Fluids Electrolytes AcidBase Balance Chapter 52 1 Body

Fluids, Electrolytes & Acid-Base Balance Chapter 52 1

Body fluid Intracellular Extracellular 2

3 Types of Extracellular Fluids Interstitial Intravascular Transcellular Movement 3
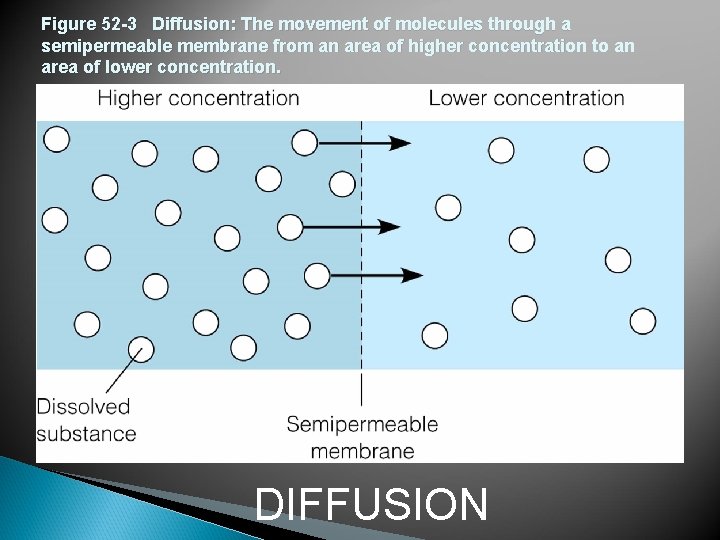
Figure 52 -3 Diffusion: The movement of molecules through a semipermeable membrane from an area of higher concentration to an area of lower concentration. DIFFUSION
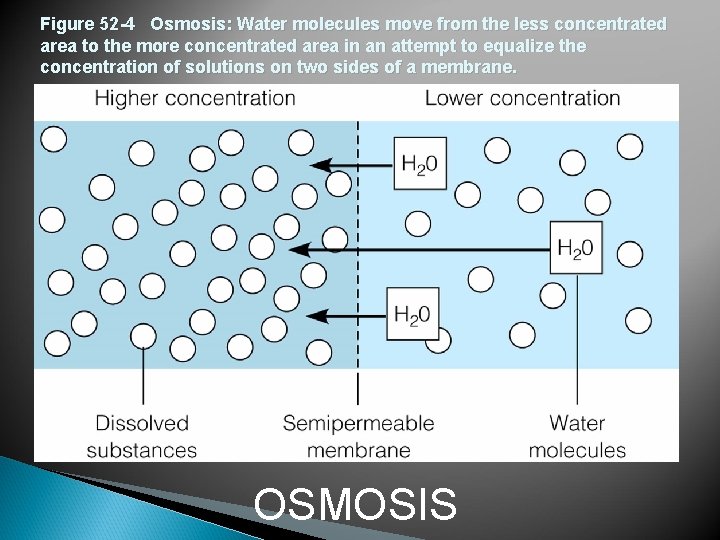
Figure 52 -4 Osmosis: Water molecules move from the less concentrated area to the more concentrated area in an attempt to equalize the concentration of solutions on two sides of a membrane. OSMOSIS
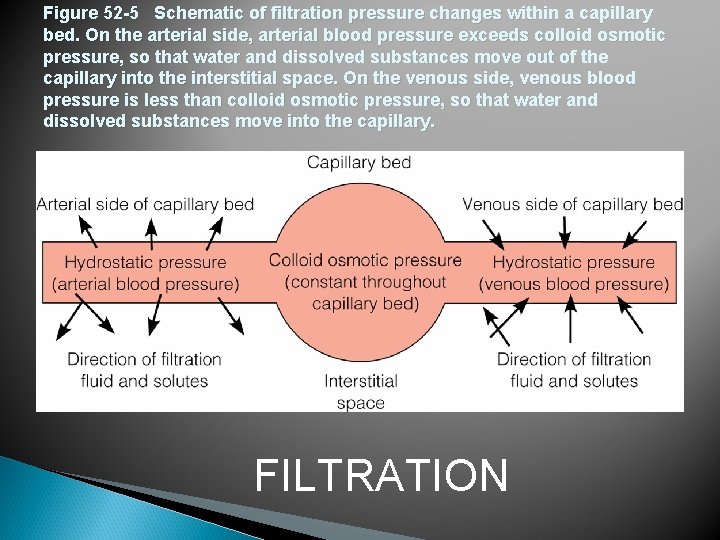
Figure 52 -5 Schematic of filtration pressure changes within a capillary bed. On the arterial side, arterial blood pressure exceeds colloid osmotic pressure, so that water and dissolved substances move out of the capillary into the interstitial space. On the venous side, venous blood pressure is less than colloid osmotic pressure, so that water and dissolved substances move into the capillary. FILTRATION
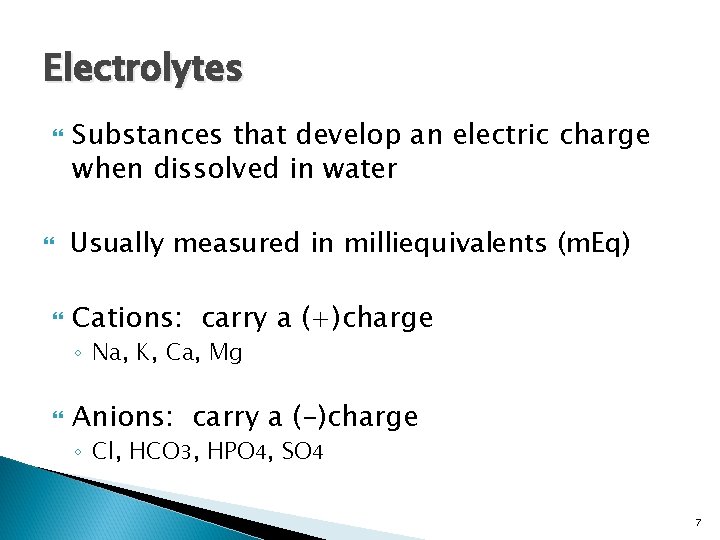
Electrolytes Substances that develop an electric charge when dissolved in water Usually measured in milliequivalents (m. Eq) Cations: carry a (+)charge ◦ Na, K, Ca, Mg Anions: carry a (-)charge ◦ Cl, HCO 3, HPO 4, SO 4 7

