Fluorescent imaging of Zinc in rat hippocampus Chintha
















- Slides: 33

Fluorescent imaging of Zinc in rat hippocampus Chintha Bastian Dr. Yang Li

Zinc in the brain Brief review most of which was discussed by Josh Ketterman n Indicators used for imaging n

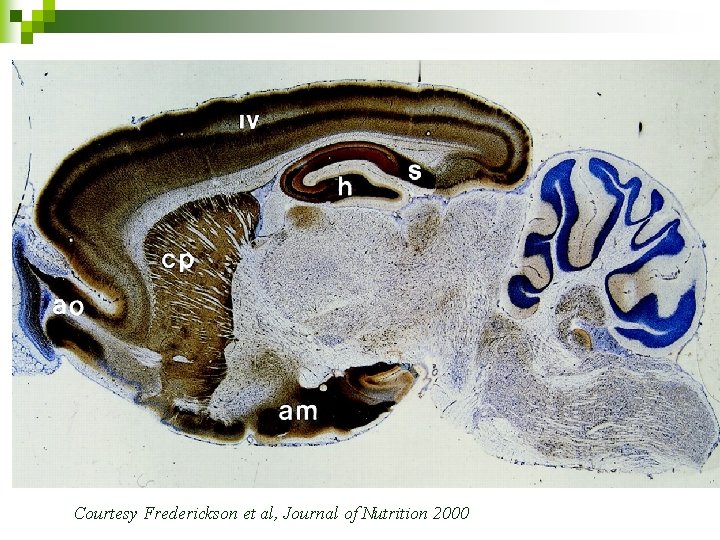
Overview n Histochemically reactive Zn 2+ is found in a subset of glutamatergic nerve terminals throughout mammalian cortex and limbic region (Frederickson et al. 2000) n Studies have shown most of the histochemically staining Zn 2+ localized within synaptic vesicles of glutamatergic neurons. (Frederickson et al. 1983; Huang 1967; Perez- Clausell and Danscher 1985)

Courtesy Frederickson et al, Journal of Nutrition 2000

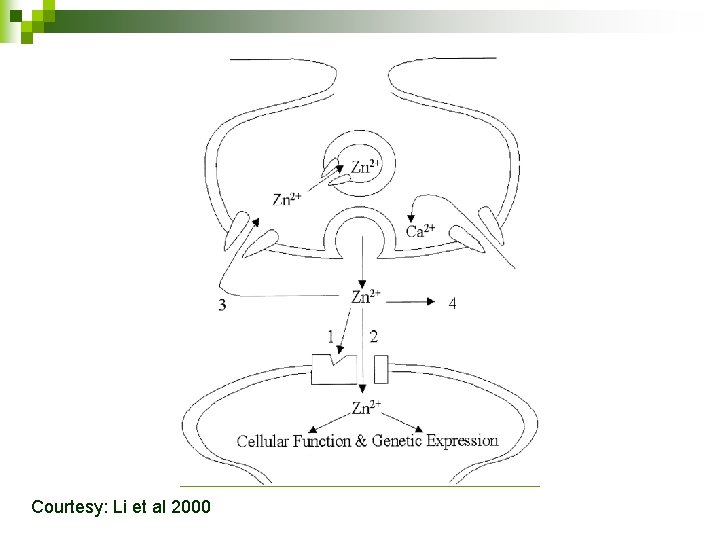
Rapid Translocation of Zn 2+ From Presynaptic Terminals Into Postsynaptic Hippocampal Neurons After Physiological Stimulation Yang Li, Christopher J. Hough, Sang Won Suh, John M. Sarvey and Christopher J. Frederickson Journal of Neurophysiology • VOL 86 • NOVEMBER 2001

Why do we study the role of Zinc in our lab? Zinc has been indicated in many pathological processes in the brain like n Epilepsy n Alzheimer's disease n Ischemia to the brain n Also, LTP and Memory!

