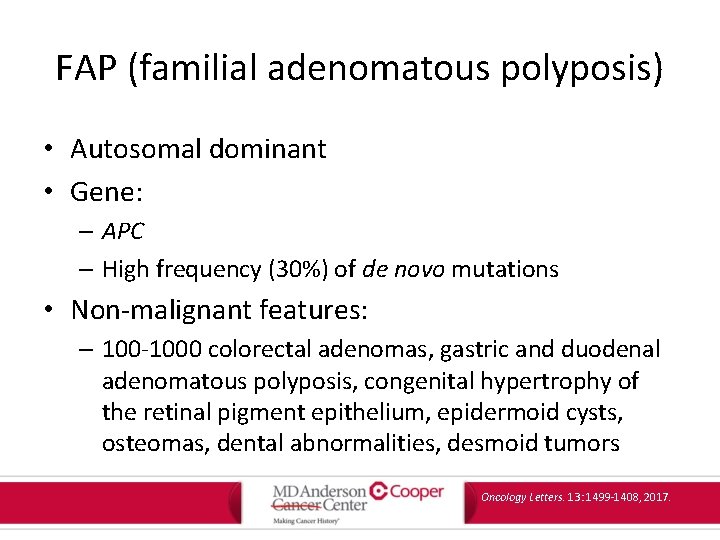
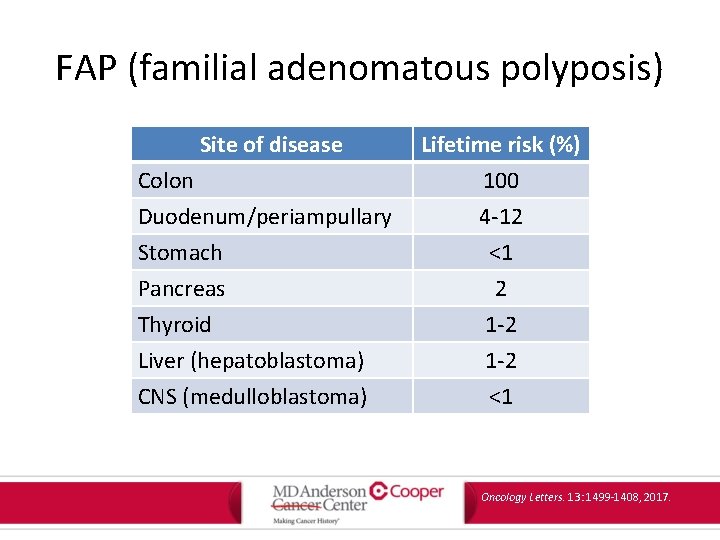
FAP (familial adenomatous polyposis) • Autosomal dominant • Gene: – APC – High frequency (30%) of de novo mutations • Non-malignant features: – 100 -1000 colorectal adenomas, gastric and duodenal adenomatous polyposis, congenital hypertrophy of the retinal pigment epithelium, epidermoid cysts, osteomas, dental abnormalities, desmoid tumors Oncology Letters. 13: 1499 -1408, 2017.

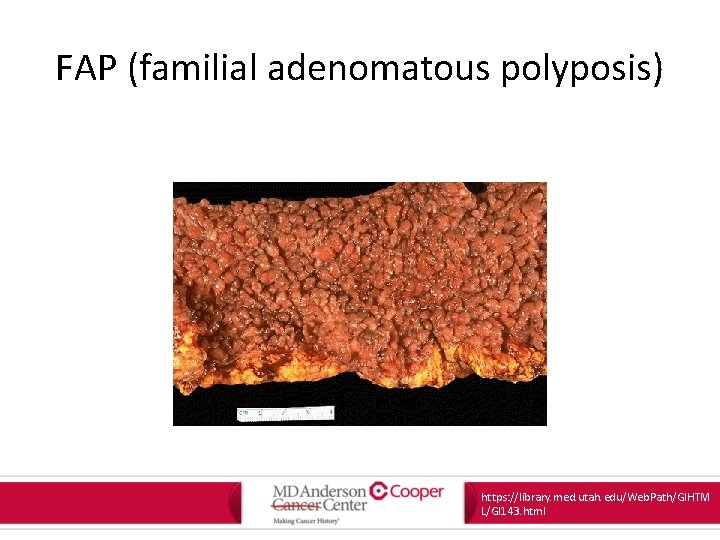
FAP (familial adenomatous polyposis) https: //library. med. utah. edu/Web. Path/GIHTM L/GI 143. html

