Fluorescence Fluorescent corals Jablonski Diagram Fluorescence From v
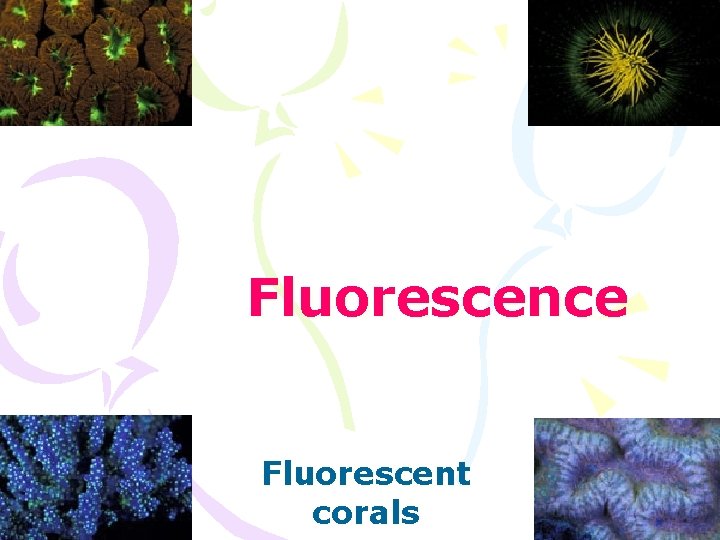
Fluorescence Fluorescent corals
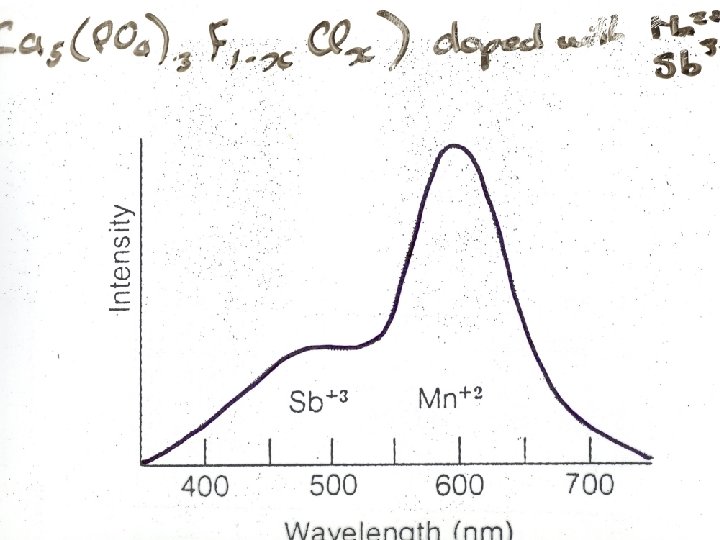
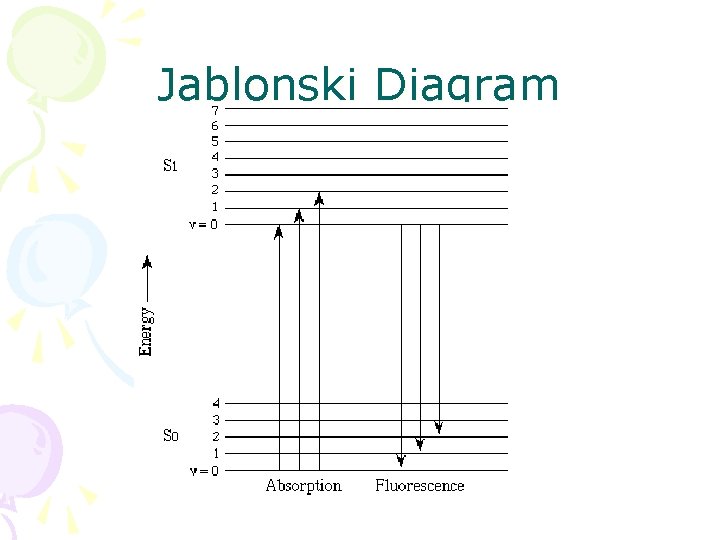
Jablonski Diagram

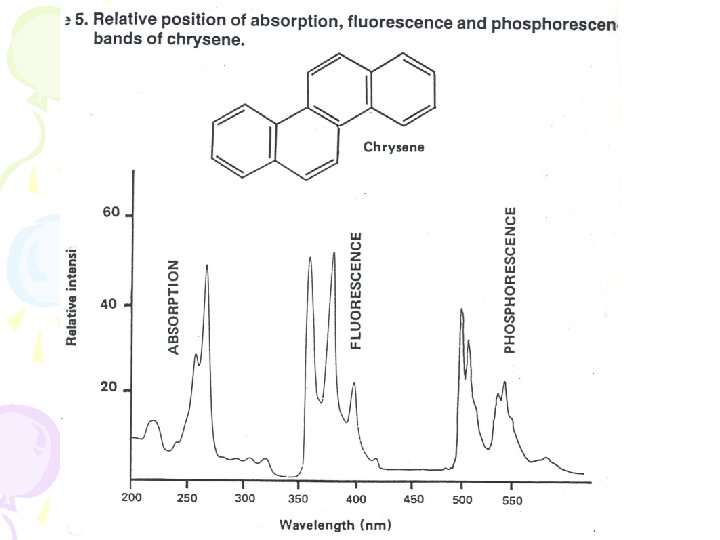
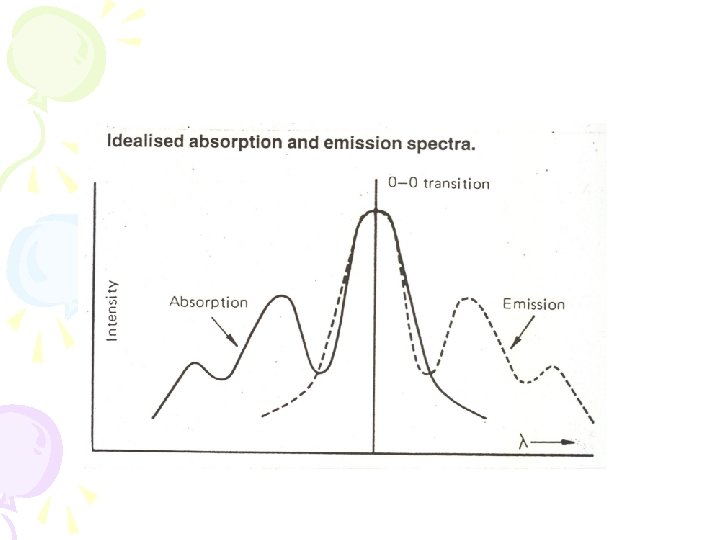
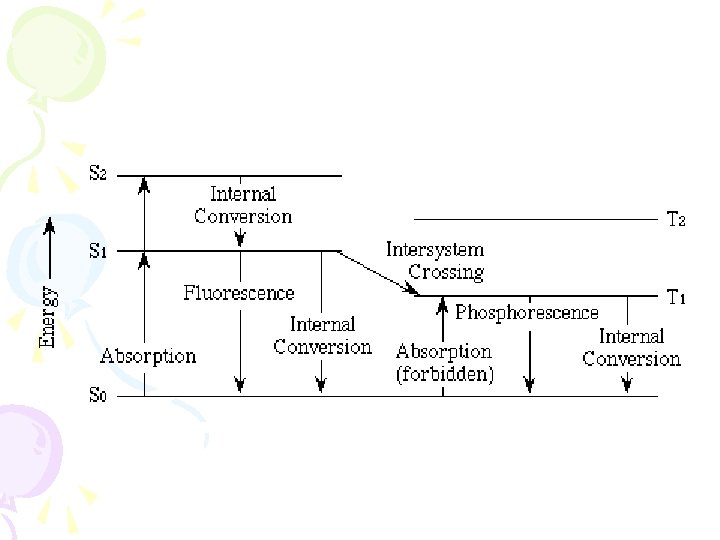
• Fluorescence • From v = 0 down • Absorption • From v = 0 up • So expect the emission and absorption spectra to overlap here • Mostly don’t – because of changes of energy due to solvent interactions
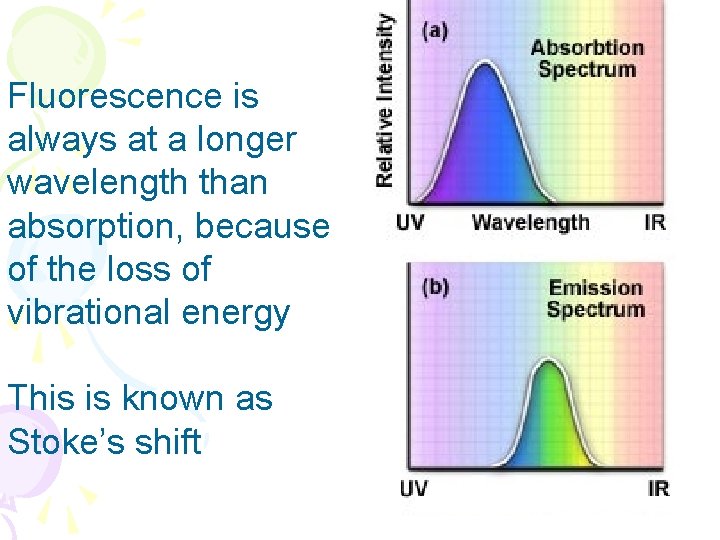
Fluorescence is always at a longer wavelength than absorption, because of the loss of vibrational energy This is known as Stoke’s shift
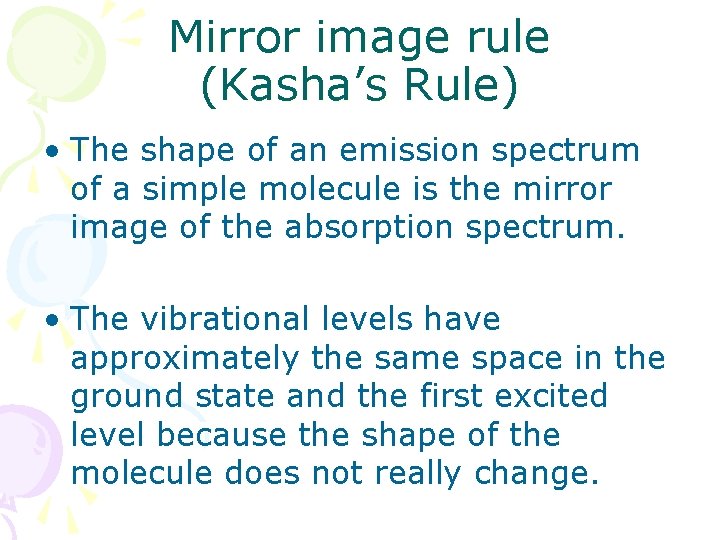
