Fluidized Bubbling Bed Reactor Model For Silane Pyrolysis
Fluidized Bubbling Bed Reactor Model For Silane Pyrolysis In Solar Grade Silicon Production Yue Huang 1, Palghat. A. Ramachandran 1, Milorad. P. Dudukovic 1, Milind S. Kulkarni 2 Chemical Reaction Engineering Laboratory (CREL), Department of Energy, Environmental & Chemical Engineering, Campus Box 1198, Washington University in St. Louis, MO 63130 2 MEMC Electronic Materials, Inc. , 501 Pearl Drive, St. Peters, MO 63376 1
Solar Energy l clean, green, renewable: environmentally friendly l tremendous source: sunlight intensity on the earth 1000 W/m 2 At some time in the future (50 years or more) fossil fuels will be depleted and humans will have to turn to other energy sources and solar cells will be a big part of generating electricity.
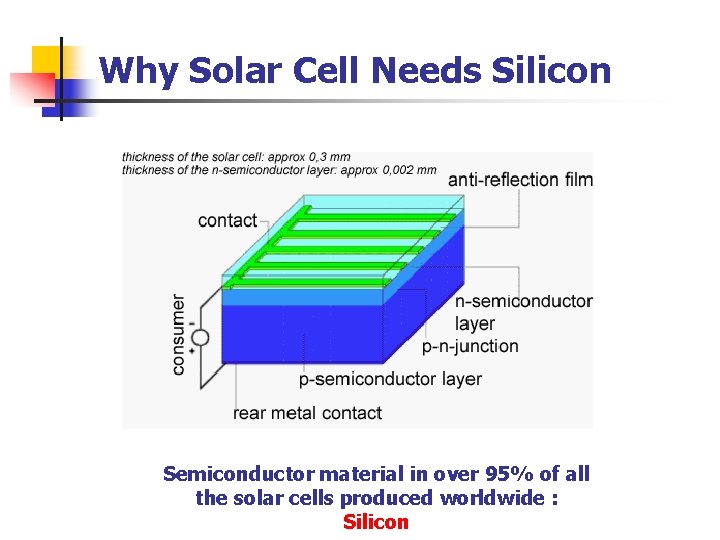