Experimental animals Adult male Sprague Dawley rats n Weight >150 g n Brain slices prepared using a Vibratome n

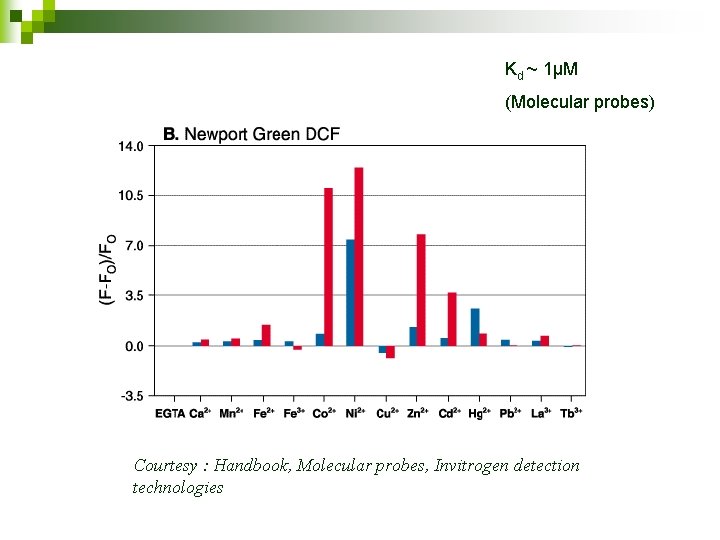
Newport green dipotassium salt cell impermeable for extracellular imaging n Dissociation constant Kd = ~1μM n Sensitive to Zn 2+ n

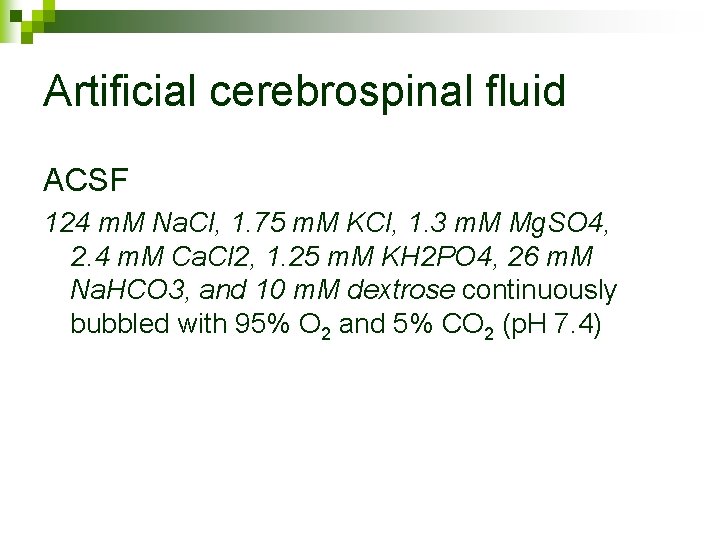
Artificial cerebrospinal fluid ACSF 124 m. M Na. Cl, 1. 75 m. M KCl, 1. 3 m. M Mg. SO 4, 2. 4 m. M Ca. Cl 2, 1. 25 m. M KH 2 PO 4, 26 m. M Na. HCO 3, and 10 m. M dextrose continuously bubbled with 95% O 2 and 5% CO 2 (p. H 7. 4)

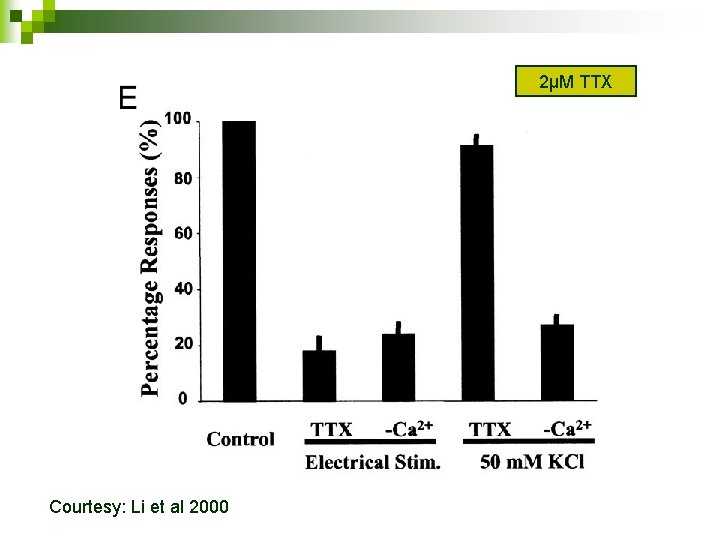
Stimulation parameters used 0. 5 -m. A pulses at 100 Hz for 5 sec. n