Mirror image rule (Kasha’s Rule) • The shape of an emission spectrum of a simple molecule is the mirror image of the absorption spectrum. • The vibrational levels have approximately the same space in the ground state and the first excited level because the shape of the molecule does not really change.
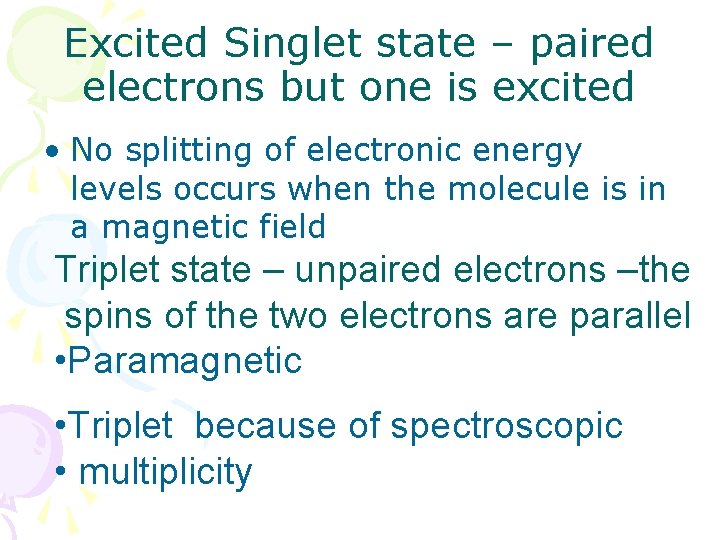
Excited Singlet state – paired electrons but one is excited • No splitting of electronic energy levels occurs when the molecule is in a magnetic field Triplet state – unpaired electrons –the spins of the two electrons are parallel • Paramagnetic • Triplet because of spectroscopic • multiplicity
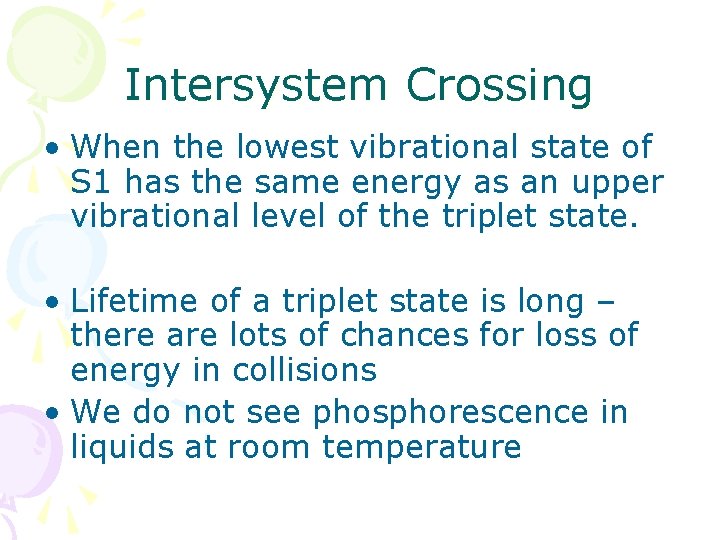
Intersystem Crossing • When the lowest vibrational state of S 1 has the same energy as an upper vibrational level of the triplet state. • Lifetime of a triplet state is long – there are lots of chances for loss of energy in collisions • We do not see phosphorescence in liquids at room temperature
