FLUORESCENT LABELING OF BIOMOLECULES WITH ORGANIC PROBES Soundarya
FLUORESCENT LABELING OF BIOMOLECULES WITH ORGANIC PROBES Soundarya Vaithianathan Graduate Student Department of Medicinal Chemistry, School of Pharmacy, VCU. email: vaithianaths@mymail. vcu. edu February 12 th, 2010. 1
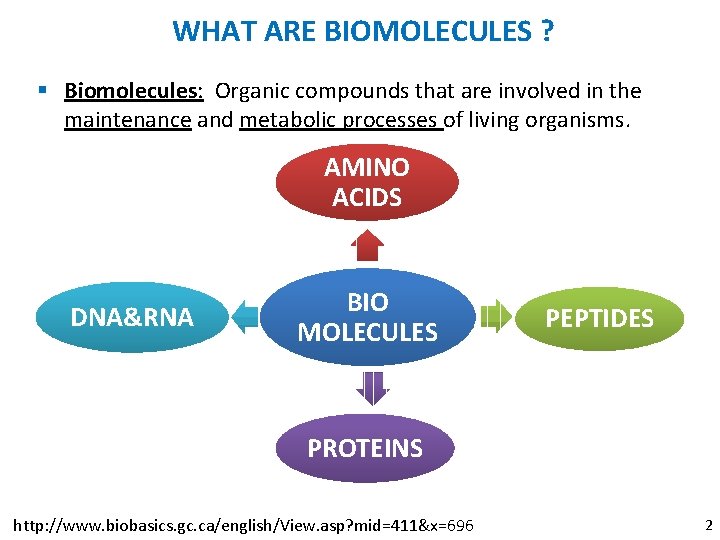
WHAT ARE BIOMOLECULES ? § Biomolecules: Organic compounds that are involved in the maintenance and metabolic processes of living organisms. AMINO ACIDS DNA&RNA BIO MOLECULES PEPTIDES PROTEINS http: //www. biobasics. gc. ca/english/View. asp? mid=411&x=696 2
BIOMOLECULES § Implicated in various disease conditions such as those with impaired amino acid metabolism. § E. g. Phenylketonuria § Peptide based pharmaceuticals, important class of therapeutic agent. § Used to treat many diseases. § E. g. Interferon used in treat Multiple Sclerosis. NEED TO BE DETECTED !! Gatti, M. ; Gioia, M. G. ; Andreatta, P. ; Pentassuglia, G. J. Pharm. Biomed. Anal. 2004, 35, 339 -348. Bennett, F. A. ; Barlow, D. J. ; Dodoo, A. N. O. ; Hider, R. C. ; Lansley, A. B. ; Lawrence, M. J. ; Marriott, C. ; Bansal, S. S. Anal. Biochem. 1999, 270, 15 -23. 3
BIOMOLECULES § Problems in detection: 1. Structural similarity between § Analyte § Degradation product § Endogenous component § Impurities 2. Lack of selectivity 3. Sensitivity requirements not met 4. Lack of an effective method for detection in biological matrices FLUORESCENT LABELING Bennett, F. A. ; Barlow, D. J. ; Dodoo, A. N. O. ; Hider, R. C. . ; Lansley, A. B. ; Lawrence, M. J. ; Marriott, C. ; Bansal, S. S. Anal. Biochem. 1999, 270, 15 -23. Wang, W. ; Li, H. Tetrahedron Lett. 2004, 45, 8479 -8481. 4
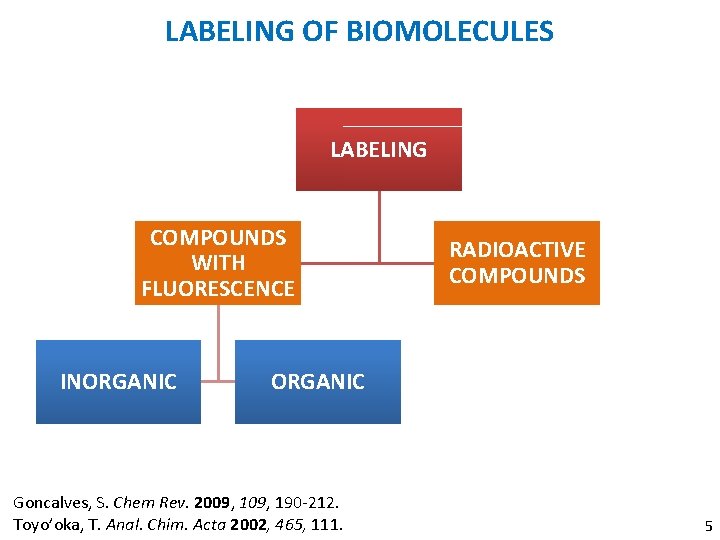
LABELING OF BIOMOLECULES LABELING COMPOUNDS WITH FLUORESCENCE INORGANIC RADIOACTIVE COMPOUNDS ORGANIC Goncalves, S. Chem Rev. 2009, 190 -212. Toyo’oka, T. Anal. Chim. Acta 2002, 465, 111. 5
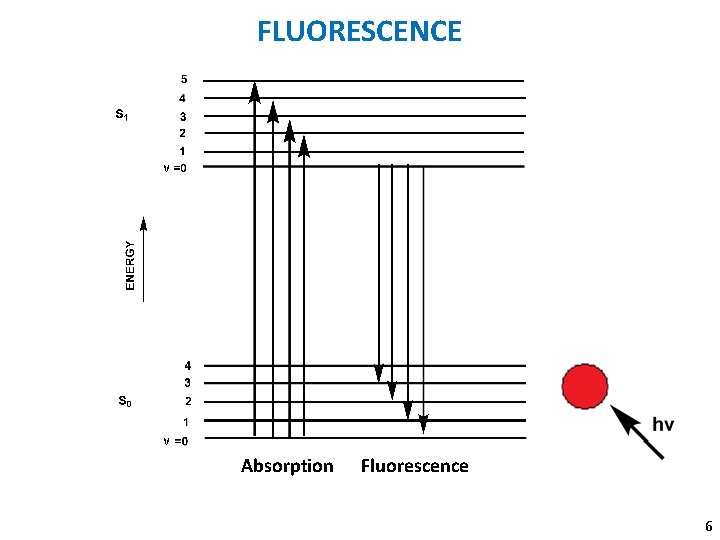
FLUORESCENCE ν ν Absorption Fluorescence 6
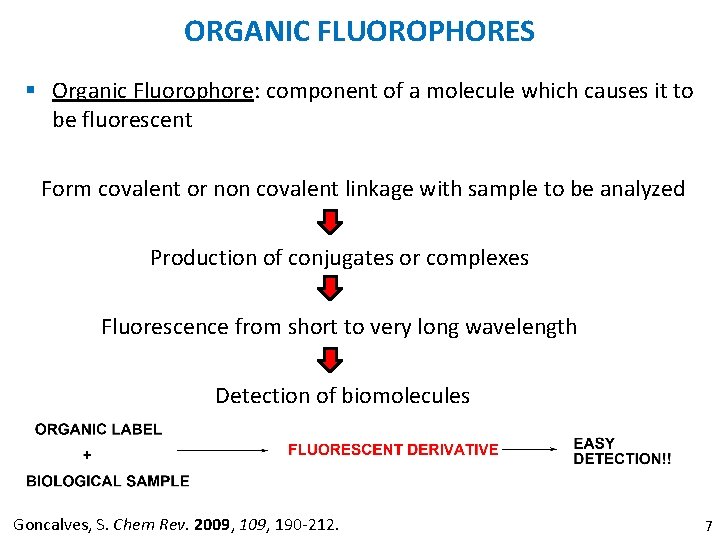
ORGANIC FLUOROPHORES § Organic Fluorophore: component of a molecule which causes it to be fluorescent Form covalent or non covalent linkage with sample to be analyzed Production of conjugates or complexes Fluorescence from short to very long wavelength Detection of biomolecules Goncalves, S. Chem Rev. 2009, 190 -212. 7
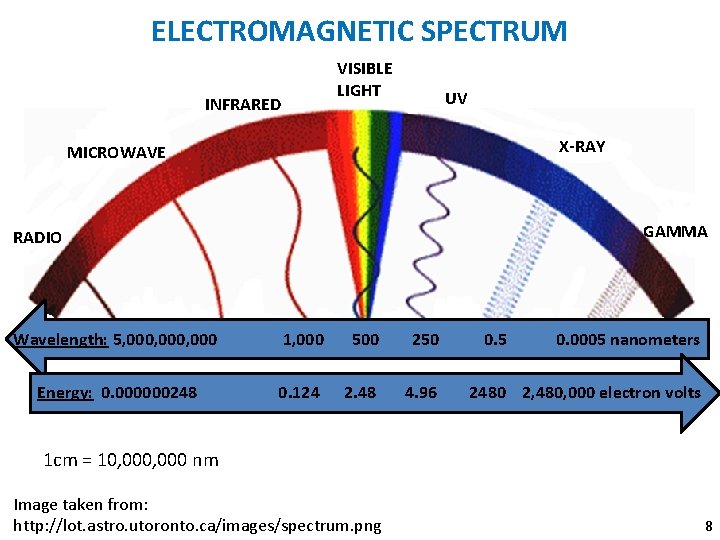
ELECTROMAGNETIC SPECTRUM VISIBLE LIGHT INFRARED UV X-RAY MICROWAVE nan RADIO GAMMA Wavelength: 5, 000, 000 1, 000 500 250 Energy: 0. 000000248 0. 124 2. 48 4. 96 0. 5 0. 0005 nanometers 2480 2, 480, 000 electron volts 1 cm = 10, 000 nm Image taken from: http: //lot. astro. utoronto. ca/images/spectrum. png 8
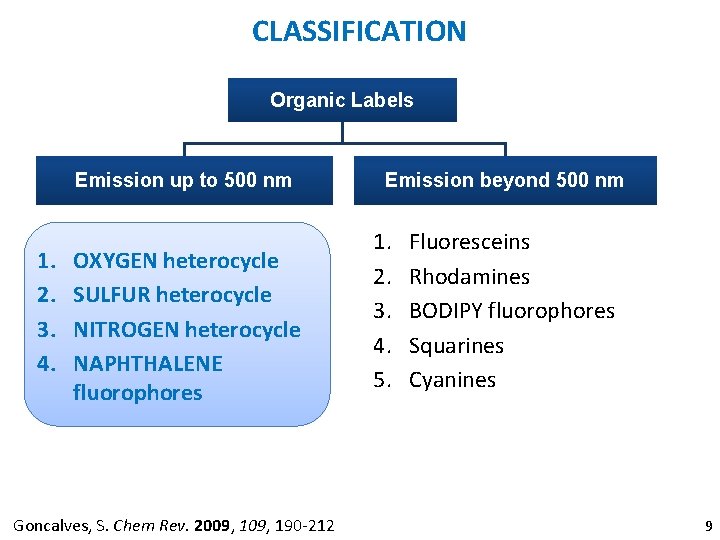