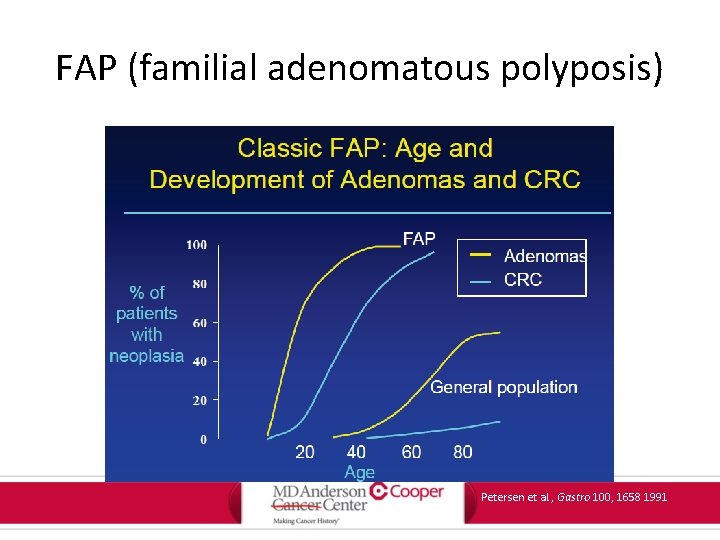
FAP (familial adenomatous polyposis) Petersen et al. , Gastro 100, 1658 1991

FAP (familial adenomatous polyposis) Site of disease Colon Duodenum/periampullary Stomach Pancreas Thyroid Liver (hepatoblastoma) CNS (medulloblastoma) Lifetime risk (%) 100 4 -12 <1 2 1 -2 <1 Oncology Letters. 13: 1499 -1408, 2017.

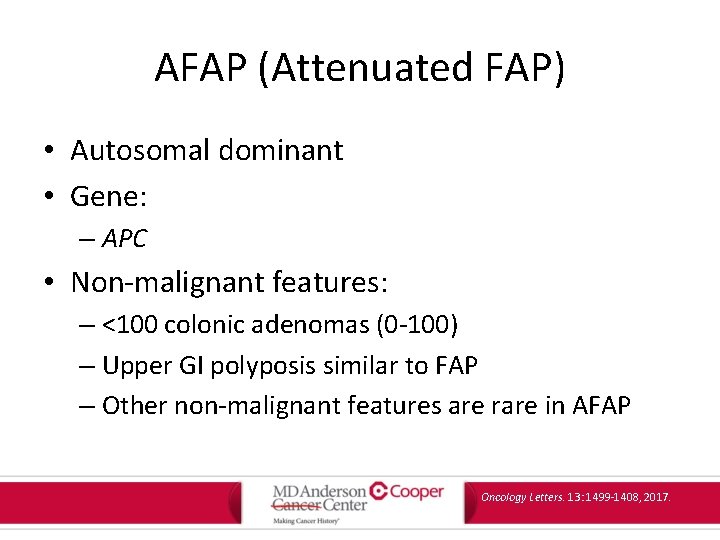
AFAP (Attenuated FAP) • Autosomal dominant • Gene: – APC • Non-malignant features: – <100 colonic adenomas (0 -100) – Upper GI polyposis similar to FAP – Other non-malignant features are rare in AFAP Oncology Letters. 13: 1499 -1408, 2017.