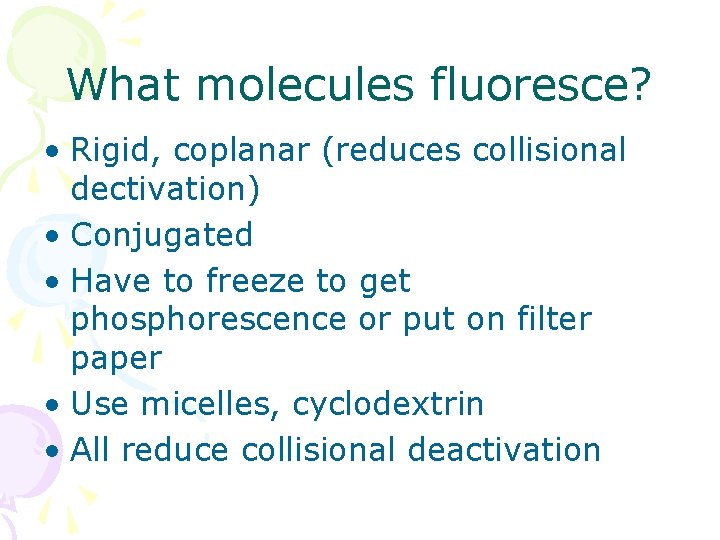
What molecules fluoresce? • Rigid, coplanar (reduces collisional dectivation) • Conjugated • Have to freeze to get phosphorescence or put on filter paper • Use micelles, cyclodextrin • All reduce collisional deactivation
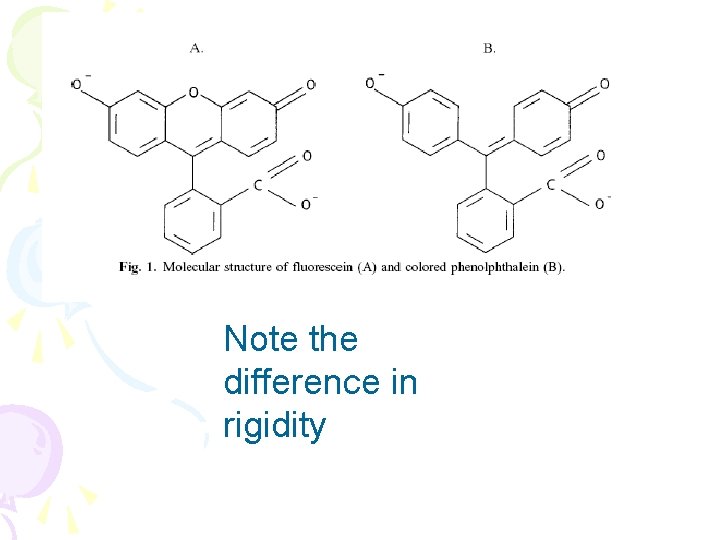
Note the difference in rigidity

Applications
Advantages of Fluorescence over Absorption • Greater selectivity and freedom from spectral interferences – Fewer species which luminesce – Can vary the absorption (excitation) and emission wavelengths • Lower LOD than Absorption for same compound • F is linear with conc over 3 -4 orders of magnitude