CLASSIFICATION Organic Labels Emission up to 500 nm 1. 2. 3. 4. OXYGEN heterocycle SULFUR heterocycle NITROGEN heterocycle NAPHTHALENE fluorophores Goncalves, S. Chem Rev. 2009, 190 -212 Emission beyond 500 nm 1. 2. 3. 4. 5. Fluoresceins Rhodamines BODIPY fluorophores Squarines Cyanines 9
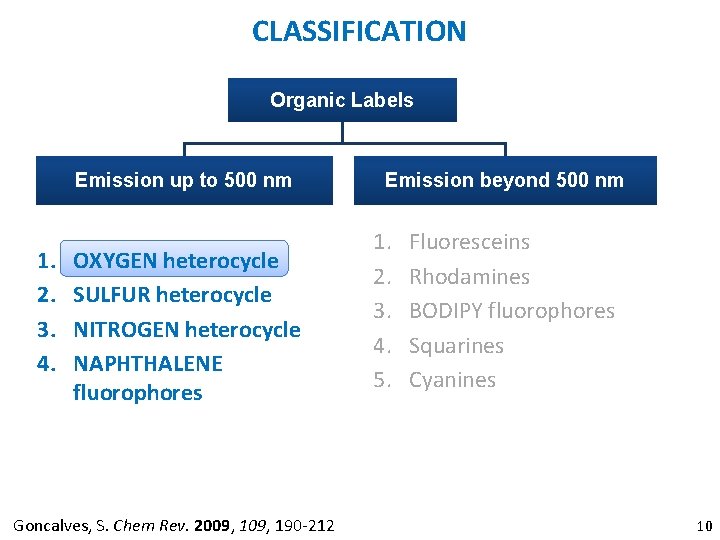
CLASSIFICATION Organic Labels Emission up to 500 nm 1. 2. 3. 4. OXYGEN heterocycle SULFUR heterocycle NITROGEN heterocycle NAPHTHALENE fluorophores Goncalves, S. Chem Rev. 2009, 190 -212 Emission beyond 500 nm 1. 2. 3. 4. 5. Fluoresceins Rhodamines BODIPY fluorophores Squarines Cyanines 10
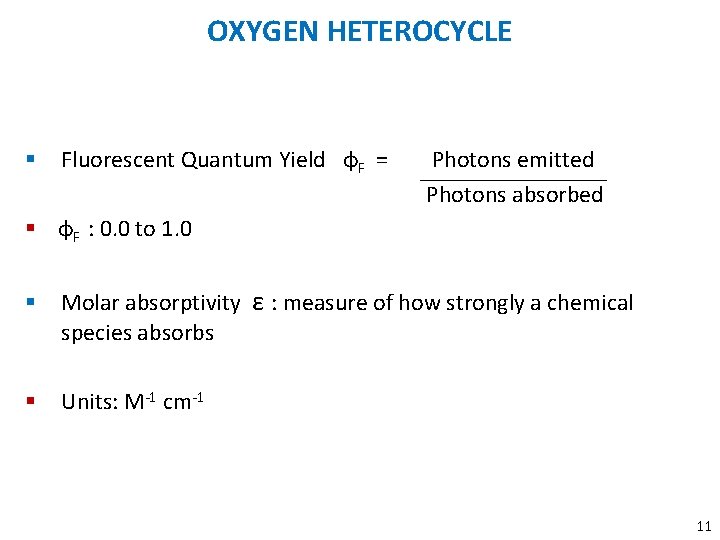
OXYGEN HETEROCYCLE § Fluorescent Quantum Yield φF = Photons emitted Photons absorbed § φF : 0. 0 to 1. 0 § Molar absorptivity ε : measure of how strongly a chemical species absorbs § Units: M-1 cm-1 11
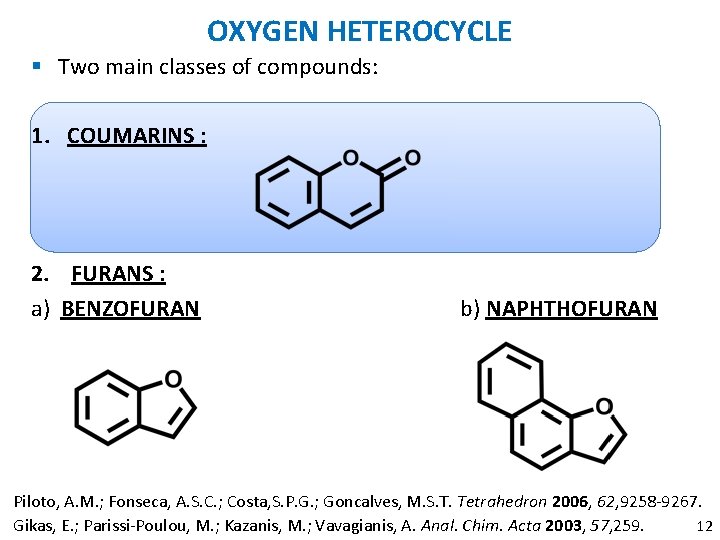
OXYGEN HETEROCYCLE § Two main classes of compounds: 1. COUMARINS : 2. FURANS : a) BENZOFURAN b) NAPHTHOFURAN Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 -9267. 12 Gikas, E. ; Parissi-Poulou, M. ; Kazanis, M. ; Vavagianis, A. Anal. Chim. Acta 2003, 57, 259.
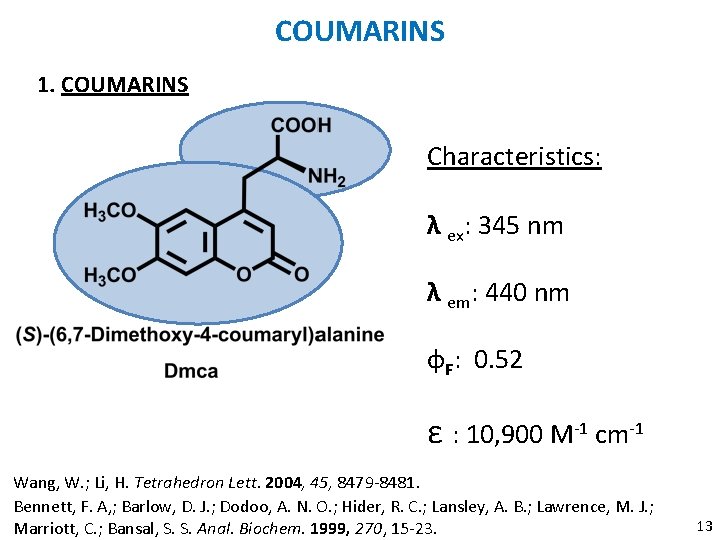
COUMARINS 1. COUMARINS Characteristics: λ ex: 345 nm λ em: 440 nm ɸF: 0. 52 ε : 10, 900 M-1 cm-1 Wang, W. ; Li, H. Tetrahedron Lett. 2004, 45, 8479 -8481. Bennett, F. A, ; Barlow, D. J. ; Dodoo, A. N. O. ; Hider, R. C. ; Lansley, A. B. ; Lawrence, M. J. ; Marriott, C. ; Bansal, S. S. Anal. Biochem. 1999, 270, 15 -23. 13
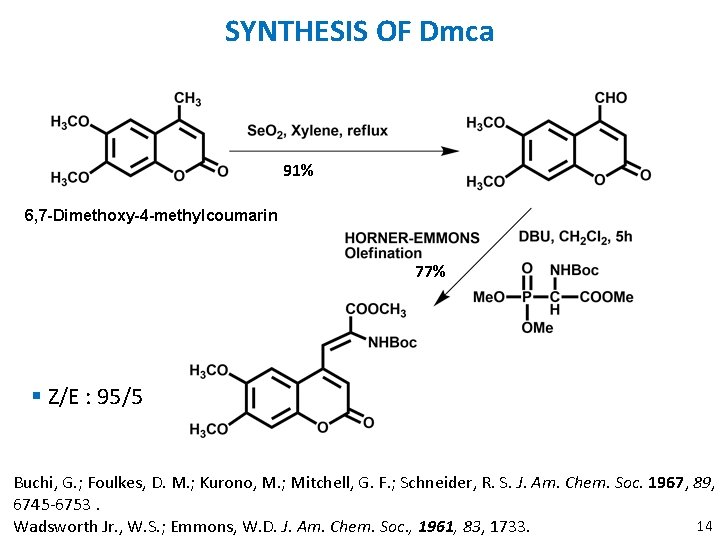
SYNTHESIS OF Dmca 91% 6, 7 -Dimethoxy-4 -methylcoumarin 77% § Z/E : 95/5 Buchi, G. ; Foulkes, D. M. ; Kurono, M. ; Mitchell, G. F. ; Schneider, R. S. J. Am. Chem. Soc. 1967, 89, 6745 -6753. 14 Wadsworth Jr. , W. S. ; Emmons, W. D. J. Am. Chem. Soc. , 1961, 83, 1733.
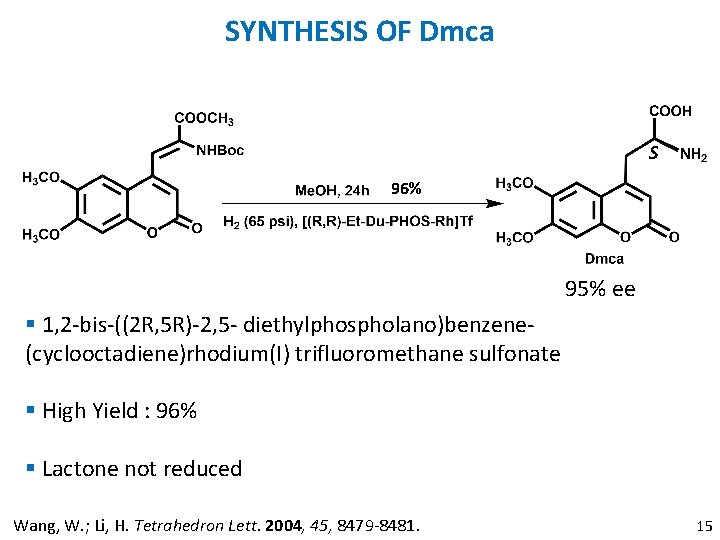
SYNTHESIS OF Dmca S 96% 95% ee § 1, 2 -bis-((2 R, 5 R)-2, 5 - diethylphospholano)benzene(cyclooctadiene)rhodium(I) trifluoromethane sulfonate § High Yield : 96% § Lactone not reduced Wang, W. ; Li, H. Tetrahedron Lett. 2004, 45, 8479 -8481. 15
ADVANTAGES OF Dmca § Coumarin side chain is fluorescent. § Selective determination. § High detection sensitivity. § Easy incorporation into peptide sequence. § Treatment with HBr/TFA does not alter stability. Bennett, F. A, ; Barlow, D. J. ; Dodoo, A. N. O. ; Hider, R. C. ; Lansley, A. B. ; Lawrence, M. J. ; Marriott, C. ; Bansal, S. S. Anal. Biochem. 1999, 270, 15 -23. 16
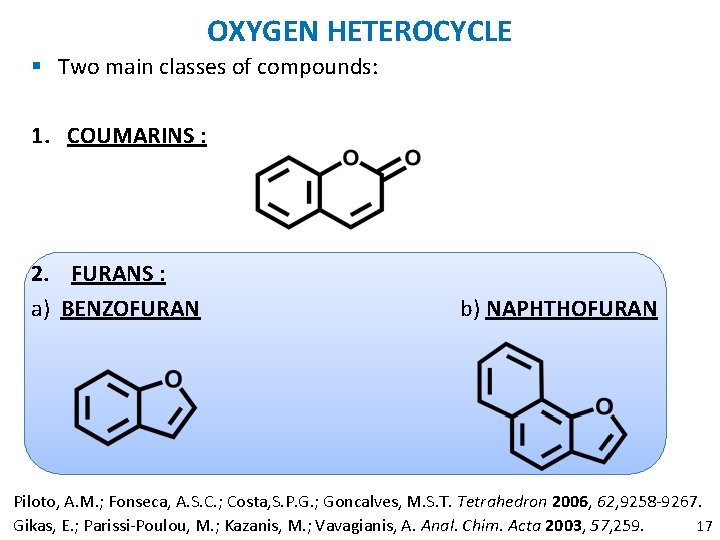
OXYGEN HETEROCYCLE § Two main classes of compounds: 1. COUMARINS : 2. FURANS : a) BENZOFURAN b) NAPHTHOFURAN Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 -9267. 17 Gikas, E. ; Parissi-Poulou, M. ; Kazanis, M. ; Vavagianis, A. Anal. Chim. Acta 2003, 57, 259.