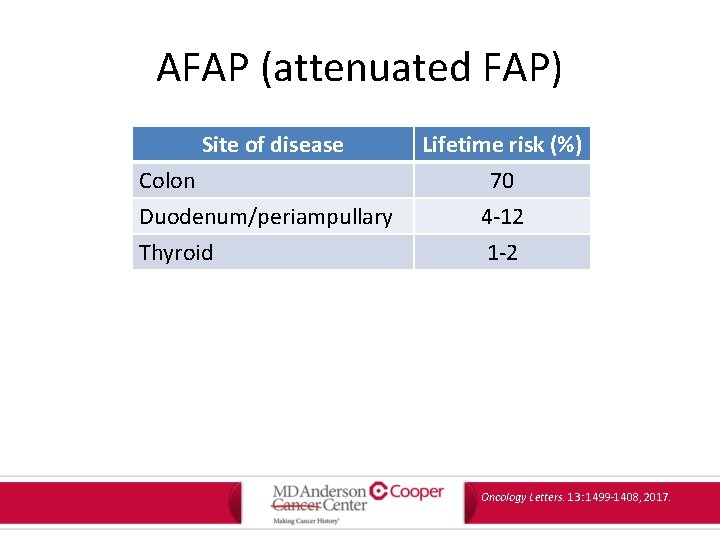
AFAP (attenuated FAP) Site of disease Colon Duodenum/periampullary Thyroid Lifetime risk (%) 70 4 -12 1 -2 Oncology Letters. 13: 1499 -1408, 2017.

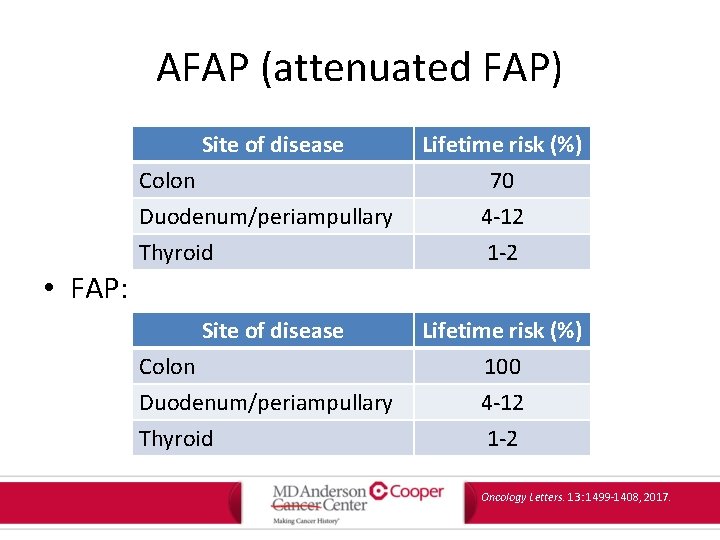
AFAP (attenuated FAP) Site of disease Colon Duodenum/periampullary Thyroid Lifetime risk (%) 70 4 -12 1 -2 • FAP: Site of disease Colon Duodenum/periampullary Thyroid Lifetime risk (%) 100 4 -12 1 -2 Oncology Letters. 13: 1499 -1408, 2017.

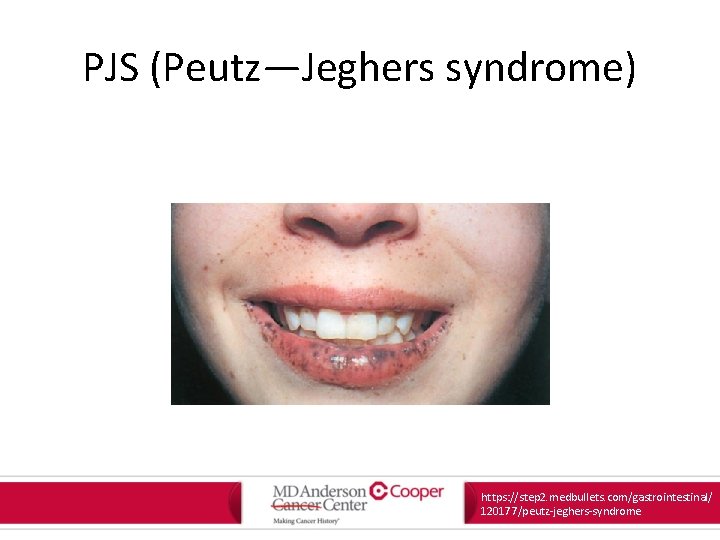
PJS (Peutz—Jeghers syndrome) • Autosomal dominant • Gene: – STK 11 • Non-malignant features: – Mucocutaneous pigmentations – Gastrointestinal hamartomatous (Peutz-Jegher) polyps Oncology Letters. 13: 1499 -1408, 2017.