Lower LOD than Absorption for same compound • Fluorescence is read directly by detector • Absorption is a ratio • F is linear with conc over 3 -4 orders of magnitude (extending to lower conc range)
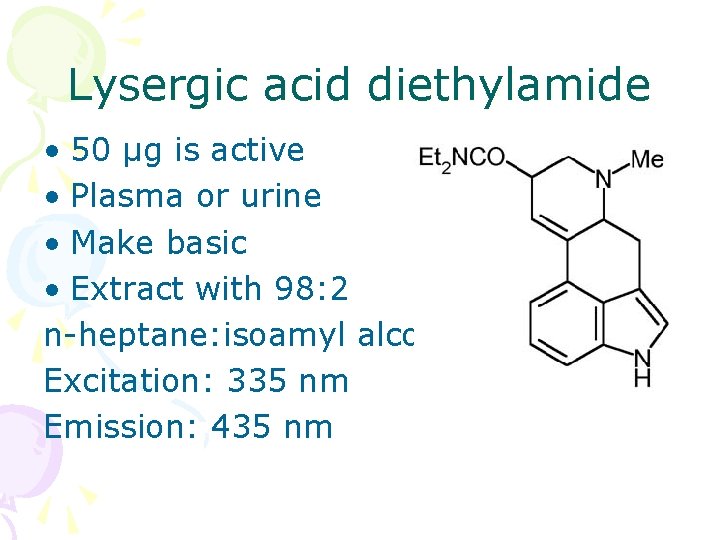
Lysergic acid diethylamide • 50 µg is active • Plasma or urine • Make basic • Extract with 98: 2 n-heptane: isoamyl alcohol Excitation: 335 nm Emission: 435 nm

Phosphorescence • • Radiative relaxation from T 1 to G Is forbidden – so has long lifetime 10 -6 – 10 sec To make a glow-in-the-dark toy, what you want is a phosphor that is energized by normal light and that has a very long persistence. Two phosphors that have these properties are Zinc Sulfide and Strontium Aluminate is newer -- it's what you see in the "super" glow-in-the-dark toys. It has a much longer persistence than Zinc Sulfide does. The phosphor is mixed into a plastic and molded to make most glow-in-the-dark stuff.