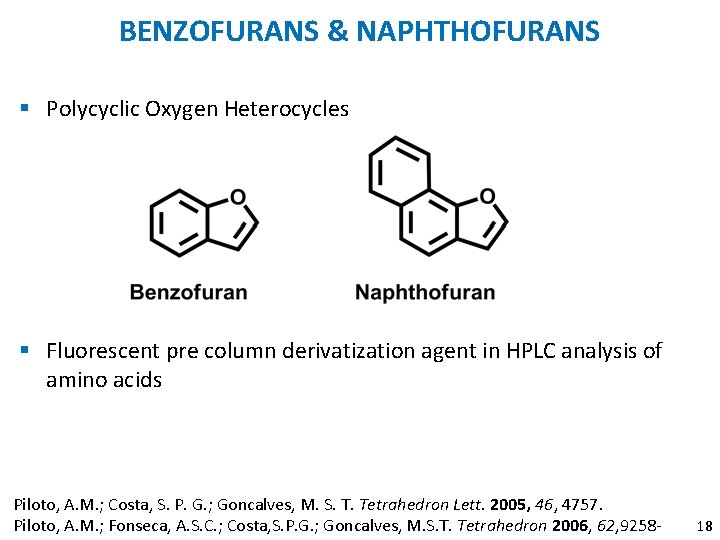
BENZOFURANS & NAPHTHOFURANS § Polycyclic Oxygen Heterocycles § Fluorescent pre column derivatization agent in HPLC analysis of amino acids Piloto, A. M. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron Lett. 2005, 46, 4757. Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 - 18
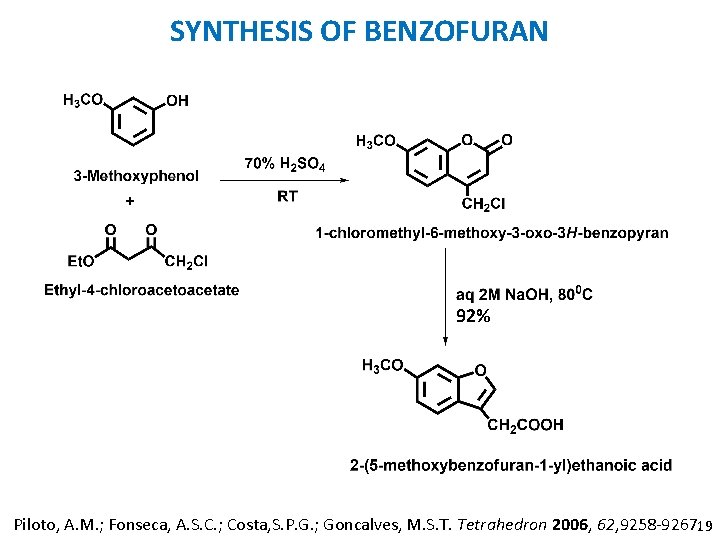
SYNTHESIS OF BENZOFURAN 92% Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 -9267. 19
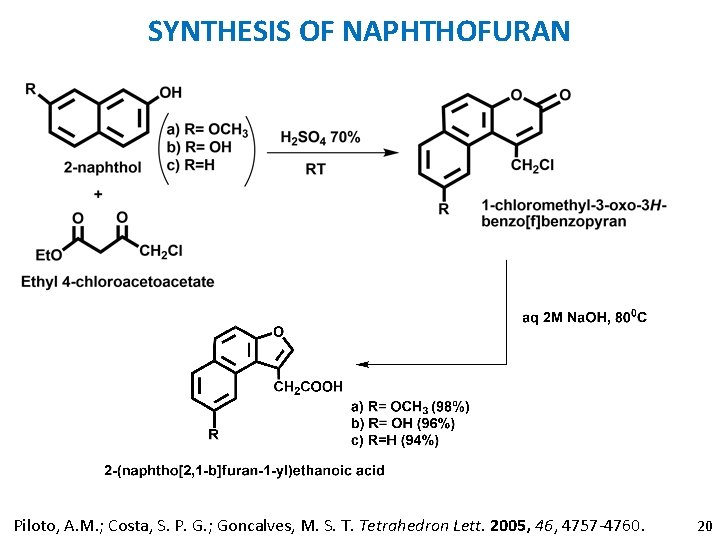
SYNTHESIS OF NAPHTHOFURAN Piloto, A. M. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron Lett. 2005, 46, 4757 -4760. 20
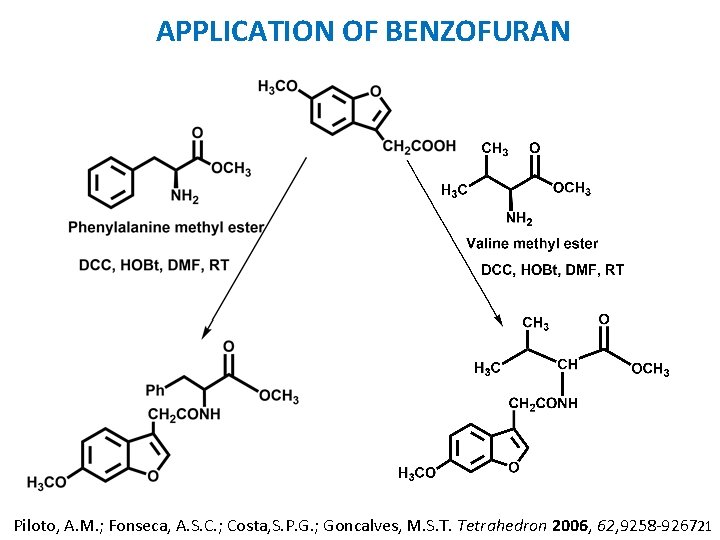
APPLICATION OF BENZOFURAN Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 -9267. 21
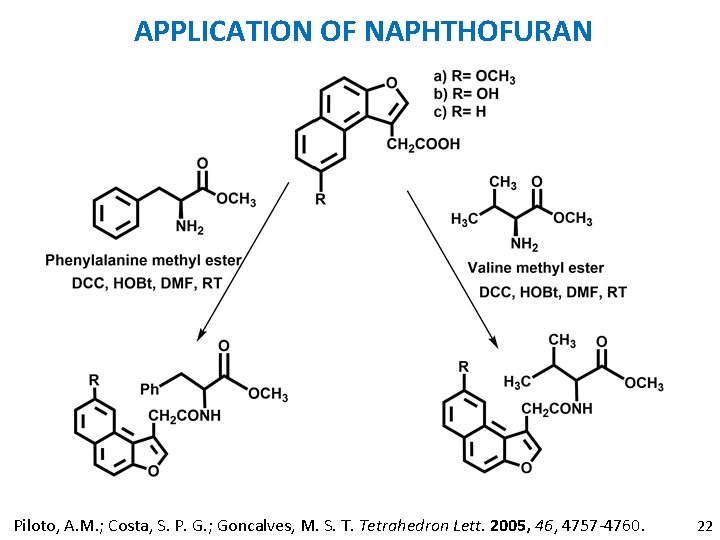
APPLICATION OF NAPHTHOFURAN Piloto, A. M. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron Lett. 2005, 46, 4757 -4760. 22
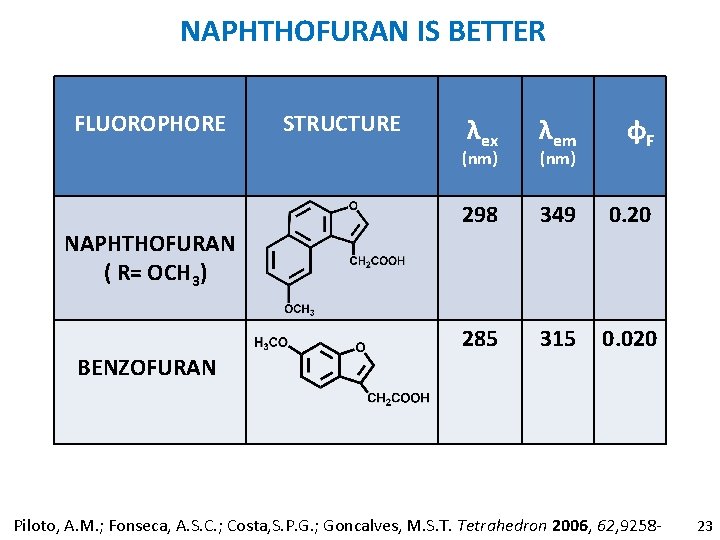
NAPHTHOFURAN IS BETTER FLUOROPHORE STRUCTURE λex λem φF 298 349 0. 20 285 315 0. 020 (nm) NAPHTHOFURAN ( R= OCH 3) BENZOFURAN (nm) Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 - 23
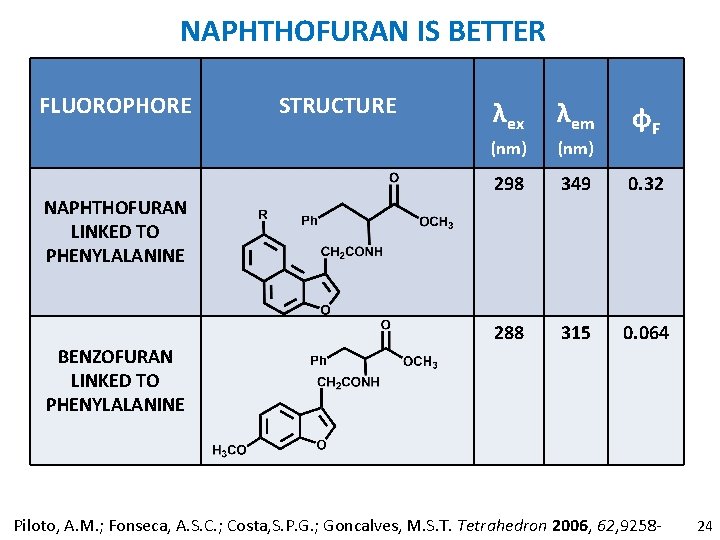
NAPHTHOFURAN IS BETTER FLUOROPHORE NAPHTHOFURAN LINKED TO PHENYLALANINE BENZOFURAN LINKED TO PHENYLALANINE STRUCTURE λex λem (nm) 298 349 0. 32 288 315 0. 064 φF Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 - 24
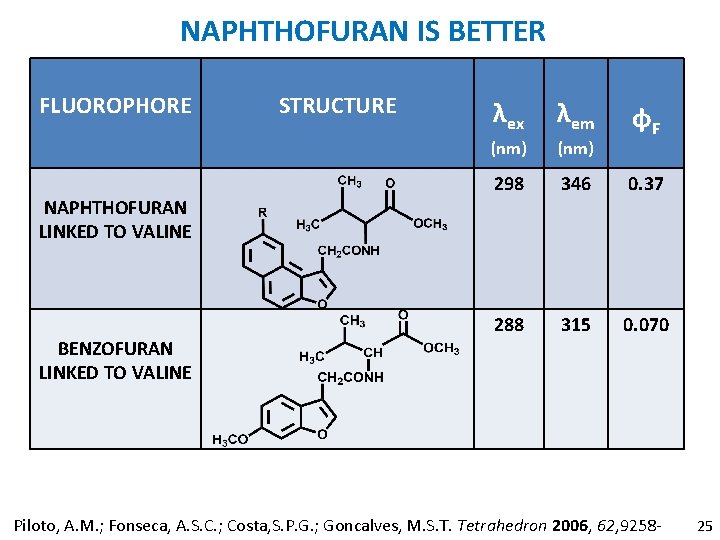
NAPHTHOFURAN IS BETTER FLUOROPHORE NAPHTHOFURAN LINKED TO VALINE BENZOFURAN LINKED TO VALINE STRUCTURE λex λem (nm) 298 346 0. 37 288 315 0. 070 φF Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 - 25
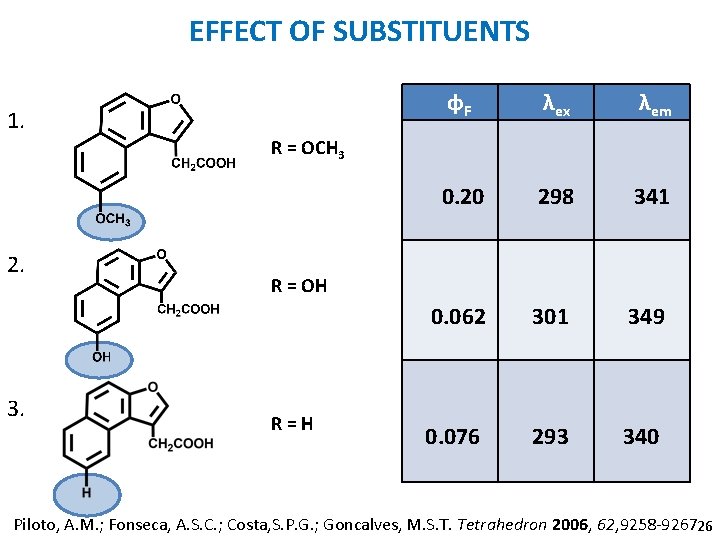
EFFECT OF SUBSTITUENTS 1. ɸF λex λem 0. 20 298 341 0. 062 301 349 0. 076 293 340 R = OCH 3 2. 3. R = OH R=H Piloto, A. M. ; Fonseca, A. S. C. ; Costa, S. P. G. ; Goncalves, M. S. T. Tetrahedron 2006, 62, 9258 -9267. 26
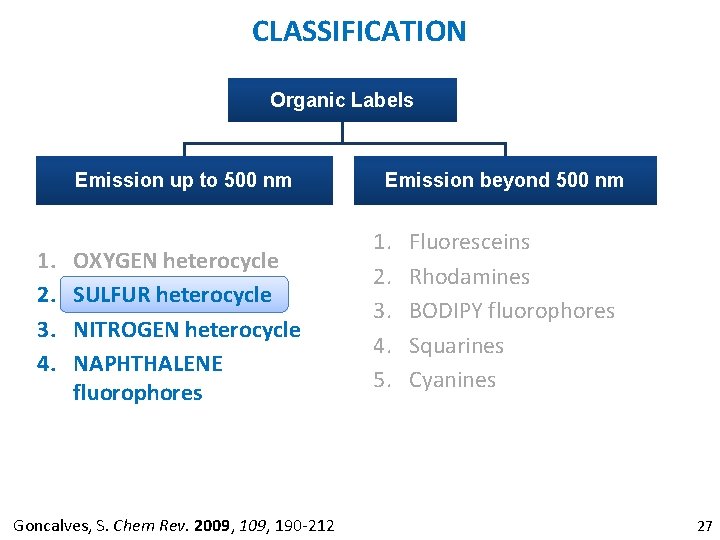