PJS (Peutz—Jeghers syndrome) https: //step 2. medbullets. com/gastrointestinal/ 120177/peutz-jeghers-syndrome

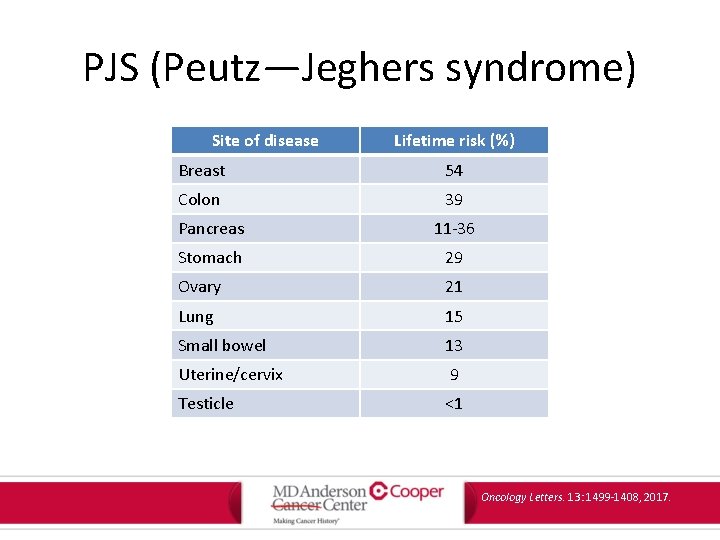
PJS (Peutz—Jeghers syndrome) Site of disease Lifetime risk (%) Breast 54 Colon 39 Pancreas 11 -36 Stomach 29 Ovary 21 Lung 15 Small bowel 13 Uterine/cervix 9 Testicle <1 Oncology Letters. 13: 1499 -1408, 2017.

JPS (juvenile polyposis syndrome) • Autosomal dominant • Genes: – SMAD 4: associated with colon (39% lifetime risk) – BMPR 1 A: associated with stomach, pancreas, small bowel (21% lifetime risk) • Non-malignant features: – Gastrointestinal hamartomatous (juvenile) polyps – Features of HHT congenital defects Oncology Letters. 13: 1499 -1408, 2017.

Hereditary Diffuse Gastric Cancer • 1994: family with 8 related members with gastric cancer at early ages (31 -65 years), over 4 generations, with autosomal dominant transmission – Diffuse gastric cancer, with multiple isolated nests of signet ring cells – “Linitus plastica” extending from proximal stomach into small intestine Cell Mol Gastroenterol Hepatol. 2017; 3: 192200.

Hereditary Diffuse Gastric Cancer • CDH 1 gene – Encodes E-cadherin – Also associated with lobular breast cancers Cell Mol Gastroenterol Hepatol. 2017; 3: 192200.

Hereditary Diffuse Gastric Cancer • Diagnosis should be considered – Families with 2 or more individuals with gastric cancer at any age – Individuals with diffuse gastric cancer before the age of 40 – Families with both diffuse gastric cancer and lobular breast cancer – Individuals with bilateral lobular breast cancer before age 50 – Individuals with gastric cancer and cleft lip or cleft palate Cell Mol Gastroenterol Hepatol. 2017; 3: 192200.

