Fo RT Follicular Radiotherapy Trial A phase III
- Slides: 1

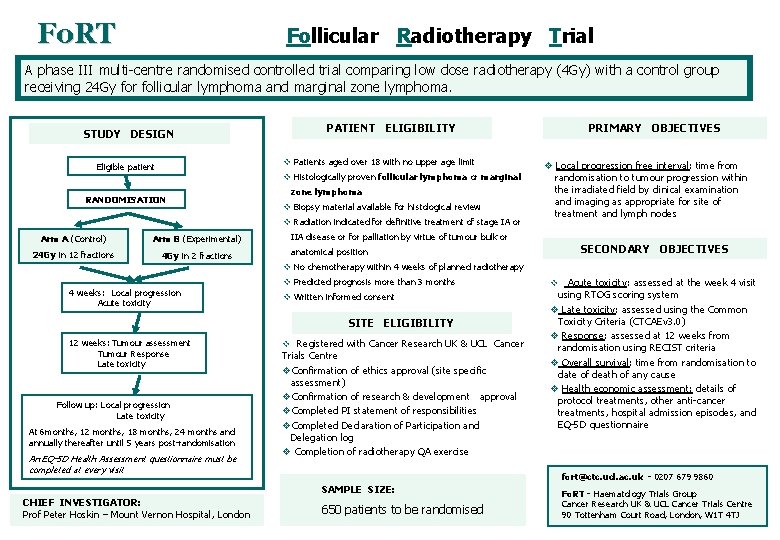
Fo. RT Follicular Radiotherapy Trial A phase III multi-centre randomised controlled trial comparing low dose radiotherapy (4 Gy) with a control group receiving 24 Gy for follicular lymphoma and marginal zone lymphoma. STUDY DESIGN PATIENT ELIGIBILITY v Patients aged over 18 with no upper age limit Eligible patient v Histologically proven follicular lymphoma or marginal RANDOMISATION zone lymphoma v Biopsy material available for histological review v Radiation indicated for definitive treatment of stage IA or Arm A (Control) Arm B (Experimental) 24 Gy in 12 fractions 4 Gy in 2 fractions PRIMARY OBJECTIVES v Local progression free interval: time from randomisation to tumour progression within the irradiated field by clinical examination and imaging as appropriate for site of treatment and lymph nodes IIA disease or for palliation by virtue of tumour bulk or SECONDARY OBJECTIVES anatomical position v No chemotherapy within 4 weeks of planned radiotherapy 4 weeks: Local progression Acute toxicity v Predicted prognosis more than 3 months v Written informed consent SITE ELIGIBILITY 12 weeks: Tumour assessment Tumour Response Late toxicity Follow up: Local progression Late toxicity At 6 months, 12 months, 18 months, 24 months and annually thereafter until 5 years post-randomisation An EQ-5 D Health Assessment questionnaire must be completed at every visit v Registered with Cancer Research UK & UCL Cancer Trials Centre v. Confirmation of ethics approval (site specific assessment) v. Confirmation of research & development approval v. Completed PI statement of responsibilities v. Completed Declaration of Participation and Delegation log v Completion of radiotherapy QA exercise fort@ctc. ucl. ac. uk - 0207 679 9860 SAMPLE SIZE: CHIEF INVESTIGATOR: Prof Peter Hoskin – Mount Vernon Hospital, London Acute toxicity: assessed at the week 4 visit using RTOG scoring system v Late toxicity: assessed using the Common Toxicity Criteria (CTCAEv 3. 0) v Response: assessed at 12 weeks from randomisation using RECIST criteria v Overall survival: time from randomisation to date of death of any cause v Health economic assessment: details of protocol treatments, other anti-cancer treatments, hospital admission episodes, and EQ-5 D questionnaire v 650 patients to be randomised Fo. RT - Haematology Trials Group Cancer Research UK & UCL Cancer Trials Centre 90 Tottenham Court Road, London, W 1 T 4 TJ