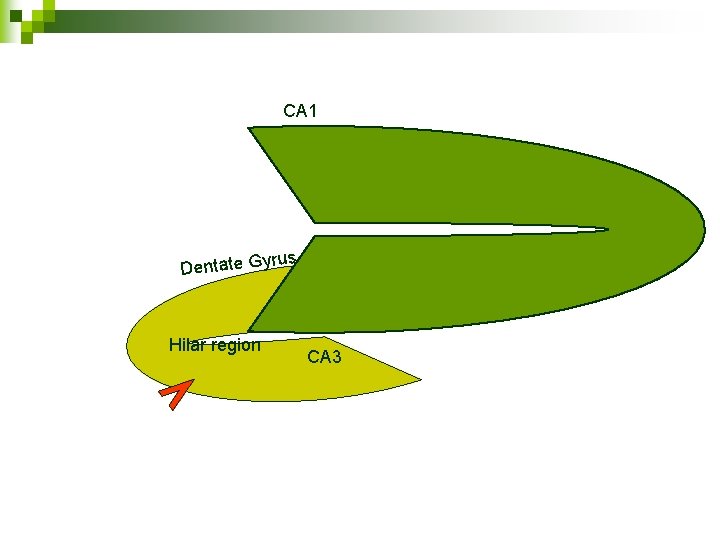
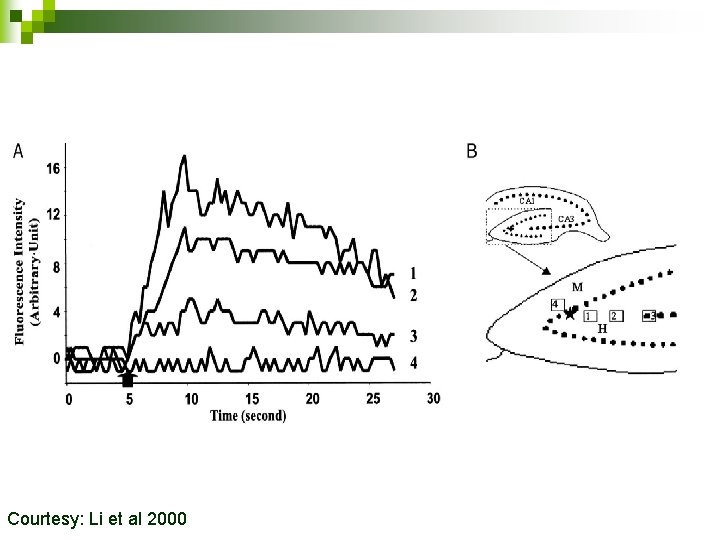
CA 1 rus Dentate Gy Hilar region CA 3

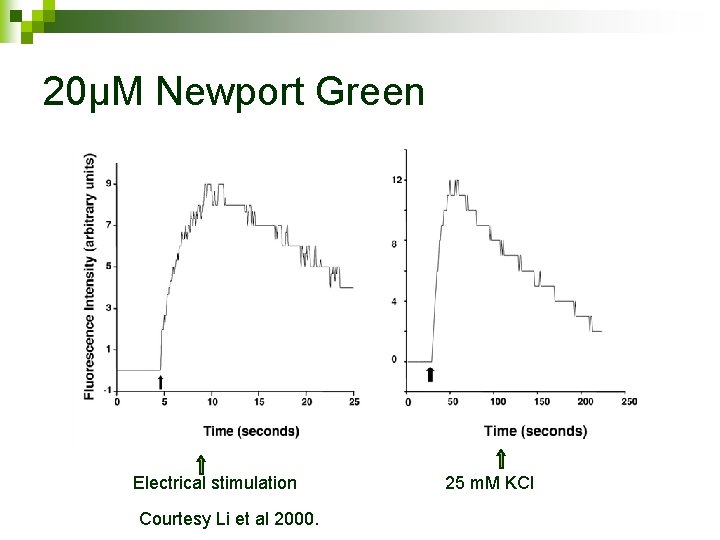
20µM Newport Green Electrical stimulation Courtesy Li et al 2000. 25 m. M KCl