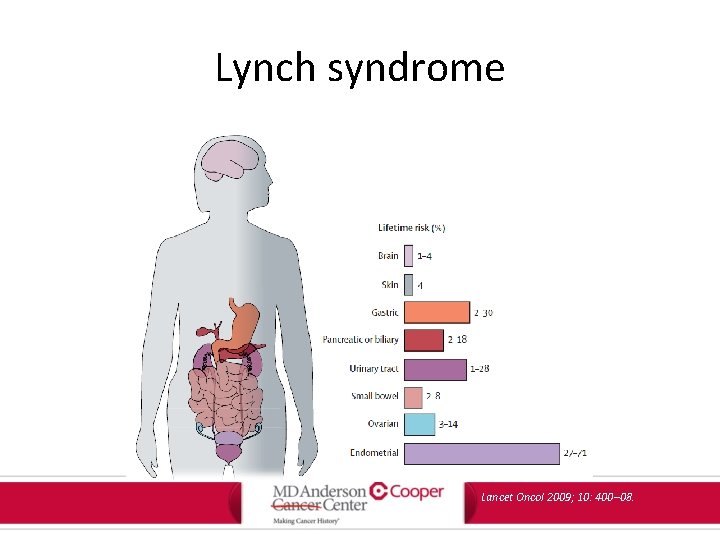
Lynch syndrome • Most common cause of inherited colorectal cancer – Hereditary nonpolyposis colorectal cancer (HNPCC) • Autosomal dominant Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome Site of disease Colon Endometrium Stomach Lifetime risk (%) 50 -80 40 -60 11 -19 Ovary Hepatobiliary tract Upper urinary tract Pancreas Small bowel CNS 9 -12 2 -7 4 -5 3 -4 1 -3 Oncology Letters. 13: 1499 -1408, 2017.

Lynch syndrome Lancet Oncol 2009; 10: 400– 08.

Lynch syndrome • Physical or non-malignant features, with the exception of keratoacanthomas and sebaceous adenomas/carcinomas, are rare Oncology Letters. 13: 1499 -1408, 2017.

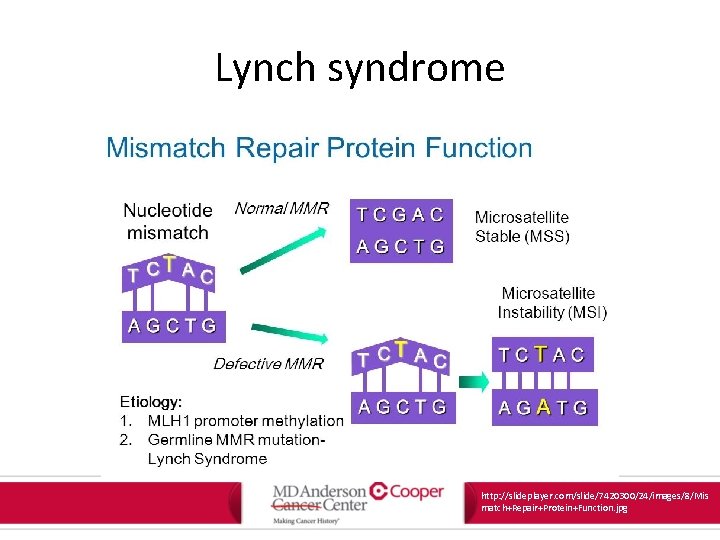
Lynch syndrome • Lynch syndrome tumors associated with changes in the length of nucleotide repeat sequences of tumor DNA • Termed “microsatellite instability” or MSI • MSI results from defective mismatch repair at the time of DNA replication – MSI and MMR used interchangeably Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome http: //slideplayer. com/slide/7420300/24/images/8/Mis match+Repair+Protein+Function. jpg