Why Solar Cell Needs Silicon Semiconductor material in over 95% of all the solar cells produced worldwide : Silicon
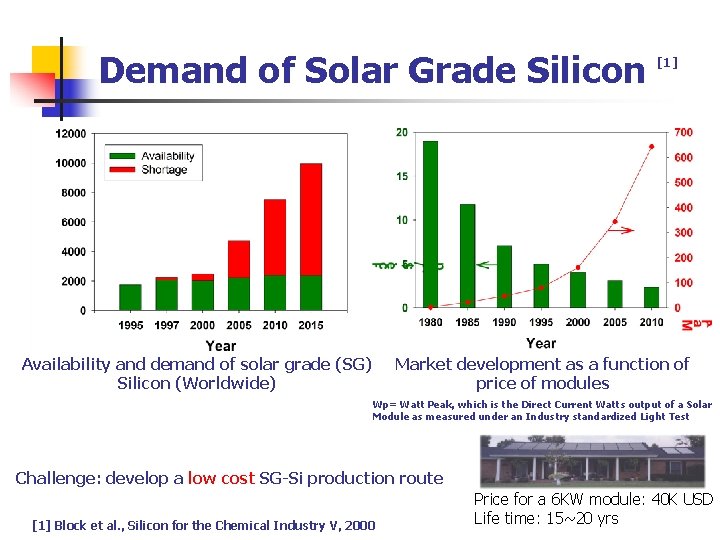
Demand of Solar Grade Silicon Availability and demand of solar grade (SG) Silicon (Worldwide) [1] Market development as a function of price of modules Wp= Watt Peak, which is the Direct Current Watts output of a Solar Module as measured under an Industry standardized Light Test Challenge: develop a low cost SG-Si production route [1] Block et al. , Silicon for the Chemical Industry V, 2000 Price for a 6 KW module: 40 K USD Life time: 15~20 yrs
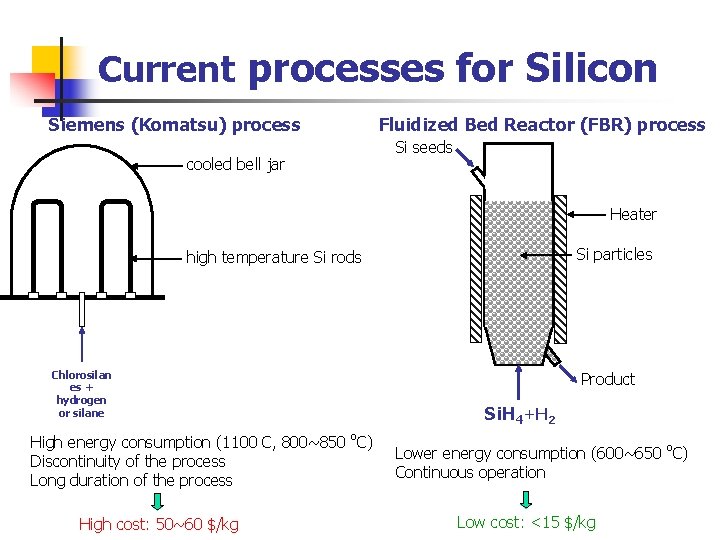
Current processes for Silicon Siemens (Komatsu) process cooled bell jar Fluidized Bed Reactor (FBR) process Si seeds Heater Si particles high temperature Si rods Chlorosilan es + hydrogen or silane High energy consumption (1100 C, 800~850 o. C) Discontinuity of the process Long duration of the process High cost: 50~60 $/kg Product Si. H 4+H 2 Lower energy consumption (600~650 o. C) Continuous operation Low cost: <15 $/kg