Phosphorescence • Occurs in solids • Which may be frozen solvents • Reduces the number of collisions • Paramagnetic species increase the likelihood of intersystem crossing • So reduce fluorescence and phosphorescence
Shape of Emission spectrum • Does not change with excitation wavelength • BUT the intensity changes • The most fluorescence will occur when a lot of light is absorbed • Can find an excitation λ by running an absorption spectrum • Use this to find λemmax and then λexmax
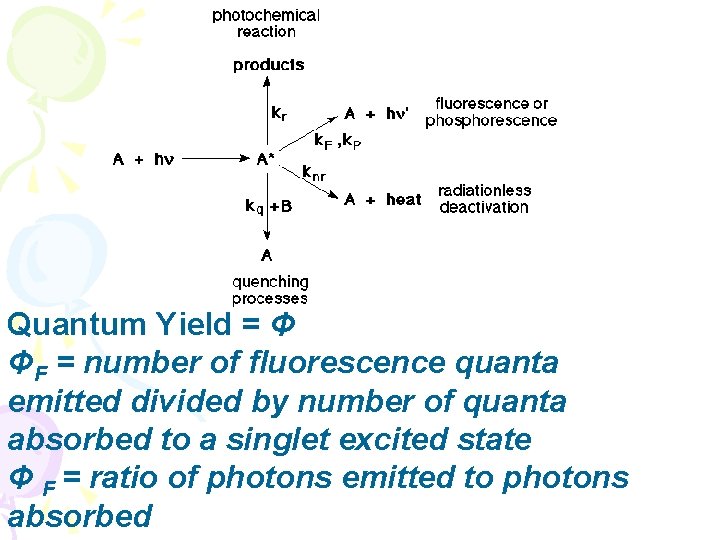
Quantum Yield = Φ ΦF = number of fluorescence quanta emitted divided by number of quanta absorbed to a singlet excited state Φ F = ratio of photons emitted to photons absorbed