Fluid Regulation Intake Thirst mechanism Output 8
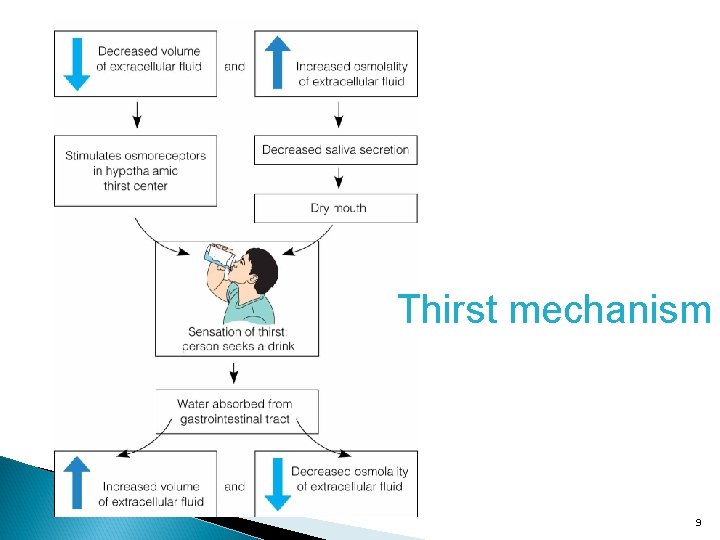
Thirst mechanism 9

Maintaining Homeostasis Kidneys Hormones ◦ Antidiuretic hormone (ADH) ◦ Renin-angiotensin-aldosterone system ◦ Atrial natriuretic factor 10

Major Electrolytes (Tab 52 -3 pg. 1456 & Tab 52 -6 pg. 1464) Sodium Potassium Calcium Magnesium Chloride Phosphate Bicarbonate Know all normal lab values 11

Sodium (Na) Function Normal range Food sources 12

Sodium (Na) Hyponatremia Hypernatremia 13
Potassium (K) Function Normal range Food sources 14