Objective of research ONLY MEMC Inc. commercialized FBR process, because l very expensive and time consuming scale-up l complex reaction mechanism l lack of engineering model for large-scale reactors OBJECTIVE
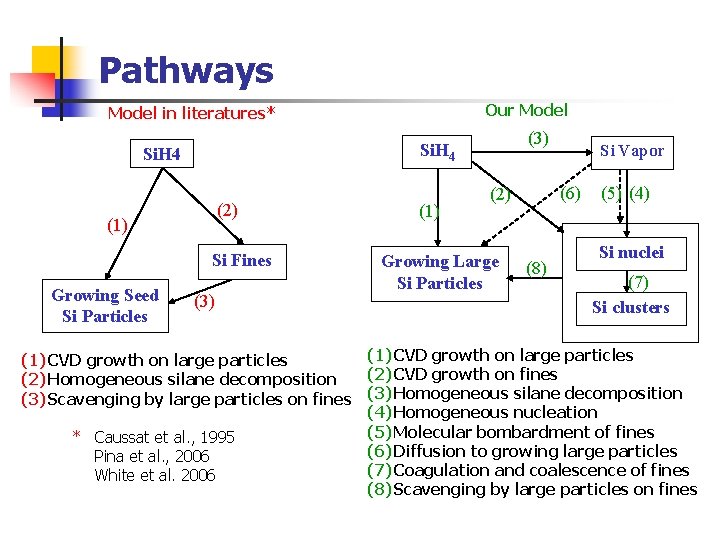
Pathways Our Model in literatures* Si. H 4 (2) (1) Si Fines Growing Seed Si Particles (3) (1) (6) (2) Growing Large Si Particles Si Vapor (8) (5) (4) Si nuclei (7) Si clusters (1) CVD growth on large particles (2) CVD growth on fines (2) Homogeneous silane decomposition (3) Scavenging by large particles on fines (3) Homogeneous silane decomposition (4) Homogeneous nucleation (5) Molecular bombardment of fines * Caussat et al. , 1995 (6) Diffusion to growing large particles Pina et al. , 2006 (7) Coagulation and coalescence of fines White et al. 2006 (8) Scavenging by large particles on fines