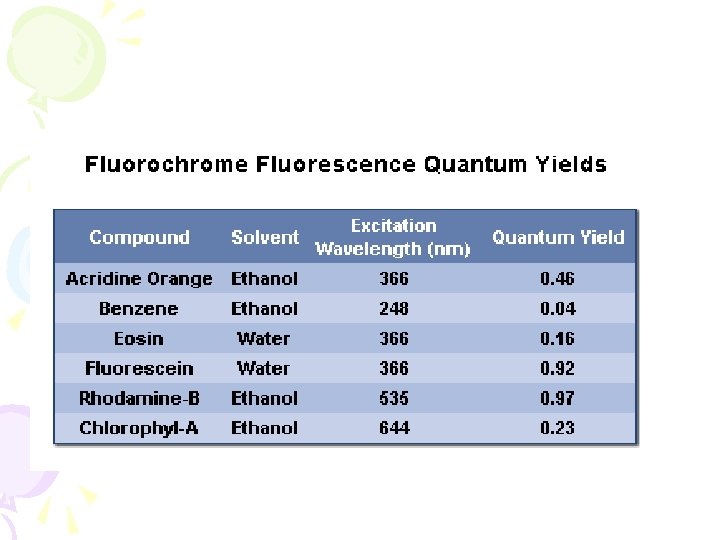
Quenching
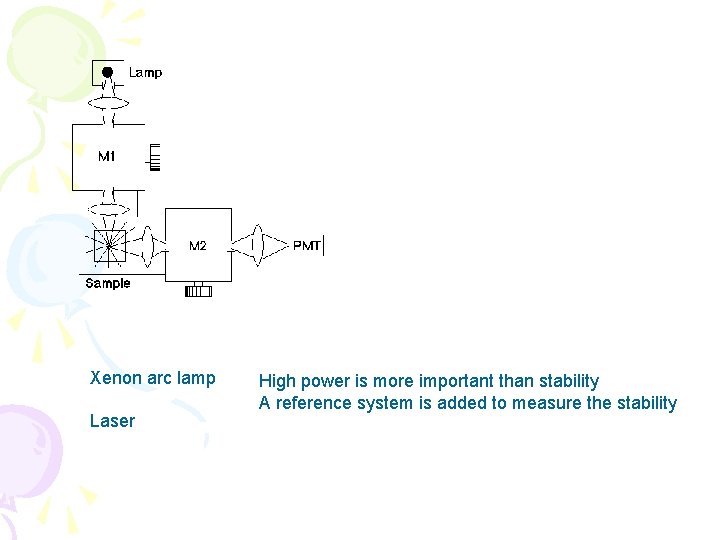
Xenon arc lamp Laser High power is more important than stability A reference system is added to measure the stability

Xenon Arc Lamp • Unstable • Some portion of initial light goes to reference detector to ratio with F signal to compensate for changes in lamp intensity • Sometimes a fluorescent standard of rhodamine is included • May have to restrict intensity of light to minimize sample decomposition (photobleaching)

• Sources of UV produce ozone. Fan disperses this and cools lamp. • Ozone is toxic but also absorbs certain wavelengths • Detector at right angles to lamp • Two