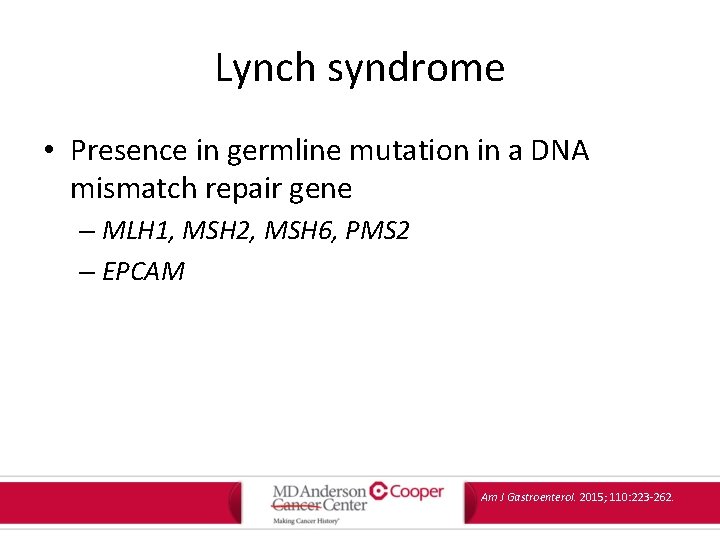
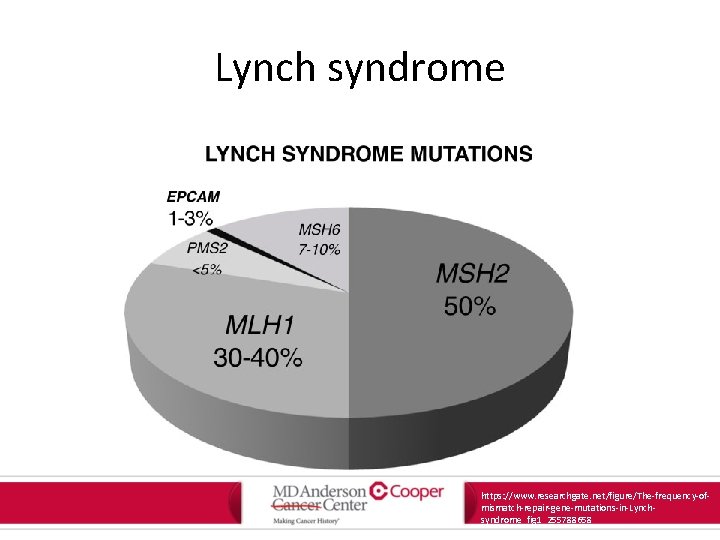
Lynch syndrome • Presence in germline mutation in a DNA mismatch repair gene – MLH 1, MSH 2, MSH 6, PMS 2 – EPCAM Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome https: //www. researchgate. net/figure/The-frequency-ofmismatch-repair-gene-mutations-in-Lynchsyndrome_fig 1_255788658

Lynch syndrome Am J Gastroenterol. 2015; 110: 223 -262.

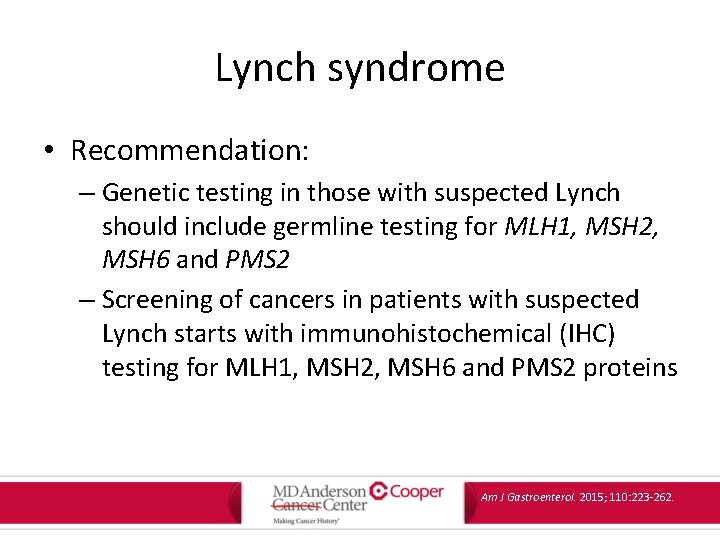
Lynch syndrome • Recommendation: – Genetic testing in those with suspected Lynch should include germline testing for MLH 1, MSH 2, MSH 6 and PMS 2 – Screening of cancers in patients with suspected Lynch starts with immunohistochemical (IHC) testing for MLH 1, MSH 2, MSH 6 and PMS 2 proteins Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome • Recommendation: – In individuals at risk for or affected with Lynch, screening for CRC by colonoscopy should be performed at least every 2 years, beginning between 20 and 25 years. – Annual colonoscopy should be considered in confirmed mutation carriers. Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome • Recommendation: – Colectomy with ileorectal anastomosis is the preferred treatment of patients affected with Lynch with colon cancer or colonic neoplasia not controllable by endoscopy. – Segmental colectomy is an option in patients unsuitable for total colectomy if regular postoperative surveillance is conducted. Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome • Recommendation: – Hysterectomy with bilateral salpingooophrectomy should be offered to women who are known Lynch carriers and who have finished child bearing, optimally at age 40 -45 years. Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome • Recommendation: – Screening for endometrial cancer and ovarian cancer should be offered to women at risk for or affected by Lynch by endometrial biopsy and transvaginal ultrasound annually, starting at age 30 -35 years before undergoing surgery, or if surgery is deferred. Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome • Recommendation: – Screening for gastric and duodenal cancer can be considered in individuals at risk for or affected with Lynch by baseline EGD with gastric biopsy at age 30 -35 years. Data for ongoing surveillance is limited, but may be appropriate every 3 -5 years. Am J Gastroenterol. 2015; 110: 223 -262.