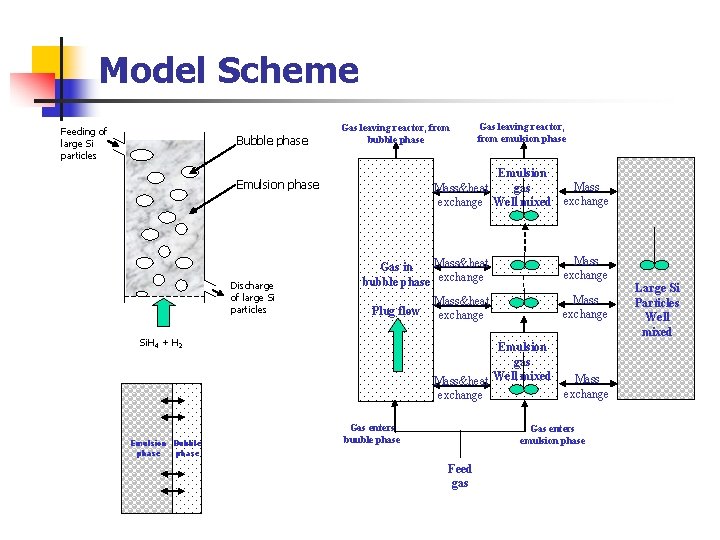
Model Scheme Feeding of large Si particles Bubble phase Gas leaving reactor, from bubble phase Emulsion gas Mass&heat exchange Well mixed Emulsion phase Discharge of large Si particles Mass exchange Mass&heat Gas in bubble phase exchange Mass&heat exchange Mass exchange Emulsion gas Mass&heat Well mixed exchange Mass exchange Plug flow Si. H 4 + H 2 Emulsion Bubble phase Gas leaving reactor, from emulsion phase Gas enters buuble phase Gas enters emulsion phase Feed gas Large Si Particles Well mixed
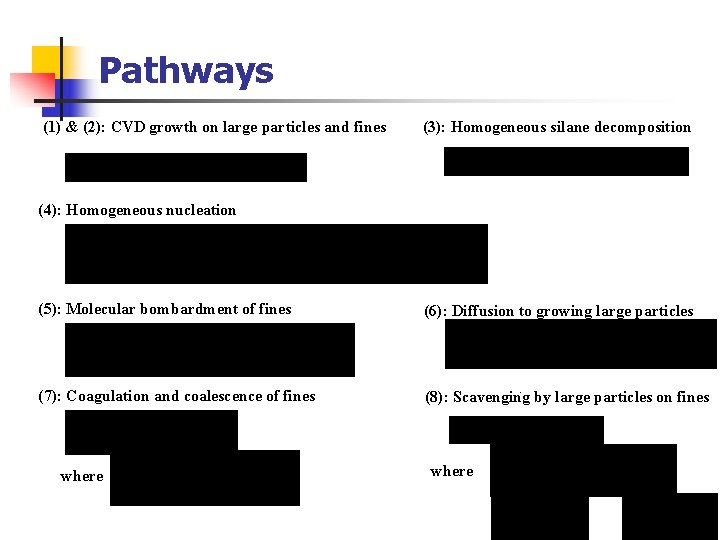
Pathways (1) & (2): CVD growth on large particles and fines (3): Homogeneous silane decomposition (4): Homogeneous nucleation (5): Molecular bombardment of fines (6): Diffusion to growing large particles (7): Coagulation and coalescence of fines (8): Scavenging by large particles on fines where