CLASSIFICATION Organic Labels Emission up to 500 nm 1. 2. 3. 4. OXYGEN heterocycle SULFUR heterocycle NITROGEN heterocycle NAPHTHALENE fluorophores Goncalves, S. Chem Rev. 2009, 190 -212 Emission beyond 500 nm 1. 2. 3. 4. 5. Fluoresceins Rhodamines BODIPY fluorophores Squarines Cyanines 27
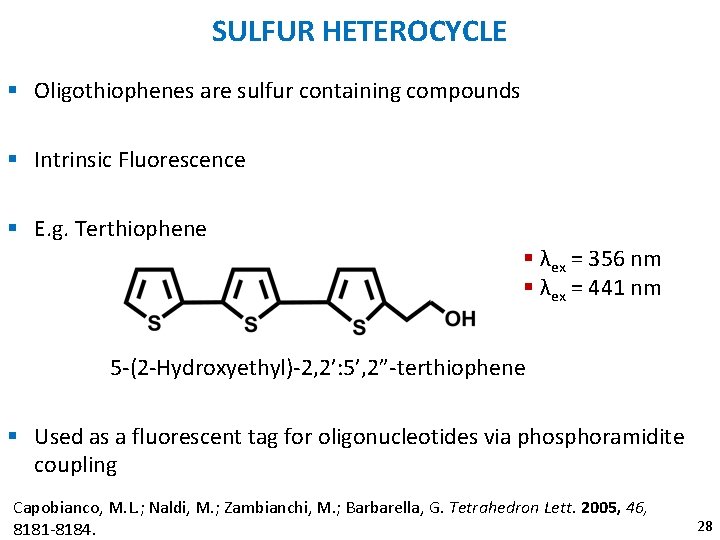
SULFUR HETEROCYCLE § Oligothiophenes are sulfur containing compounds § Intrinsic Fluorescence § E. g. Terthiophene § λex = 356 nm § λex = 441 nm 5 -(2 -Hydroxyethyl)-2, 2’: 5’, 2”-terthiophene § Used as a fluorescent tag for oligonucleotides via phosphoramidite coupling Capobianco, M. L. ; Naldi, M. ; Zambianchi, M. ; Barbarella, G. Tetrahedron Lett. 2005, 46, 8181 -8184. 28
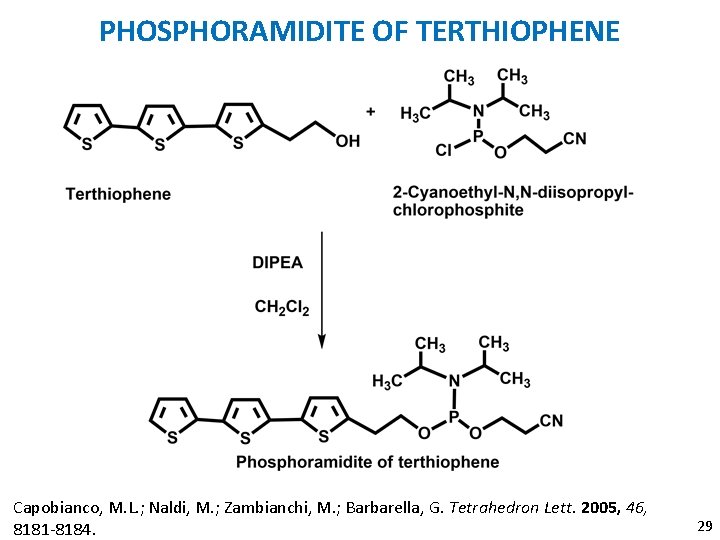
PHOSPHORAMIDITE OF TERTHIOPHENE Capobianco, M. L. ; Naldi, M. ; Zambianchi, M. ; Barbarella, G. Tetrahedron Lett. 2005, 46, 8181 -8184. 29
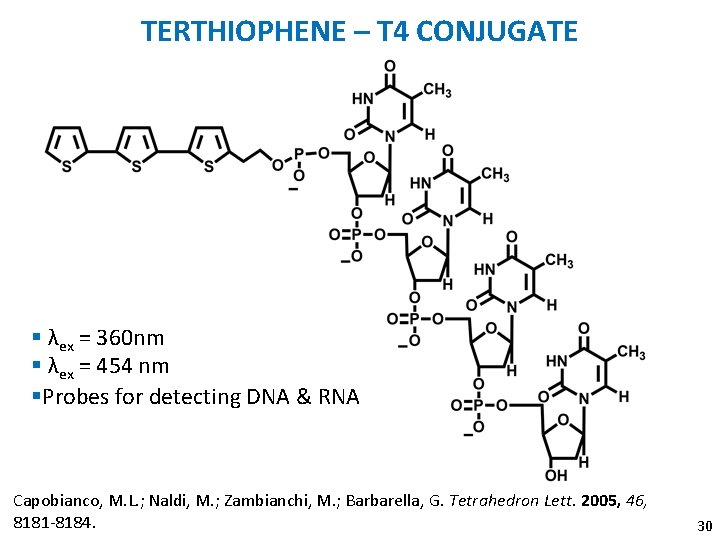
TERTHIOPHENE – T 4 CONJUGATE § λex = 360 nm § λex = 454 nm §Probes for detecting DNA & RNA Capobianco, M. L. ; Naldi, M. ; Zambianchi, M. ; Barbarella, G. Tetrahedron Lett. 2005, 46, 8181 -8184. 30
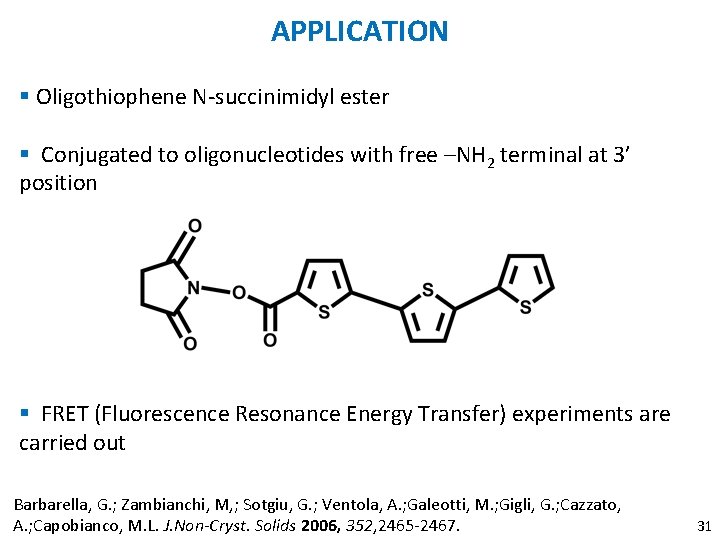
APPLICATION § Oligothiophene N-succinimidyl ester § Conjugated to oligonucleotides with free –NH 2 terminal at 3’ position § FRET (Fluorescence Resonance Energy Transfer) experiments are carried out Barbarella, G. ; Zambianchi, M, ; Sotgiu, G. ; Ventola, A. ; Galeotti, M. ; Gigli, G. ; Cazzato, A. ; Capobianco, M. L. J. Non-Cryst. Solids 2006, 352, 2465 -2467. 31
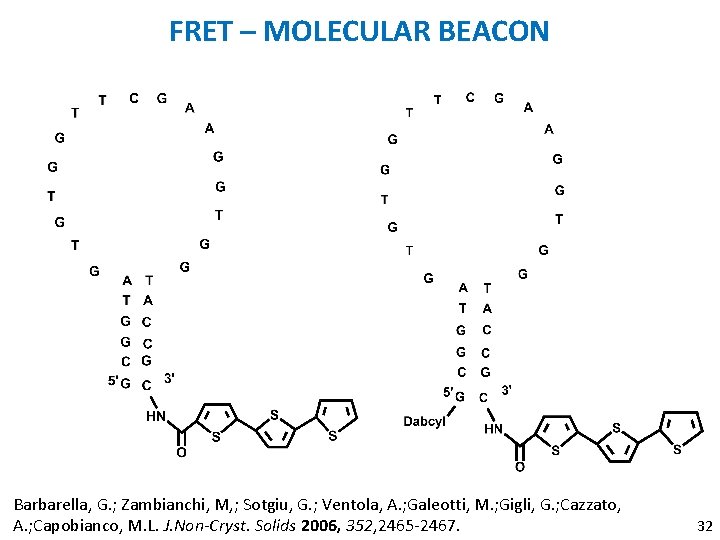
FRET – MOLECULAR BEACON Barbarella, G. ; Zambianchi, M, ; Sotgiu, G. ; Ventola, A. ; Galeotti, M. ; Gigli, G. ; Cazzato, A. ; Capobianco, M. L. J. Non-Cryst. Solids 2006, 352, 2465 -2467. 32
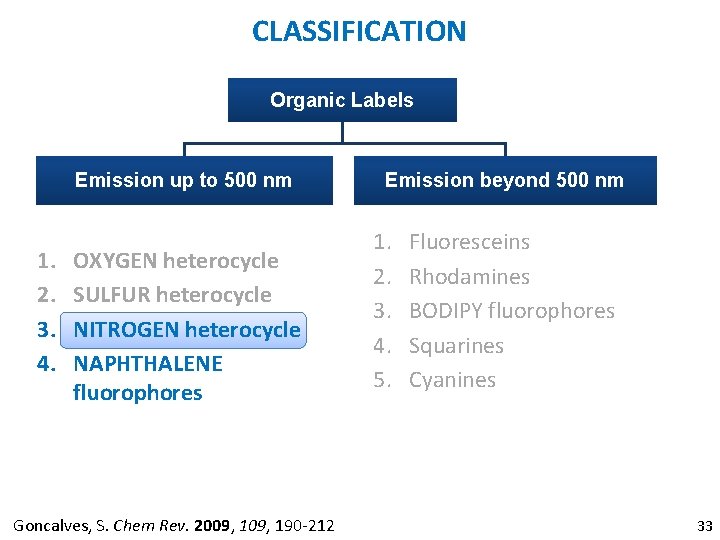
CLASSIFICATION Organic Labels Emission up to 500 nm 1. 2. 3. 4. OXYGEN heterocycle SULFUR heterocycle NITROGEN heterocycle NAPHTHALENE fluorophores Goncalves, S. Chem Rev. 2009, 190 -212 Emission beyond 500 nm 1. 2. 3. 4. 5. Fluoresceins Rhodamines BODIPY fluorophores Squarines Cyanines 33