Lynch syndrome • Recommendation: – Screening beyond population-based recommendations for cancers of the urinary tract, pancreas, prostate, and breast (in the absence of additional risk factors or family history) has limited quality data. Am J Gastroenterol. 2015; 110: 223 -262.

Hereditary Cancer Syndromes • Until 2017, identification of a cancer syndrome impacted: – Screening tests in affected individuals – Screening tests in relatives of affected individuals – Prophylactic organ removal or risk reduction – Definitive surgical management • Lacking treatment implications

Hereditary Cancer Syndromes • Until 2017: Lynch colorectal cancer treated identically to every other colorectal cancer • Cancer landscape is changing: – Translating clinical genetics into bedside clinical care

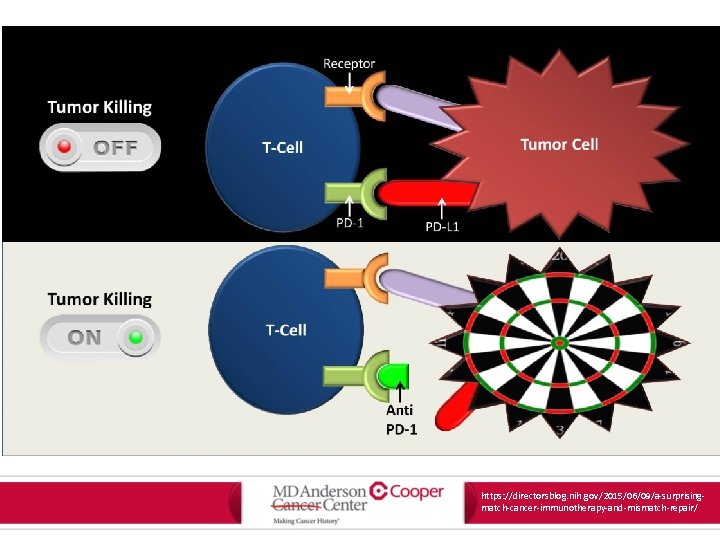
https: //directorsblog. nih. gov/2015/06/09/a-surprisingmatch-cancer-immunotherapy-and-mismatch-repair/

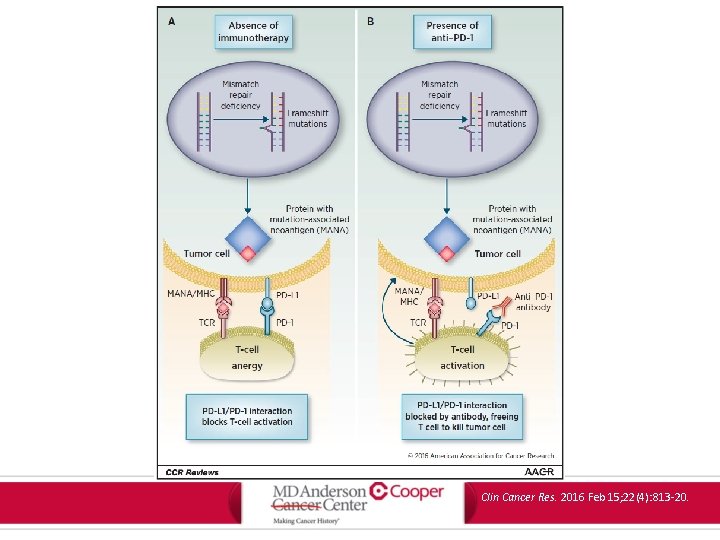
Clin Cancer Res. 2016 Feb 15; 22(4): 813 -20.