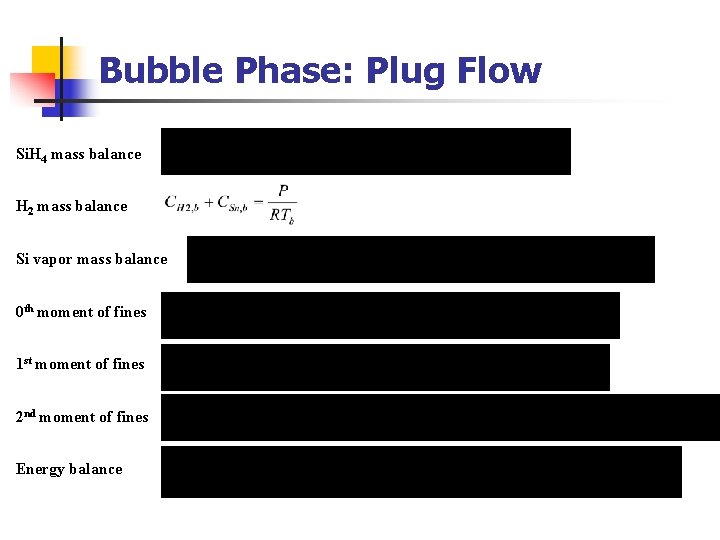
Bubble Phase: Plug Flow Si. H 4 mass balance H 2 mass balance Si vapor mass balance 0 th moment of fines 1 st moment of fines 2 nd moment of fines Energy balance
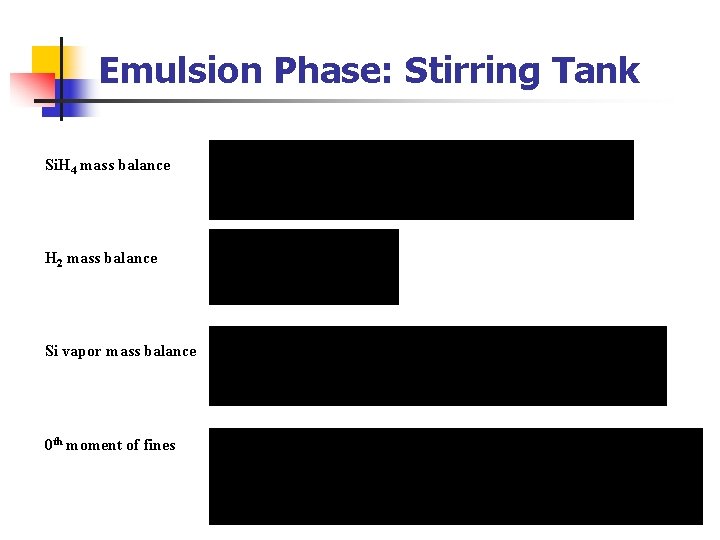
Emulsion Phase: Stirring Tank Si. H 4 mass balance H 2 mass balance Si vapor mass balance 0 th moment of fines
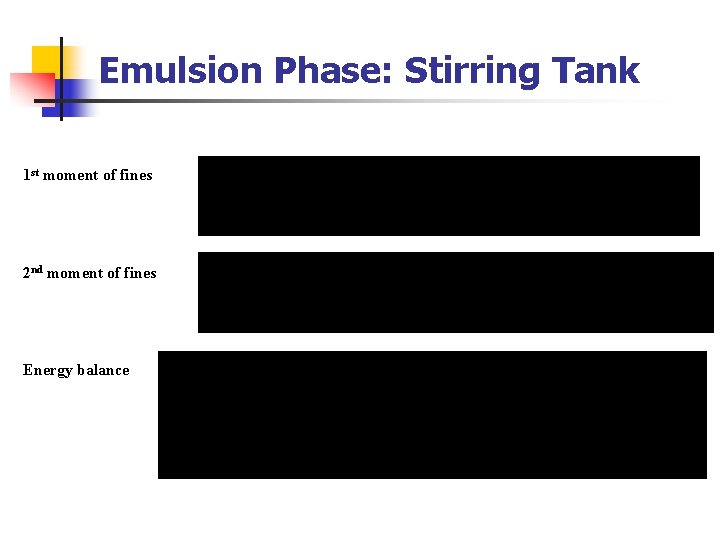
Emulsion Phase: Stirring Tank 1 st moment of fines 2 nd moment of fines Energy balance
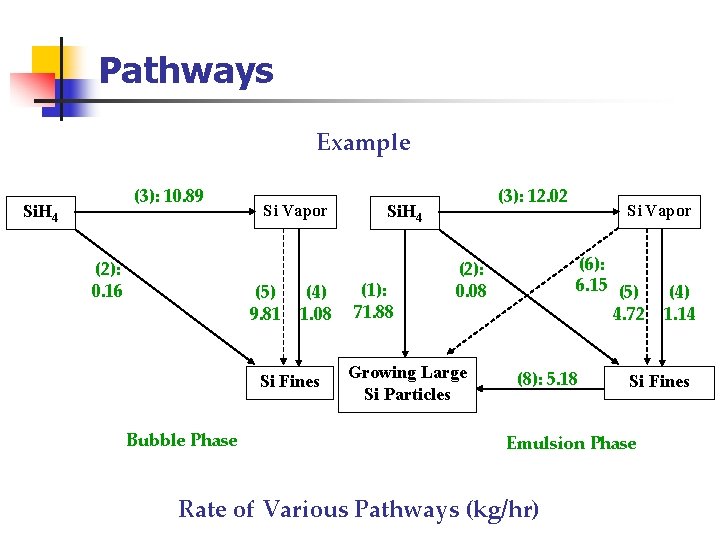
Pathways Example (3): 10. 89 Si. H 4 (2): 0. 16 Si Vapor (5) 9. 81 (4) 1. 08 Si Fines Bubble Phase (3): 12. 02 Si. H 4 (1): 71. 88 (6): 6. 15 (5) 4. 72 (2): 0. 08 Growing Large Si Particles Si Vapor (8): 5. 18 Si Fines Emulsion Phase Rate of Various Pathways (kg/hr) (4) 1. 14
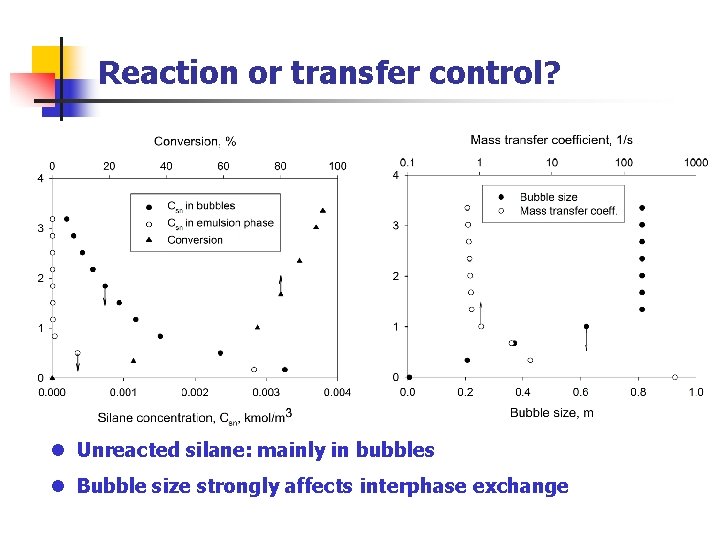