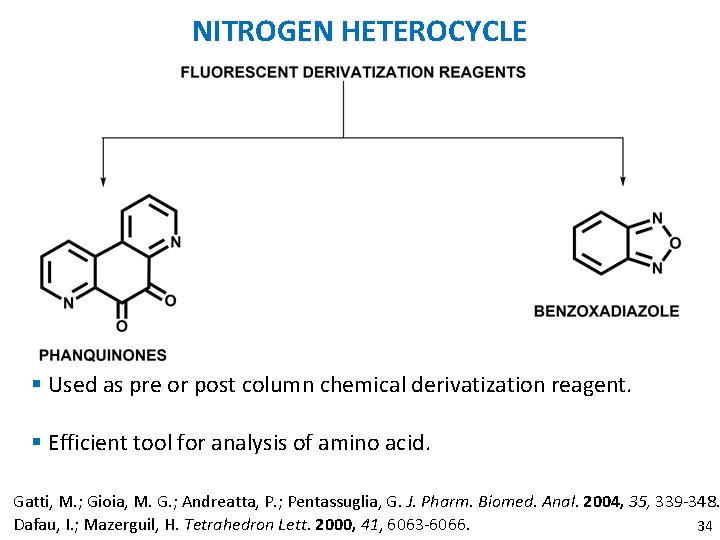
NITROGEN HETEROCYCLE § Used as pre or post column chemical derivatization reagent. § Efficient tool for analysis of amino acid. Gatti, M. ; Gioia, M. G. ; Andreatta, P. ; Pentassuglia, G. J. Pharm. Biomed. Anal. 2004, 35, 339 -348. Dafau, I. ; Mazerguil, H. Tetrahedron Lett. 2000, 41, 6063 -6066. 34
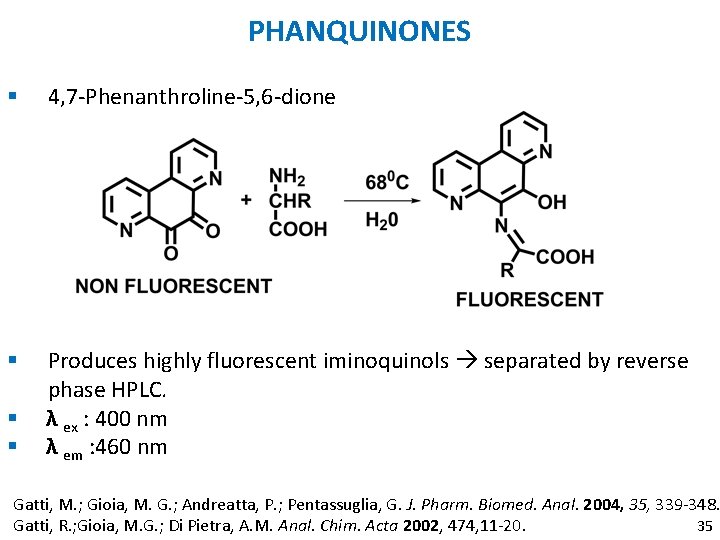
PHANQUINONES § 4, 7 -Phenanthroline-5, 6 -dione § Produces highly fluorescent iminoquinols separated by reverse phase HPLC. λ ex : 400 nm λ em : 460 nm § § Gatti, M. ; Gioia, M. G. ; Andreatta, P. ; Pentassuglia, G. J. Pharm. Biomed. Anal. 2004, 35, 339 -348. 35 Gatti, R. ; Gioia, M. G. ; Di Pietra, A. M. Anal. Chim. Acta 2002, 474, 11 -20.
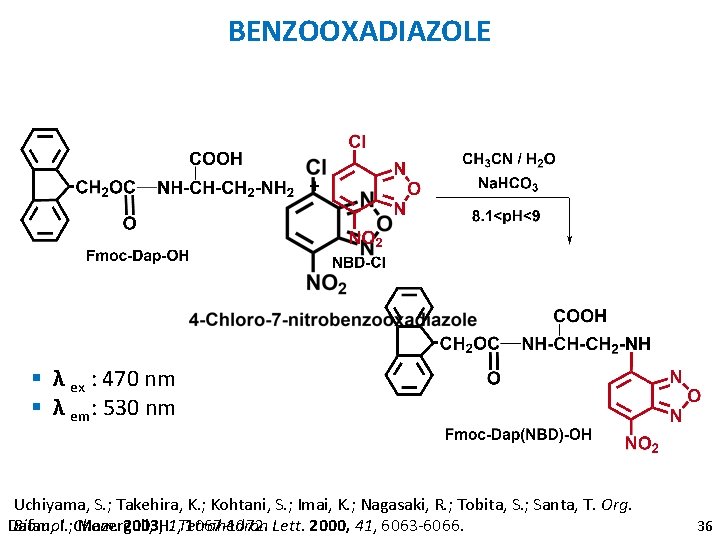
BENZOOXADIAZOLE § λ ex : 470 nm § λ em: 530 nm Uchiyama, S. ; Takehira, K. ; Kohtani, S. ; Imai, K. ; Nagasaki, R. ; Tobita, S. ; Santa, T. Org. Biomol. 2003, H. 1, Tetrahedron 1067 -1072. Lett. 2000, 41, 6063 -6066. Dafau, I. ; Chem. Mazerguil, 36
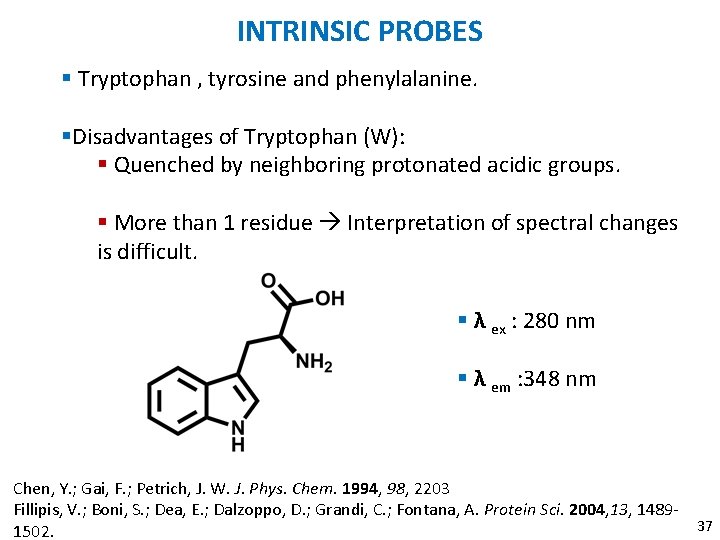
INTRINSIC PROBES § Tryptophan , tyrosine and phenylalanine. §Disadvantages of Tryptophan (W): § Quenched by neighboring protonated acidic groups. § More than 1 residue Interpretation of spectral changes is difficult. § λ ex : 280 nm § λ em : 348 nm Chen, Y. ; Gai, F. ; Petrich, J. W. J. Phys. Chem. 1994, 98, 2203 Fillipis, V. ; Boni, S. ; Dea, E. ; Dalzoppo, D. ; Grandi, C. ; Fontana, A. Protein Sci. 2004, 13, 148937 1502.
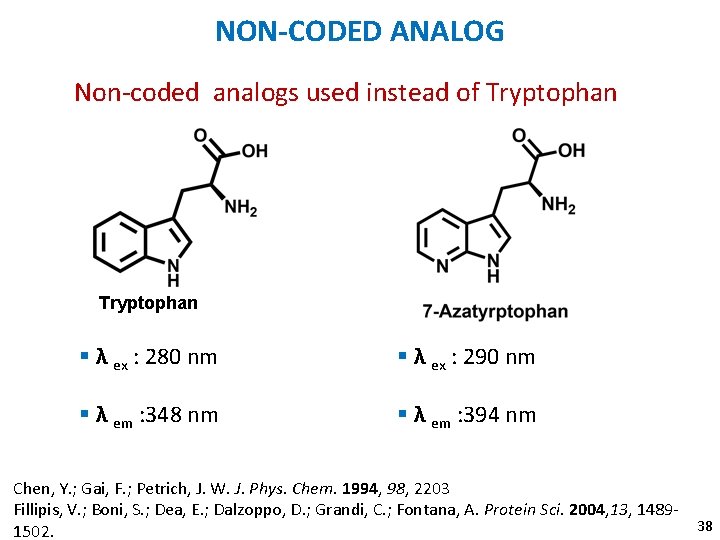
NON-CODED ANALOG Non-coded analogs used instead of Tryptophan § λ ex : 280 nm § λ ex : 290 nm § λ em : 348 nm § λ em : 394 nm Chen, Y. ; Gai, F. ; Petrich, J. W. J. Phys. Chem. 1994, 98, 2203 Fillipis, V. ; Boni, S. ; Dea, E. ; Dalzoppo, D. ; Grandi, C. ; Fontana, A. Protein Sci. 2004, 13, 148938 1502.