Stimulation induced release of Zinc when perfused with 20µM Newport Green Courtesy: Li et al 2000

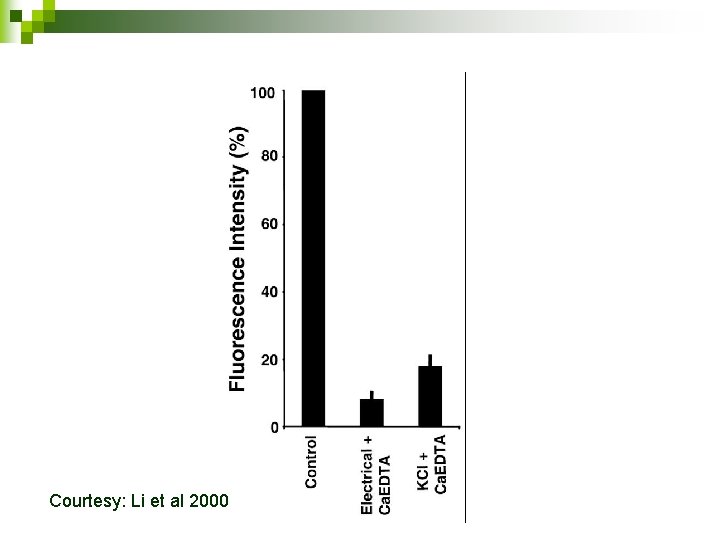
Courtesy: Li et al 2000

Courtesy: Li et al 2000

2µM TTX Courtesy: Li et al 2000

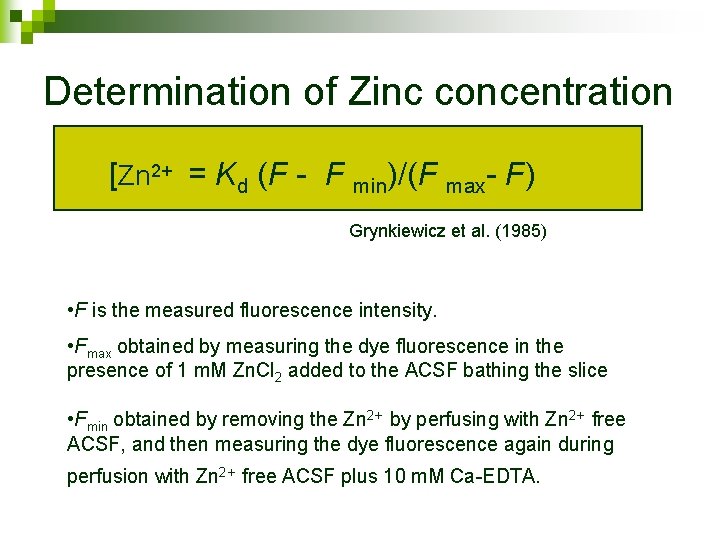
Determination of Zinc concentration [Zn 2+ = Kd (F - F min)/(F max- F) Grynkiewicz et al. (1985) • F is the measured fluorescence intensity. • Fmax obtained by measuring the dye fluorescence in the presence of 1 m. M Zn. Cl 2 added to the ACSF bathing the slice • Fmin obtained by removing the Zn 2+ by perfusing with Zn 2+ free ACSF, and then measuring the dye fluorescence again during perfusion with Zn 2+ free ACSF plus 10 m. M Ca-EDTA.

Intracellular Zinc imaging Newport Green diacetate 50µM, 0. 1% pluronic acid and 0. 5% DMSO for one hour n NG washed out with ACSF n