Potassium (K) Hypokalemia Hyperkalemia 15
Calcium (Ca) Function Normal range Food sources 16
Calcium (Ca) Hypocalcemia Hypercalcemia 17
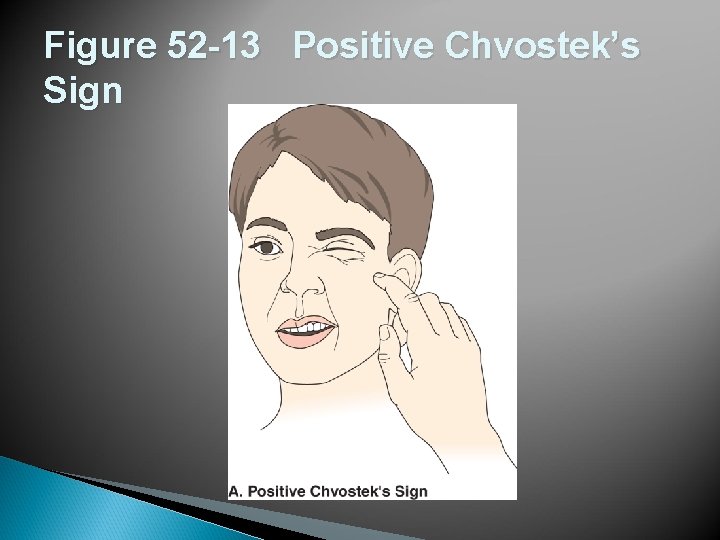
Figure 52 -13 Positive Chvostek’s Sign
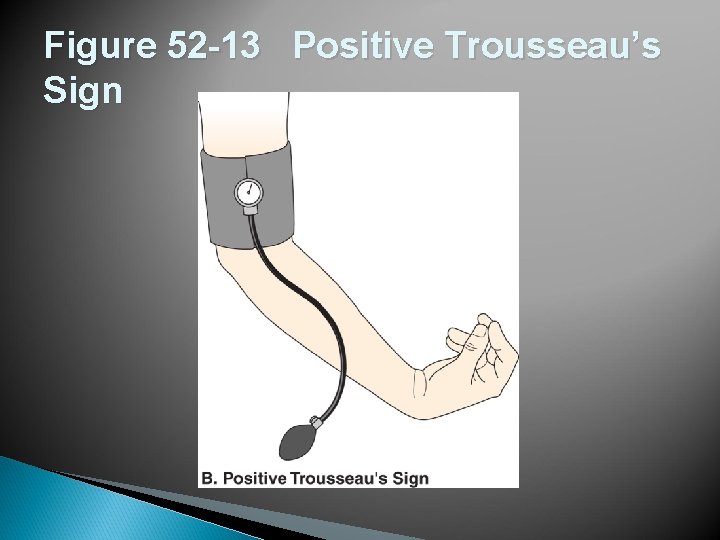
Figure 52 -13 Positive Trousseau’s Sign
Magnesium (Mg) Function Normal range Food sources 20

Magnesium (Mg) Hypomagnesemia Hypermagnesemia 21
Chloride (Cl) Function Normal range Food sources 22
Phosphate (PO 4) Function Normal range Food sources 23
Bicarbonate (HCO 3) Function Normal range Food sources 24

Factors Affecting Body Fluid, Electrolyte, and Acid-Base Balance Age Gender Body size Environmental temperature Lifestyle
Fluid Imbalances Fluid volume deficit - hypovolemia ◦ Isotonic loss of water and electrolytes ◦ Third Space Syndrome
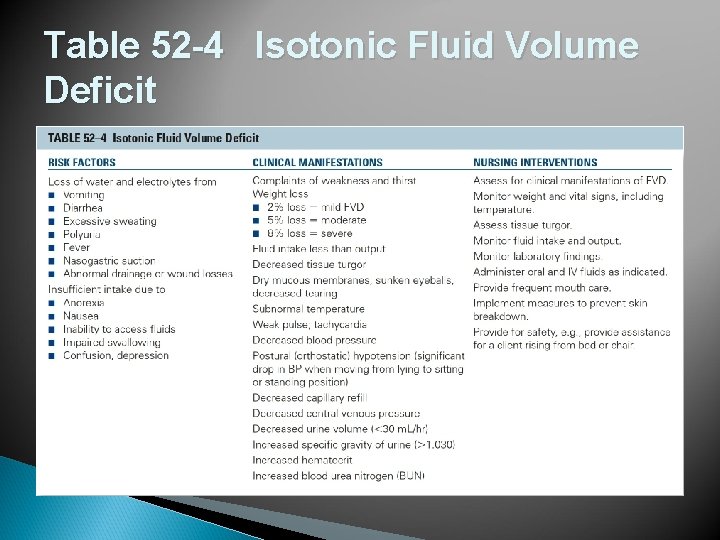
Table 52 -4 Isotonic Fluid Volume Deficit
Fluid Imbalances (cont’d) Fluid volume excess – hypervolemia Isotonic gain of water and electrolytes ◦ Edema, pitting edema
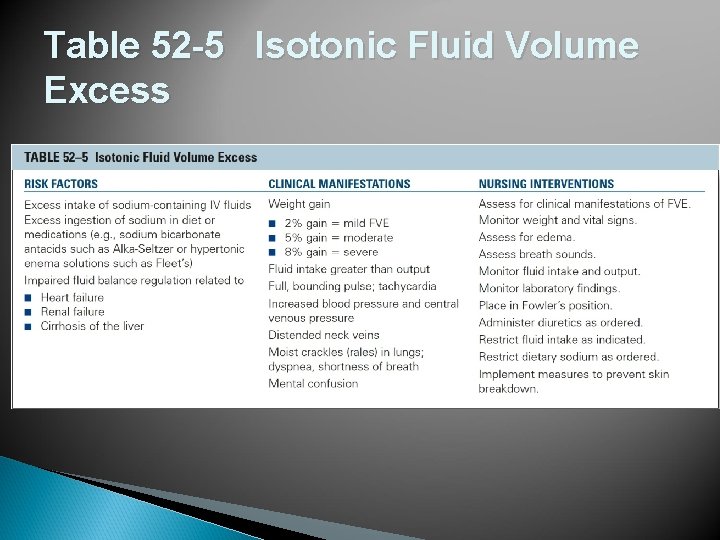
Table 52 -5 Isotonic Fluid Volume Excess
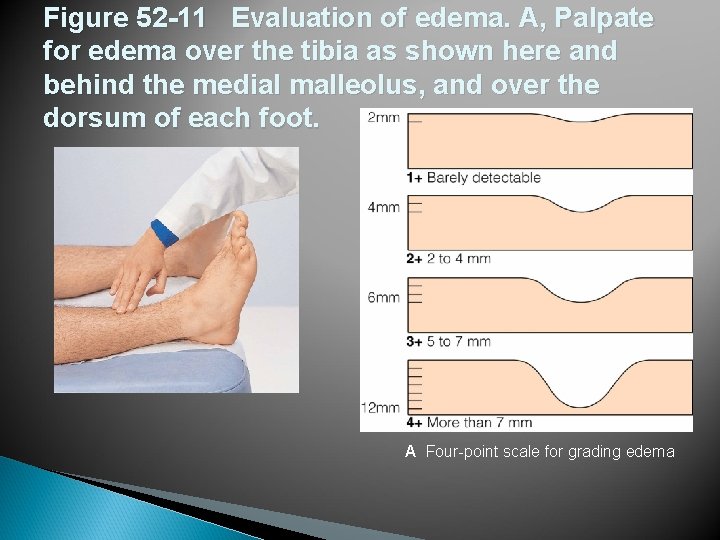
Figure 52 -11 Evaluation of edema. A, Palpate for edema over the tibia as shown here and behind the medial malleolus, and over the dorsum of each foot. A Four-point scale for grading edema