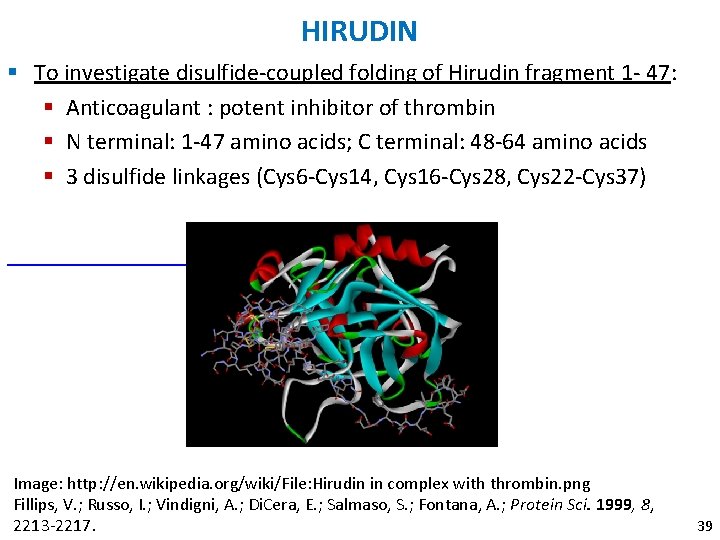
HIRUDIN § To investigate disulfide-coupled folding of Hirudin fragment 1 - 47: § Anticoagulant : potent inhibitor of thrombin § N terminal: 1 -47 amino acids; C terminal: 48 -64 amino acids § 3 disulfide linkages (Cys 6 -Cys 14, Cys 16 -Cys 28, Cys 22 -Cys 37) Image: http: //en. wikipedia. org/wiki/File: Hirudin in complex with thrombin. png Fillips, V. ; Russo, I. ; Vindigni, A. ; Di. Cera, E. ; Salmaso, S. ; Fontana, A. ; Protein Sci. 1999, 8, 2213 -2217. 39
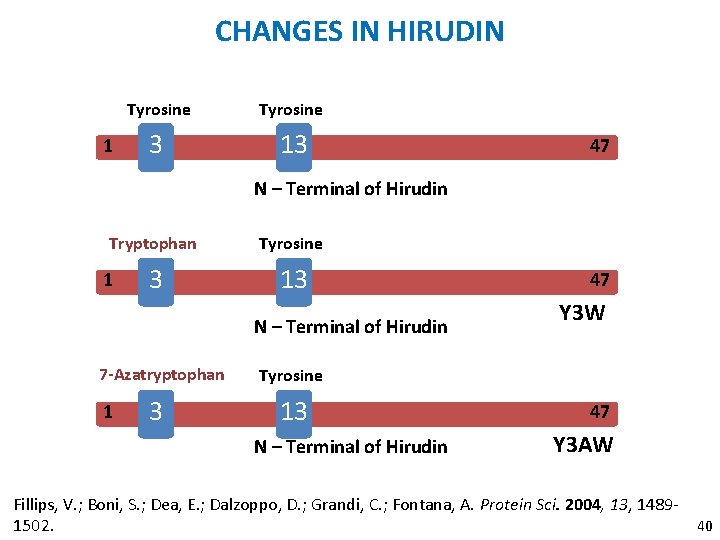
CHANGES IN HIRUDIN 1 Tyrosine 3 13 47 N – Terminal of Hirudin Tryptophan Tyrosine 3 13 1 N – Terminal of Hirudin 7 -Azatryptophan 1 3 47 Y 3 W Tyrosine 13 N – Terminal of Hirudin 47 Y 3 AW Fillips, V. ; Boni, S. ; Dea, E. ; Dalzoppo, D. ; Grandi, C. ; Fontana, A. Protein Sci. 2004, 13, 148940 1502.
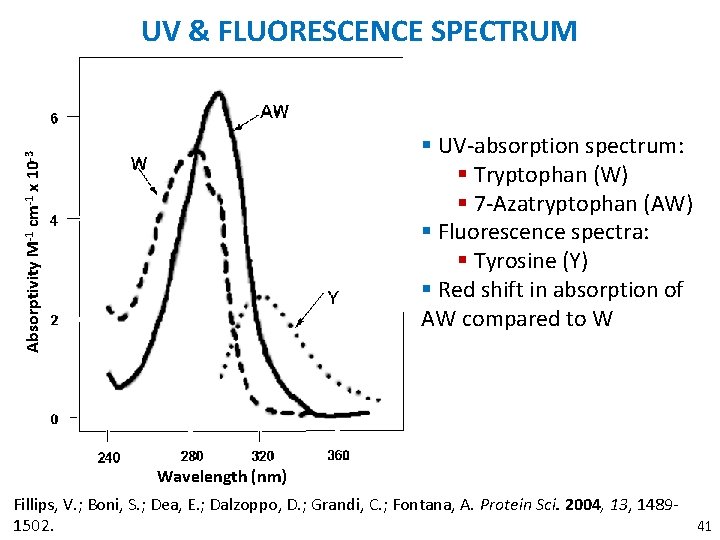
UV & FLUORESCENCE SPECTRUM Absorptivity M-1 cm-1 x 10 -3 § UV-absorption spectrum: § Tryptophan (W) § 7 -Azatryptophan (AW) § Fluorescence spectra: § Tyrosine (Y) § Red shift in absorption of AW compared to W Wavelength (nm) Fillips, V. ; Boni, S. ; Dea, E. ; Dalzoppo, D. ; Grandi, C. ; Fontana, A. Protein Sci. 2004, 13, 148941 1502.
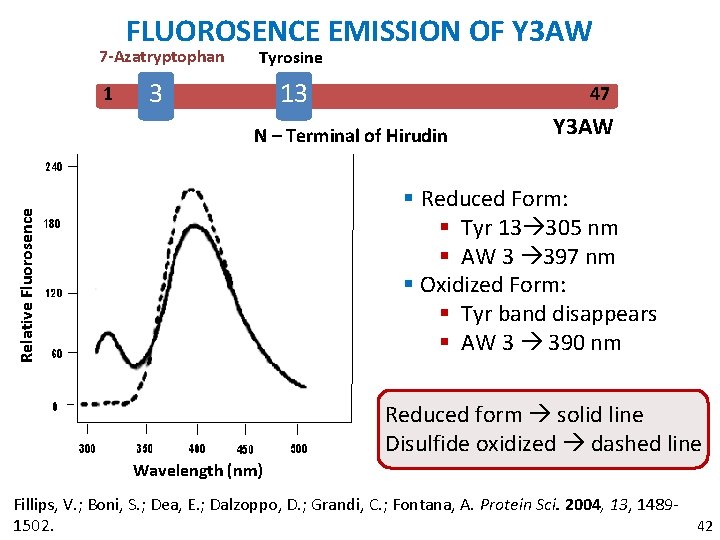
FLUOROSENCE EMISSION OF Y 3 AW 7 -Azatryptophan 1 Tyrosine 3 13 47 N – Terminal of Hirudin Y 3 AW Relative Fluorosence § Reduced Form: § Tyr 13 305 nm § AW 3 397 nm § Oxidized Form: § Tyr band disappears § AW 3 390 nm Reduced form solid line Disulfide oxidized dashed line Wavelength (nm) Fillips, V. ; Boni, S. ; Dea, E. ; Dalzoppo, D. ; Grandi, C. ; Fontana, A. Protein Sci. 2004, 13, 148942 1502.
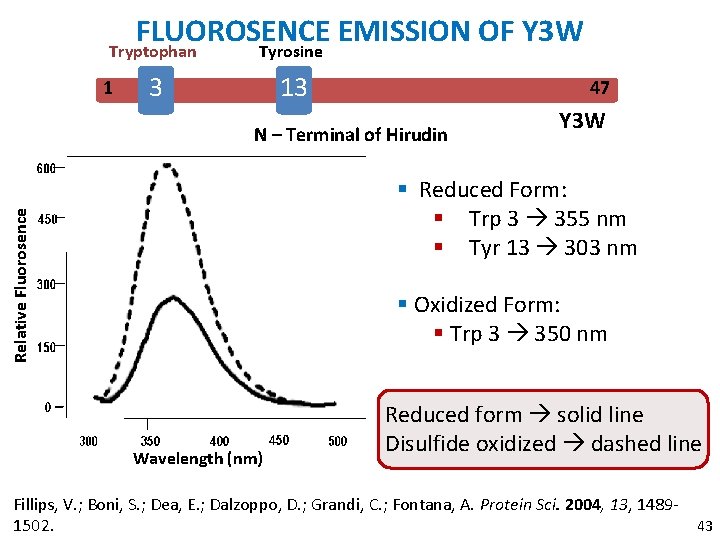
FLUOROSENCE EMISSION OF Y 3 W Tryptophan Tyrosine 3 13 1 47 N – Terminal of Hirudin Y 3 W Relative Fluorosence § Reduced Form: § Trp 3 355 nm § Tyr 13 303 nm § Oxidized Form: § Trp 3 350 nm Wavelength (nm) Reduced form solid line Disulfide oxidized dashed line Fillips, V. ; Boni, S. ; Dea, E. ; Dalzoppo, D. ; Grandi, C. ; Fontana, A. Protein Sci. 2004, 13, 148943 1502.