wavelength selectors • Slits: narrow for high resolution • Wider(5 -10 nm) to give greater sensitivity
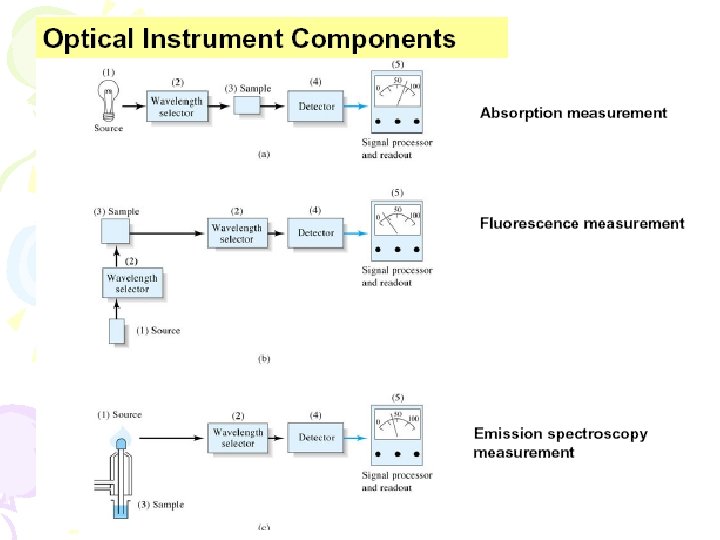
![L = k[P 0 – P] P = P 0 10 -abc (Beer’s law) L = k[P 0 – P] P = P 0 10 -abc (Beer’s law)](http://slidetodoc.com/presentation_image_h/c9407ee60c0f020e07aa7cc861e28240/image-29.jpg)
L = k[P 0 – P] P = P 0 10 -abc (Beer’s law) = k[P 0 – P 0 10 -abc] = k. P 0[1 – 10 -abc] Note: L is proportional to P 0
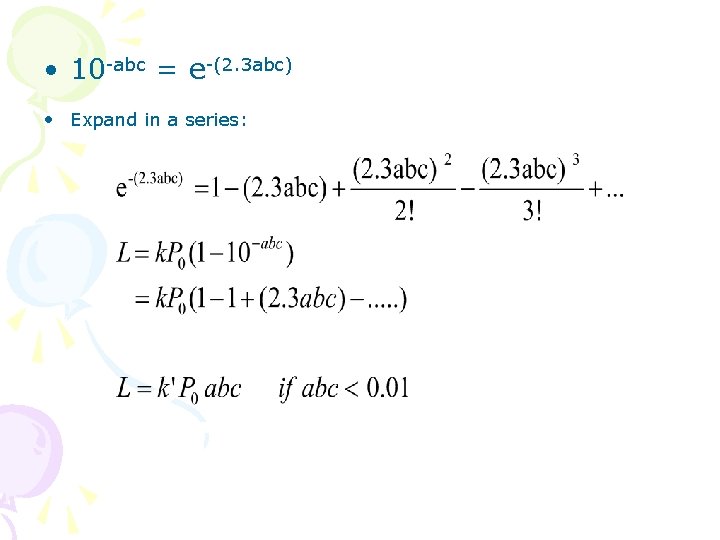
• 10 -abc = e-(2. 3 abc) • Expand in a series:
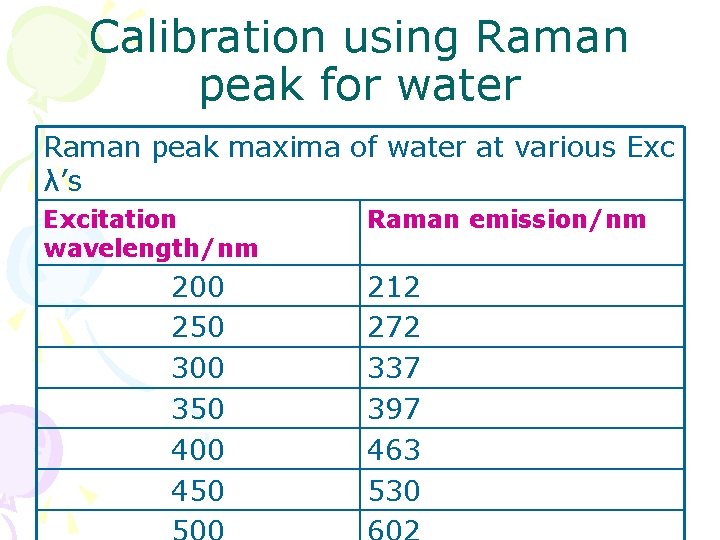
Calibration using Raman peak for water Raman peak maxima of water at various Exc λ’s Excitation wavelength/nm 200 250 300 350 400 450 Raman emission/nm 212 272 337 397 463 530