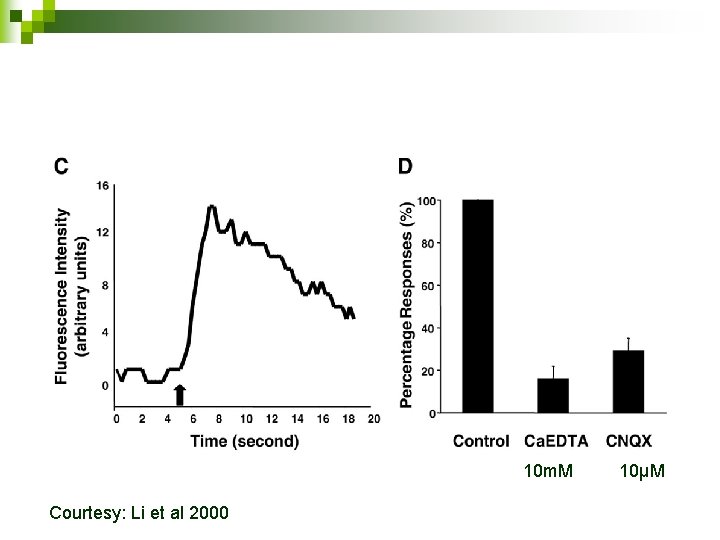
NG diacetate and stimulation (100 Hz for 10 sec) Courtesy: Li et al 2000

10 m. M Courtesy: Li et al 2000 10µM

Courtesy: Li et al 2000

Drawbacks of the paper Calcium EDTA 10 m. M Why? n Graph obtained after adding KCl to extracellular Newport Green solution n Incubated the slices in Newport Green for half an hour n

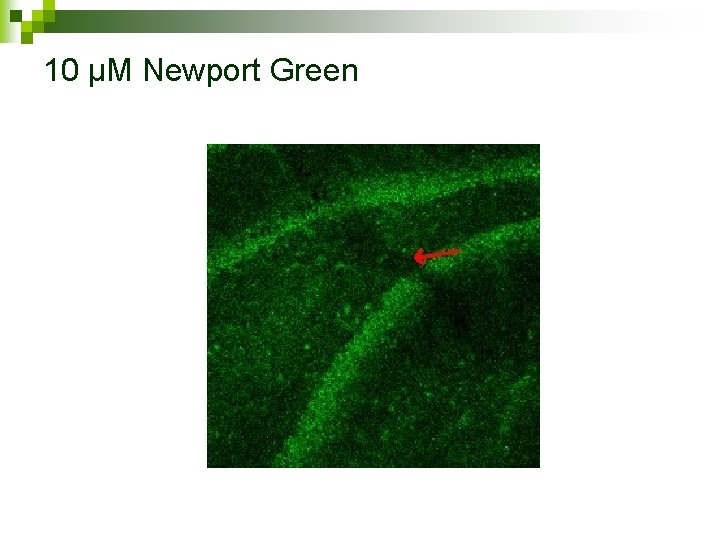
Current research Zeiss LSM 510 laser scanning inverted Confocal Microscope n 10 µM Newport Green (impermeant) shorter incubation time n 100 Hz for 10 sec n Comparing action of other dyes n

10 µM Newport Green

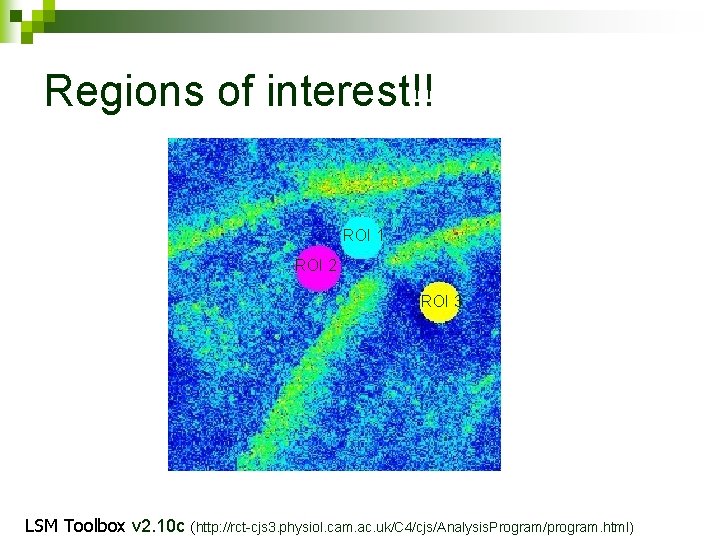
Regions of interest!! ROI 1 ROI 2 ROI 3 LSM Toolbox v 2. 10 c (http: //rct-cjs 3. physiol. cam. ac. uk/C 4/cjs/Analysis. Program/program. html)