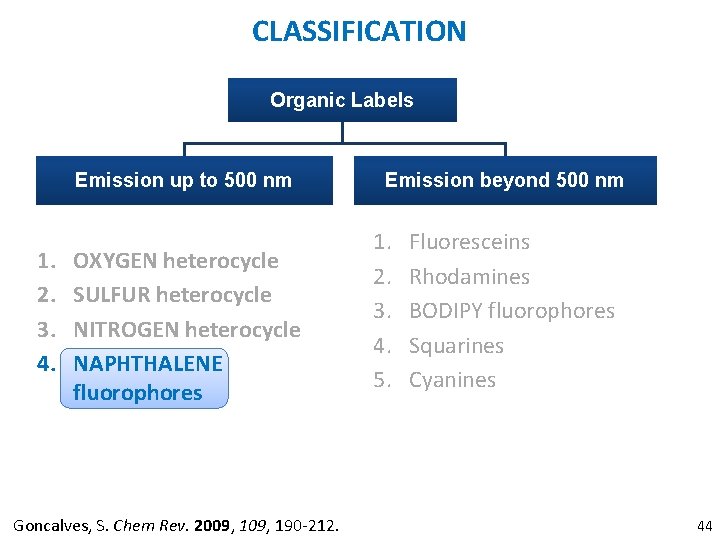
CLASSIFICATION Organic Labels Emission up to 500 nm 1. 2. 3. 4. OXYGEN heterocycle SULFUR heterocycle NITROGEN heterocycle NAPHTHALENE fluorophores Goncalves, S. Chem Rev. 2009, 190 -212. Emission beyond 500 nm 1. 2. 3. 4. 5. Fluoresceins Rhodamines BODIPY fluorophores Squarines Cyanines 44
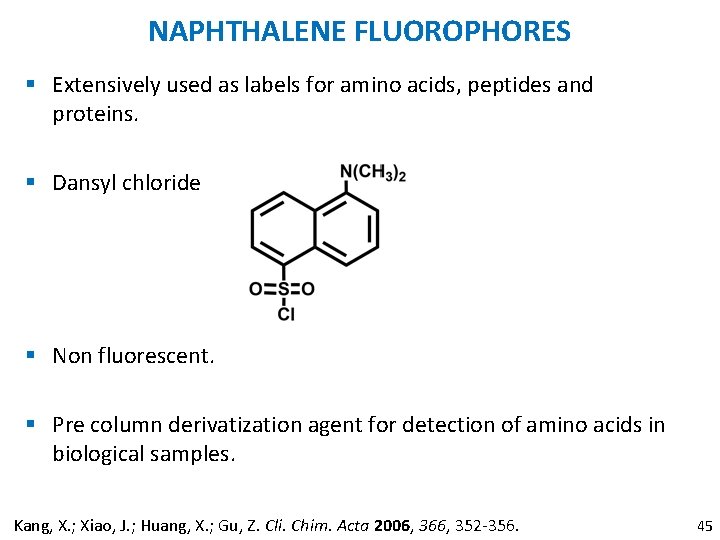
NAPHTHALENE FLUOROPHORES § Extensively used as labels for amino acids, peptides and proteins. § Dansyl chloride § Non fluorescent. § Pre column derivatization agent for detection of amino acids in biological samples. Kang, X. ; Xiao, J. ; Huang, X. ; Gu, Z. Cli. Chim. Acta 2006, 366, 352 -356. 45
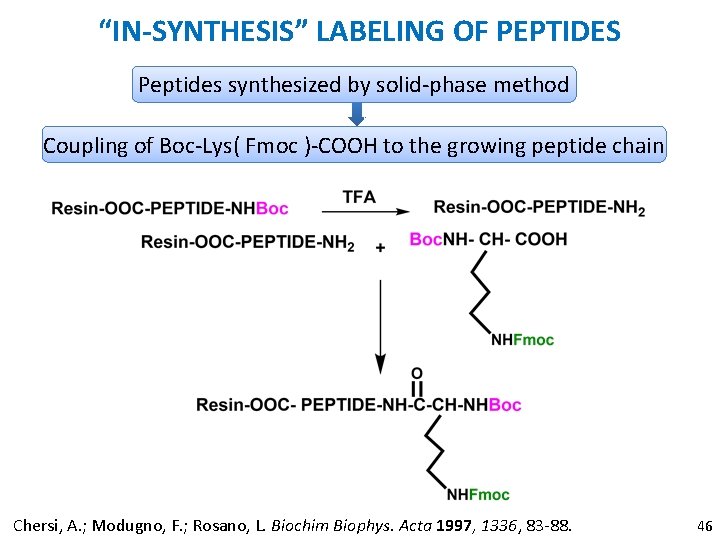
“IN-SYNTHESIS” LABELING OF PEPTIDES Peptides synthesized by solid-phase method Coupling of Boc-Lys( Fmoc )-COOH to the growing peptide chain Chersi, A. ; Modugno, F. ; Rosano, L. Biochim Biophys. Acta 1997, 1336, 83 -88. 46
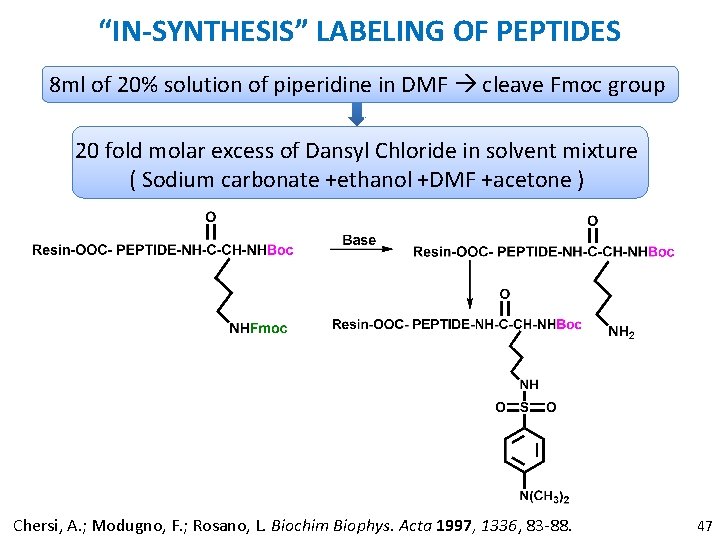
“IN-SYNTHESIS” LABELING OF PEPTIDES 8 ml of 20% solution of piperidine in DMF cleave Fmoc group 20 fold molar excess of Dansyl Chloride in solvent mixture ( Sodium carbonate +ethanol +DMF +acetone ) Chersi, A. ; Modugno, F. ; Rosano, L. Biochim Biophys. Acta 1997, 1336, 83 -88. 47
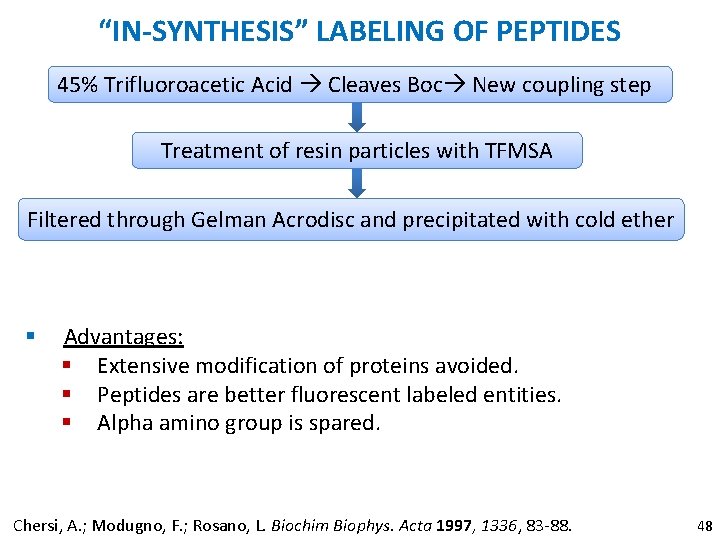
“IN-SYNTHESIS” LABELING OF PEPTIDES 45% Trifluoroacetic Acid Cleaves Boc New coupling step Treatment of resin particles with TFMSA Filtered through Gelman Acrodisc and precipitated with cold ether § Advantages: § Extensive modification of proteins avoided. § Peptides are better fluorescent labeled entities. § Alpha amino group is spared. Chersi, A. ; Modugno, F. ; Rosano, L. Biochim Biophys. Acta 1997, 1336, 83 -88. 48
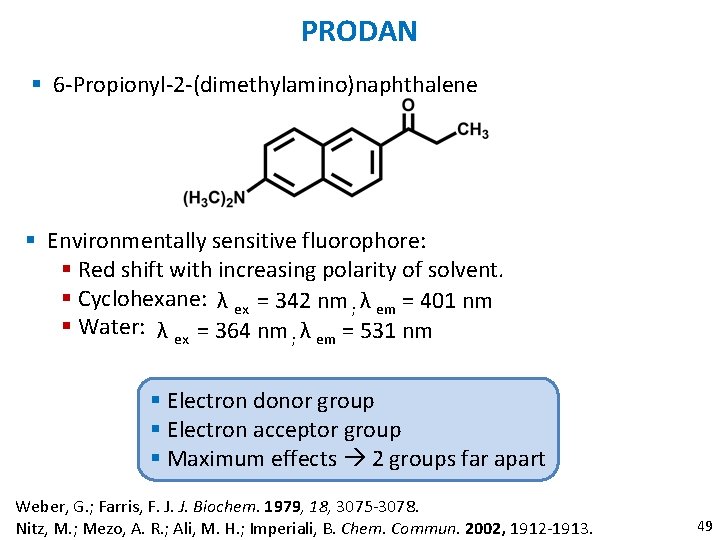
PRODAN § 6 -Propionyl-2 -(dimethylamino)naphthalene § Environmentally sensitive fluorophore: § Red shift with increasing polarity of solvent. § Cyclohexane: λ ex = 342 nm ; λ em = 401 nm § Water: λ ex = 364 nm ; λ em = 531 nm § Electron donor group § Electron acceptor group § Maximum effects 2 groups far apart Weber, G. ; Farris, F. J. J. Biochem. 1979, 18, 3075 -3078. Nitz, M. ; Mezo, A. R. ; Ali, M. H. ; Imperiali, B. Chem. Commun. 2002, 1912 -1913. 49
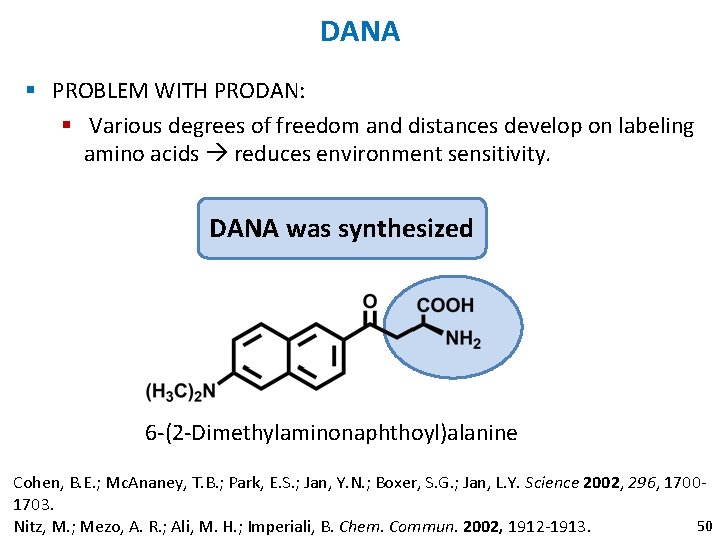
DANA § PROBLEM WITH PRODAN: § Various degrees of freedom and distances develop on labeling amino acids reduces environment sensitivity. DANA was synthesized 6 -(2 -Dimethylaminonaphthoyl)alanine Cohen, B. E. ; Mc. Ananey, T. B. ; Park, E. S. ; Jan, Y. N. ; Boxer, S. G. ; Jan, L. Y. Science 2002, 296, 17001703. 50 Nitz, M. ; Mezo, A. R. ; Ali, M. H. ; Imperiali, B. Chem. Commun. 2002, 1912 -1913.