Reaction or transfer control? l Unreacted silane: mainly in bubbles l Bubble size strongly affects interphase exchange
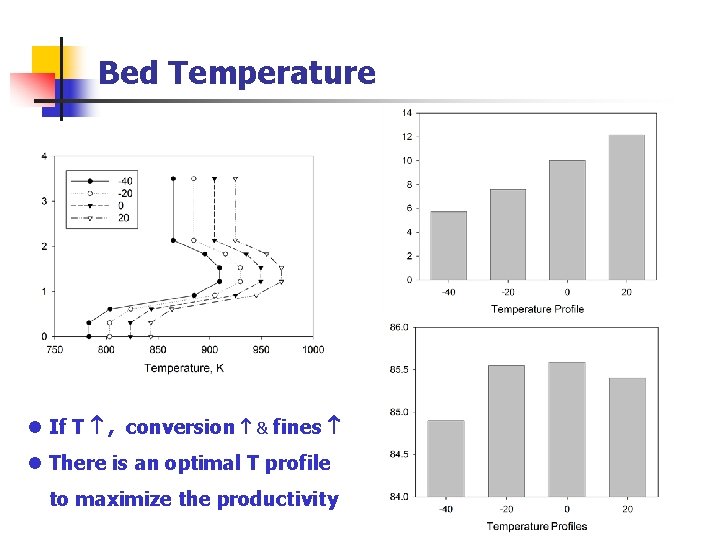
Bed Temperature l If T , conversion & fines l There is an optimal T profile to maximize the productivity
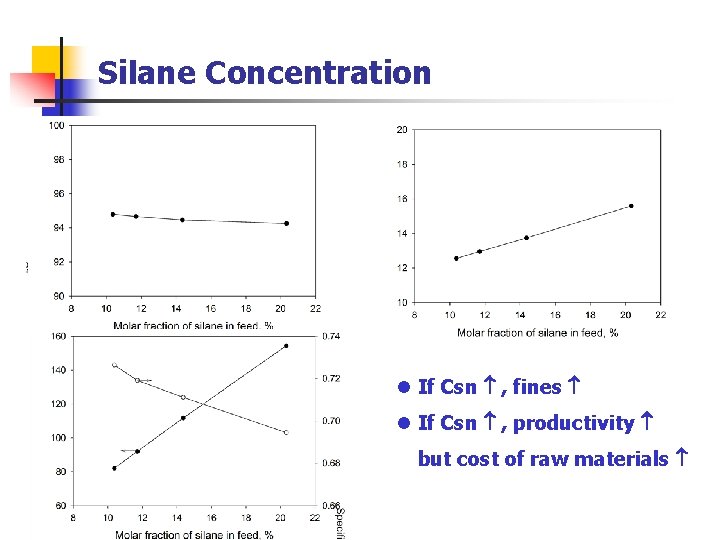
Silane Concentration l If Csn , fines l If Csn , productivity but cost of raw materials
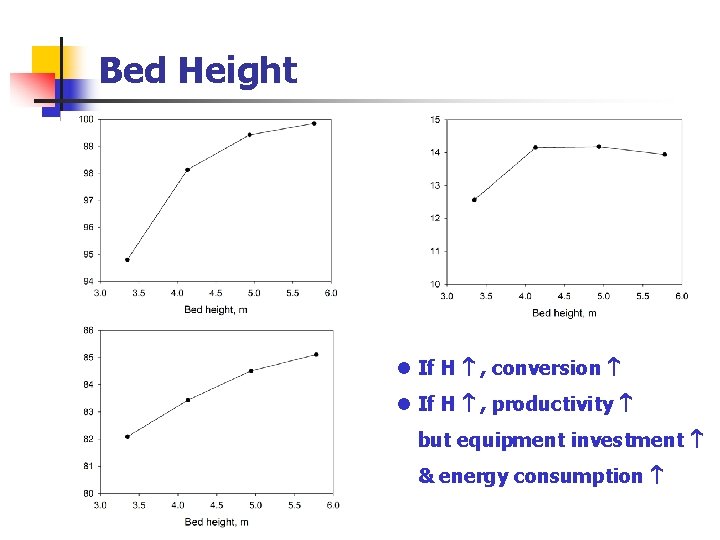
Bed Height l If H , conversion l If H , productivity but equipment investment & energy consumption
Conclusions l A phenomenological model was developed; l Mechanism of the process was investigated; l Enhancement of interphase exchange is the key to improve the reactor performance; l This study provides a good basis for optimization of operating conditions and for scale-up of reactor. Acknowledgement The financial support provided by
- Slides: 18