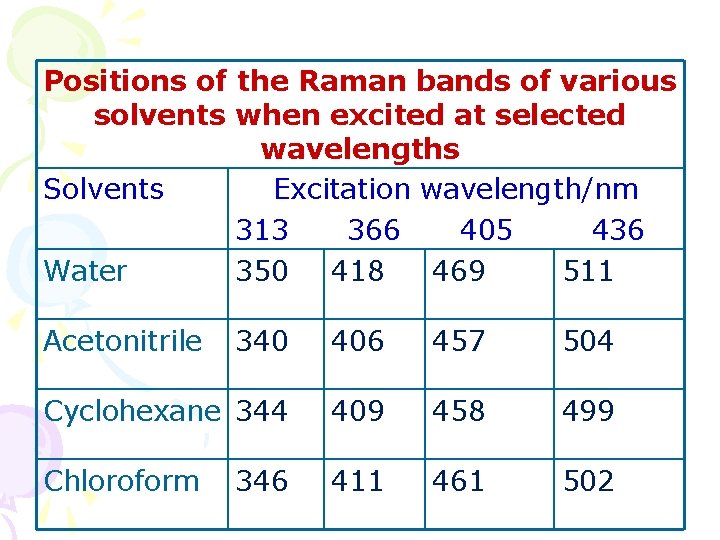
Positions of the Raman bands of various solvents when excited at selected wavelengths Solvents Excitation wavelength/nm 313 366 405 436 Water 350 418 469 511 Acetonitrile 340 406 457 504 Cyclohexane 344 409 458 499 Chloroform 411 461 502 346
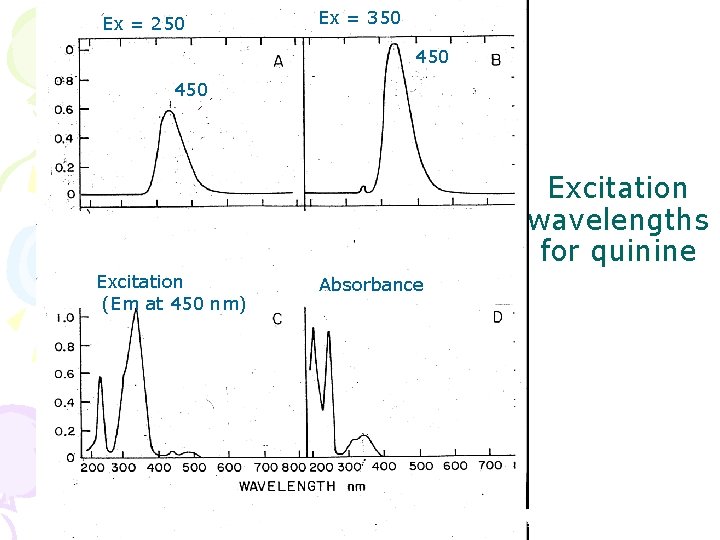
Ex = 250 Ex = 350 450 Excitation wavelengths for quinine Excitation (Em at 450 nm) Absorbance
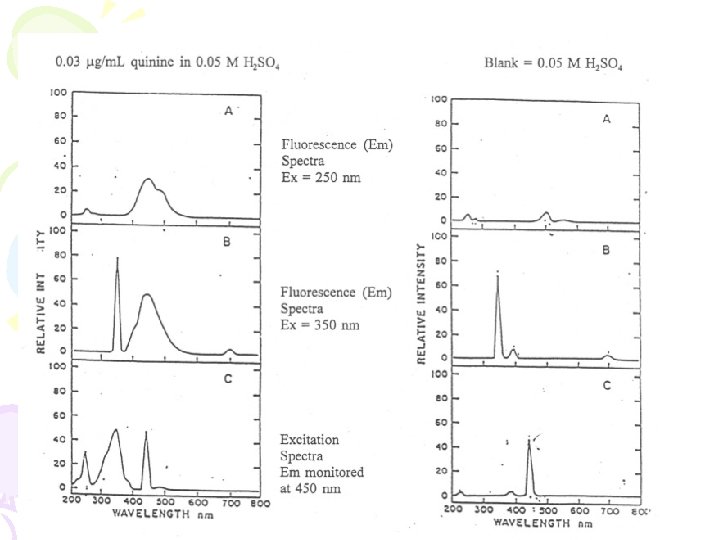
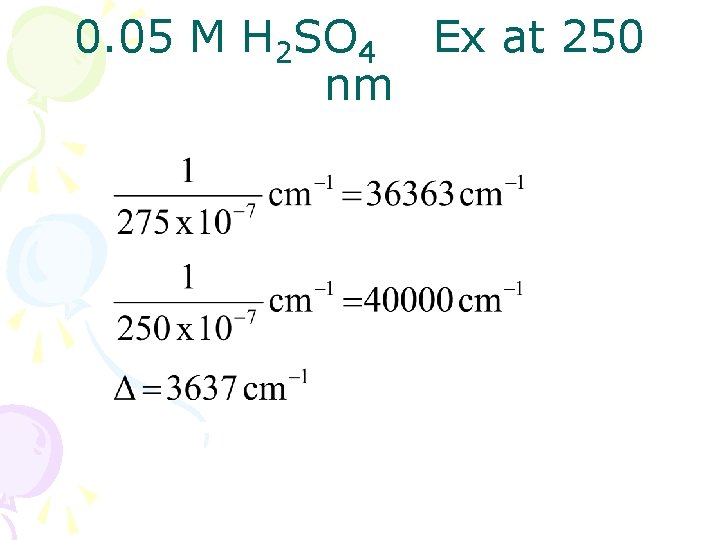
0. 05 M H 2 SO 4 Ex at 250 nm
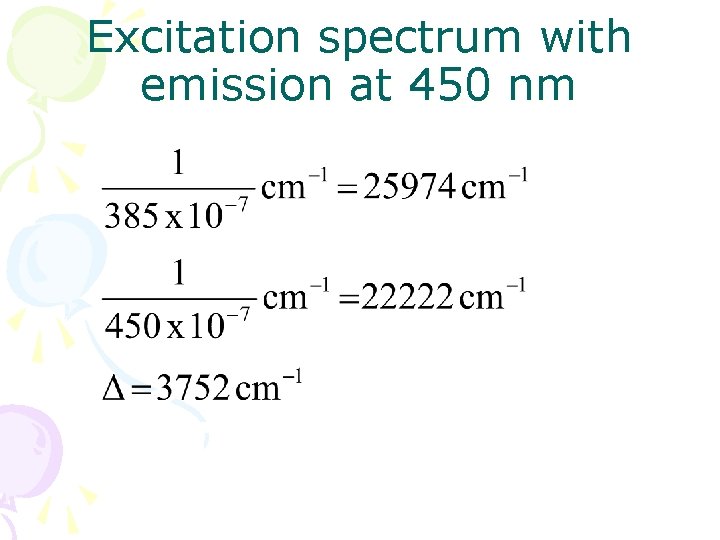
Excitation spectrum with emission at 450 nm
- Slides: 36