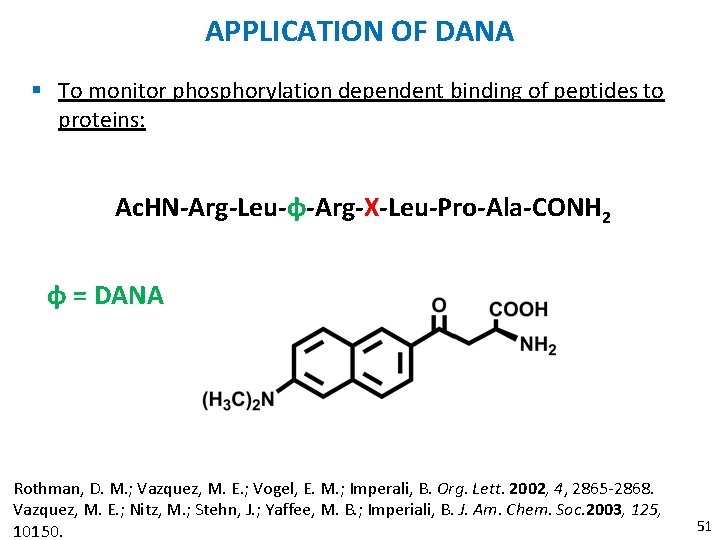
APPLICATION OF DANA § To monitor phosphorylation dependent binding of peptides to proteins: Ac. HN-Arg-Leu-ɸ-Arg-X-Leu-Pro-Ala-CONH 2 ɸ = DANA Rothman, D. M. ; Vazquez, M. E. ; Vogel, E. M. ; Imperali, B. Org. Lett. 2002, 4, 2865 -2868. Vazquez, M. E. ; Nitz, M. ; Stehn, J. ; Yaffee, M. B. ; Imperiali, B. J. Am. Chem. Soc. 2003, 125, 10150. 51
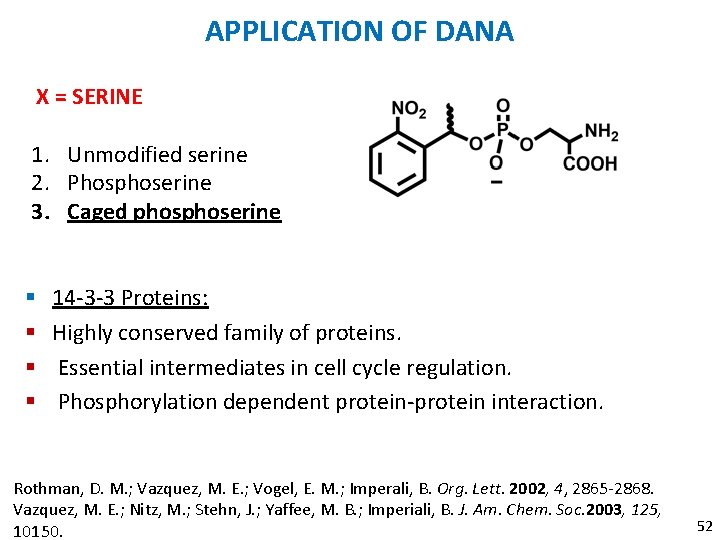
APPLICATION OF DANA X = SERINE 1. Unmodified serine 2. Phosphoserine 3. Caged phoserine § § 14 -3 -3 Proteins: Highly conserved family of proteins. Essential intermediates in cell cycle regulation. Phosphorylation dependent protein-protein interaction. Rothman, D. M. ; Vazquez, M. E. ; Vogel, E. M. ; Imperali, B. Org. Lett. 2002, 4, 2865 -2868. Vazquez, M. E. ; Nitz, M. ; Stehn, J. ; Yaffee, M. B. ; Imperiali, B. J. Am. Chem. Soc. 2003, 125, 10150. 52
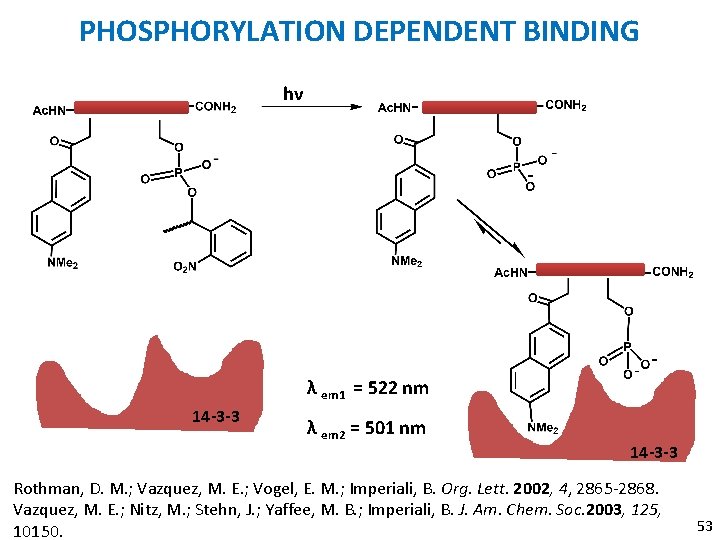
PHOSPHORYLATION DEPENDENT BINDING hν λ em 1 = 522 nm 14 -3 -3 λ em 2 = 501 nm 14 -3 -3 Rothman, D. M. ; Vazquez, M. E. ; Vogel, E. M. ; Imperiali, B. Org. Lett. 2002, 4, 2865 -2868. Vazquez, M. E. ; Nitz, M. ; Stehn, J. ; Yaffee, M. B. ; Imperiali, B. J. Am. Chem. Soc. 2003, 125, 10150. 53
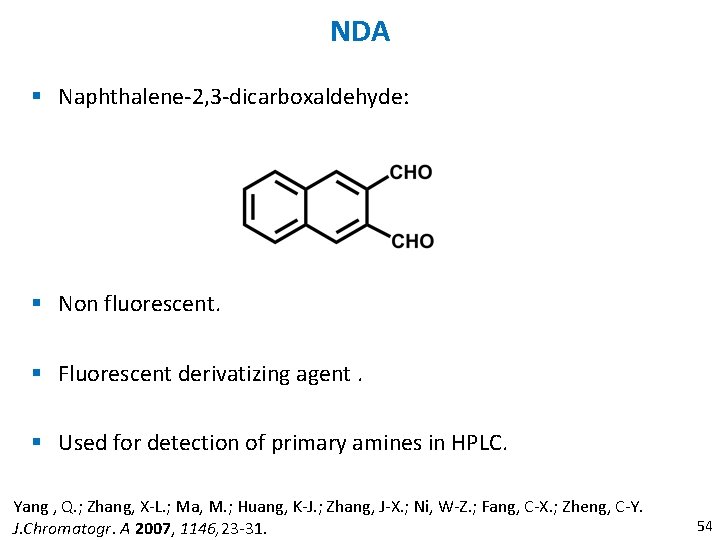
NDA § Naphthalene-2, 3 -dicarboxaldehyde: § Non fluorescent. § Fluorescent derivatizing agent. § Used for detection of primary amines in HPLC. Yang , Q. ; Zhang, X-L. ; Ma, M. ; Huang, K-J. ; Zhang, J-X. ; Ni, W-Z. ; Fang, C-X. ; Zheng, C-Y. J. Chromatogr. A 2007, 1146, 23 -31. 54
DETECTION OF DEGRADATION PRODUCTS OF MELANIN § Melanin: §Color of skin, eye and hair in mammals is due to melanin. § Uses: § Powerful antioxidant. § Photo protective pigment. § Free radical scavengers. Yang , Q. ; Zhang, X-L. ; Ma, M. ; Huang, K-J. ; Zhang, J-X. ; Ni, W-Z. ; Fang, C-X. ; Zheng, C-Y. J. Chromatogr. A 2007, 1146, 23 -31. 55
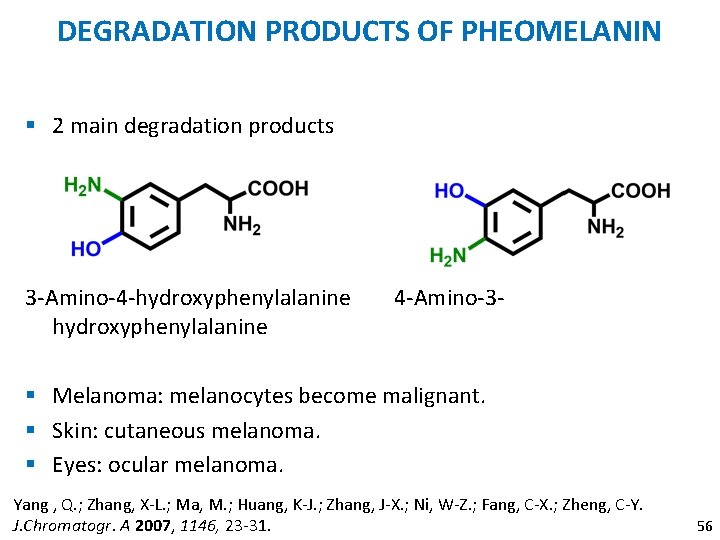
DEGRADATION PRODUCTS OF PHEOMELANIN § 2 main degradation products 3 -Amino-4 -hydroxyphenylalanine 4 -Amino-3 - § Melanoma: melanocytes become malignant. § Skin: cutaneous melanoma. § Eyes: ocular melanoma. Yang , Q. ; Zhang, X-L. ; Ma, M. ; Huang, K-J. ; Zhang, J-X. ; Ni, W-Z. ; Fang, C-X. ; Zheng, C-Y. J. Chromatogr. A 2007, 1146, 23 -31. 56
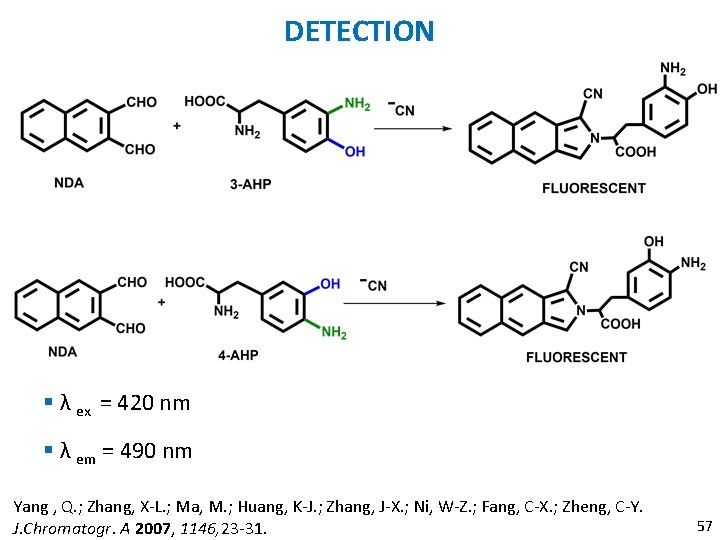
DETECTION § λ ex = 420 nm § λ em = 490 nm Yang , Q. ; Zhang, X-L. ; Ma, M. ; Huang, K-J. ; Zhang, J-X. ; Ni, W-Z. ; Fang, C-X. ; Zheng, C-Y. J. Chromatogr. A 2007, 1146, 23 -31. 57
SUMMARY § Biomolecules important mediators of various physiological processes § Number of short comings in the methods to detect biomolecules, fluorescent labels were used. § Compounds with Oxygen heterocycle, Sulfur heterocycle, Nitrogen heterocycle and Naphthalene were used as organic fluorescent labels. § Fluorophore + Biomolecules = Fluorescent derivative § Use: § Pre-column derivatization agent § Metabolic products § Degradation products 58

ACKNOWLEDGEMENTS § Dr. Yan Zhang § The Zhang Group § Department of Medicinal Chemistry, School of Pharmacy, Virginia Commonwealth University. 59
- Slides: 59