Fluid Imbalances (cont’d) Dehydration Overhydration

Assessing Nursing history Physical assessment Clinical measurement Review of laboratory test results

Physical Assessment Skin Mucus membranes Eyes Fontanels (infants) Cardiovascular system Respiratory system Neurologic
Clinical Measurements Daily weights Vital signs Fluid intake/output
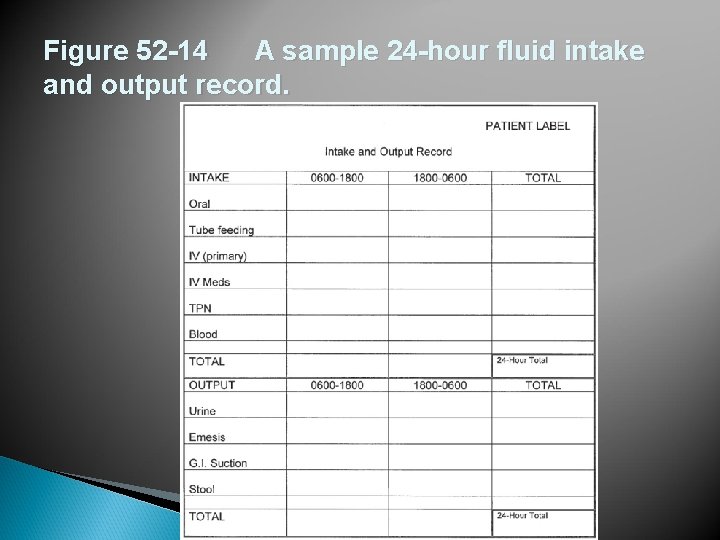
Figure 52 -14 A sample 24 -hour fluid intake and output record.
Laboratory Data Serum electrolytes (Box 52 -5 pg. 1473) Complete Blood Count ◦ Hematocrit Osmolarity Urine Specific Gravity Urine p. H Arterial blood gases (ABGs) ◦ Acid base balance

NANDA Nursing Diagnoses Deficient Fluid Volume Excess Fluid Volume Risk for Imbalanced Fluid Volume Risk for Deficient Fluid volume Impaired Gas Exchange

Planning: General Goals Maintain or restore normal fluid balance Maintain or restore normal balance of electrolytes Maintain or restore gas exchange and oxygenation Prevent associated risks ◦ Tissue breakdown, decreased cardiac output, confusion, other neurologic signs
Implementing Promoting wellness Enteral fluid/electrolyte replacement Fluid intake modification ◦ Practice guidelines pg. 1479 Oral supplements 39

QUESTIONS? ? 40
- Slides: 40