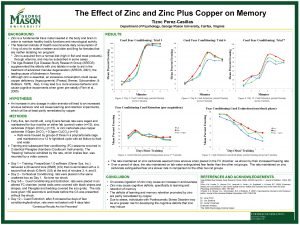
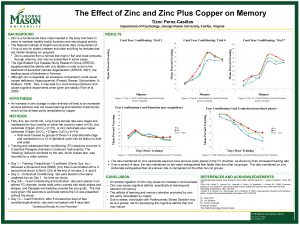
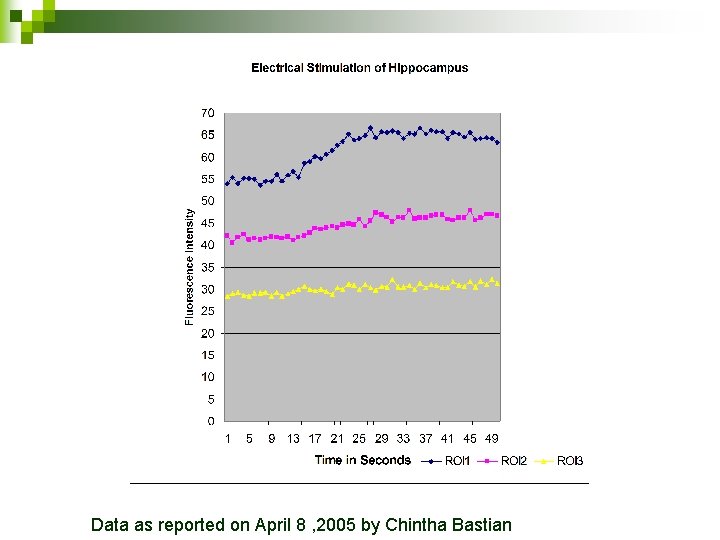
Data as reported on April 8 , 2005 by Chintha Bastian

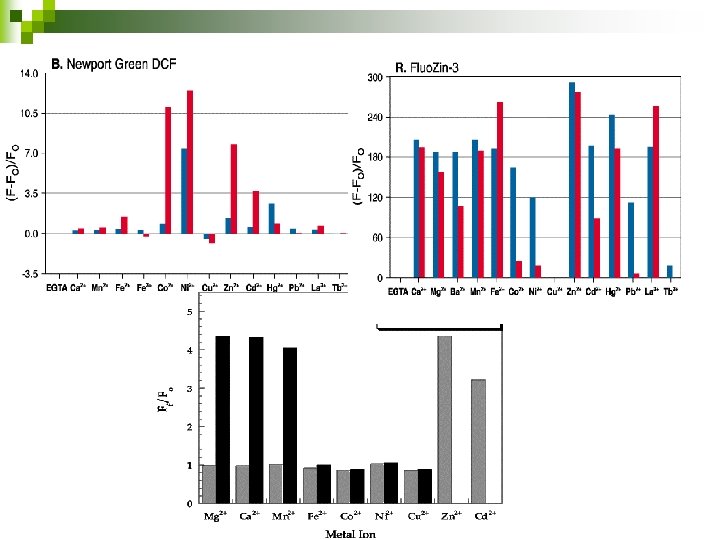
Kd ~ 1µM (Molecular probes) Courtesy : Handbook, Molecular probes, Invitrogen detection technologies

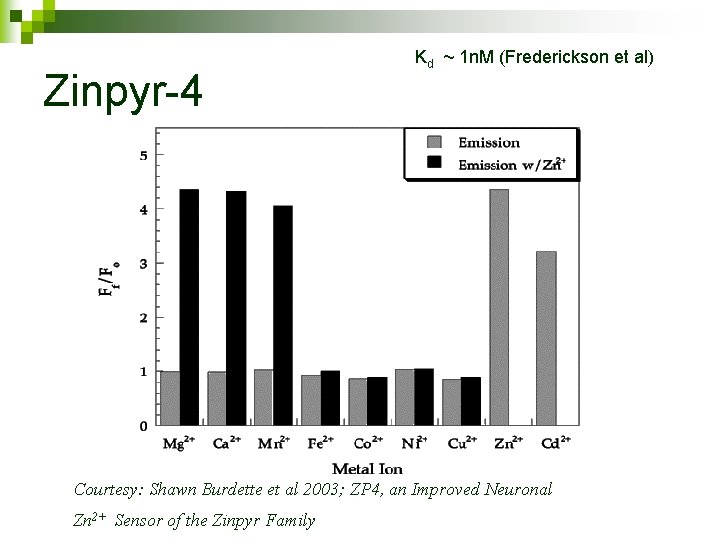
Zinpyr-4 Kd ~ 1 n. M (Frederickson et al) Courtesy: Shawn Burdette et al 2003; ZP 4, an Improved Neuronal Zn 2+ Sensor of the Zinpyr Family