N Engl J Med. 2015; 272: 2509 -2520.

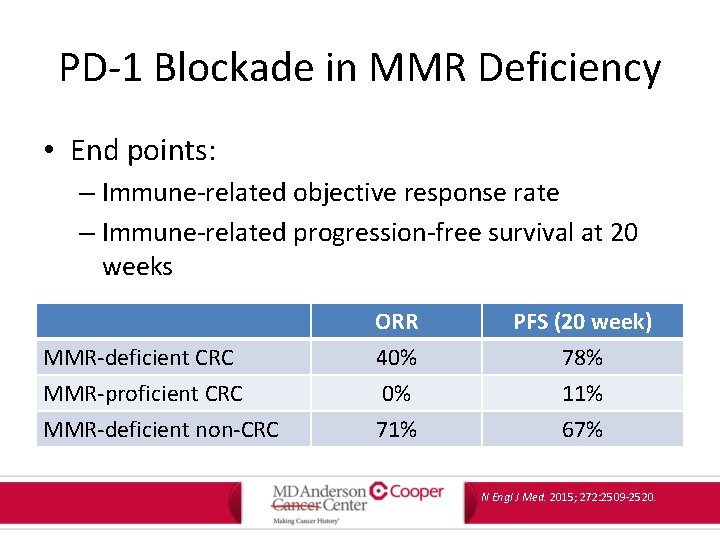
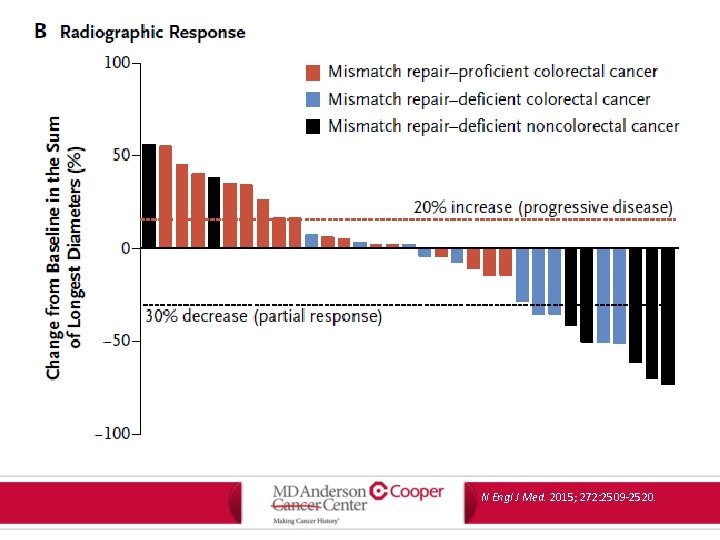
PD-1 Blockade in MMR Deficiency • Phase 2 trial of pembrolizumab (anti-PD 1 checkpoint inhibitor) in 41 patients with progressive metastatic carcinoma with or without mismatch-repair deficiency – Mismatch-repair deficient m. CRC – Mismatch-repair proficient m. CRC – Mismatch-repair deficient non-CRC N Engl J Med. 2015; 272: 2509 -2520.

PD-1 Blockade in MMR Deficiency • End points: – Immune-related objective response rate – Immune-related progression-free survival at 20 weeks MMR-deficient CRC MMR-proficient CRC MMR-deficient non-CRC ORR 40% 0% 71% PFS (20 week) 78% 11% 67% N Engl J Med. 2015; 272: 2509 -2520.

N Engl J Med. 2015; 272: 2509 -2520.

N Engl J Med. 2015; 272: 2509 -2520.

PD-1 Blockade in MMR Deficiency N Engl J Med. 2015; 272: 2509 -2520.