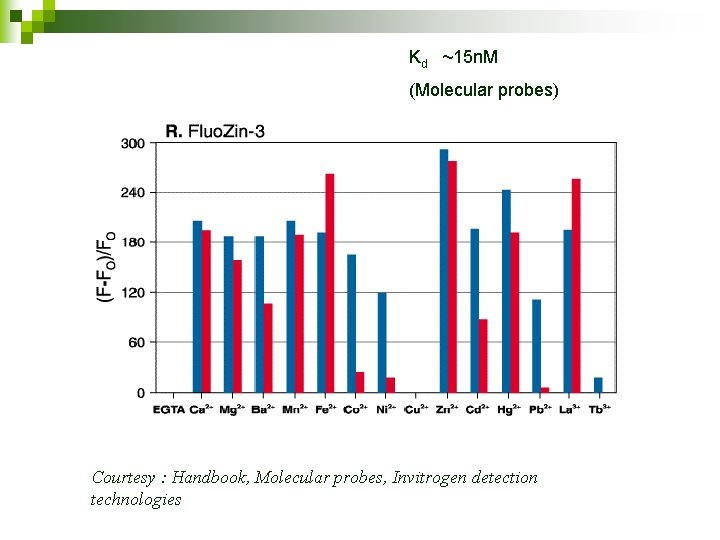
Kd ~15 n. M (Molecular probes) Courtesy : Handbook, Molecular probes, Invitrogen detection technologies


Future studies Role of Zinc in epilepsy causative , contributive or curative? n Recurrent mossy fibers releasing Zinc in the molecular layer n Translocation of Zinc n

Thanks! Dr. Yang Li n Josh Ketterman n Christian Stork n Jennifer Martin n Yanli Ding n

Questions? ? ?
 Example of a chemical change
Example of a chemical change Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Frc control system
Frc control system Rat bag and bad rat
Rat bag and bad rat Trisynaptic circuit
Trisynaptic circuit Stria olfactoria medialis
Stria olfactoria medialis Hippocampus funkcia
Hippocampus funkcia Spatial cognition examples
Spatial cognition examples Blood supply of brain
Blood supply of brain Hippocampus reading foundation
Hippocampus reading foundation Hippocampus mnemonic
Hippocampus mnemonic Fluorescent labeling
Fluorescent labeling Zoe fluorescent cell imager
Zoe fluorescent cell imager Fluorescent optic yellow sign meaning
Fluorescent optic yellow sign meaning Flourescent optic yellow signs
Flourescent optic yellow signs Compact fluorescent lamp
Compact fluorescent lamp Fluorometer
Fluorometer Diamond zinc blende structure
Diamond zinc blende structure Dr junaid mughal
Dr junaid mughal Occurrence of zinc
Occurrence of zinc Zinc finger structure
Zinc finger structure Electrolyse iodure de zinc
Electrolyse iodure de zinc Zinc dissolves in hydrochloric acid to yield hydrogen gas
Zinc dissolves in hydrochloric acid to yield hydrogen gas Dot
Dot Zinc toxicity
Zinc toxicity Minizinc examples
Minizinc examples Base cavitaria
Base cavitaria Zinc ethyl silicate primer
Zinc ethyl silicate primer Magnesium + copper oxide
Magnesium + copper oxide Deficiencia de zinc en café
Deficiencia de zinc en café Mucocompressive impression material examples
Mucocompressive impression material examples Zinc phosphate cement setting reaction
Zinc phosphate cement setting reaction Diarrhea in children
Diarrhea in children Disadvantages of flotation technique
Disadvantages of flotation technique