FLUID PROPERTIES Chapter 2 CE 319 F Elementary
















- Slides: 49

FLUID PROPERTIES Chapter 2 CE 319 F: Elementary Mechanics of Fluids 1

Fluid Properties • Define “characteristics” of a specific fluid • Properties expressed by basic “dimensions” – length, mass (or force), time, temperature • Dimensions quantified by basic “units” We will consider systems of units, important fluid properties (not all), and the dimensions associated with those properties. 2

Systeme International (SI) • • Length = meters (m) Mass = kilograms (kg) Time = second (s) Force = Newton (N) – Force required to accelerate 1 kg @ 1 m/s 2 – Acceleration due to gravity (g) = 9. 81 m/s 2 – Weight of 1 kg at earth’s surface = W = mg = 1 kg (9. 81 m/s 2) = 9. 81 kg-m/s 2 = 9. 81 N • Temperature = Kelvin (o. K) – 273. 15 o. K = freezing point of water – o. K = 273. 15 + o. C 3

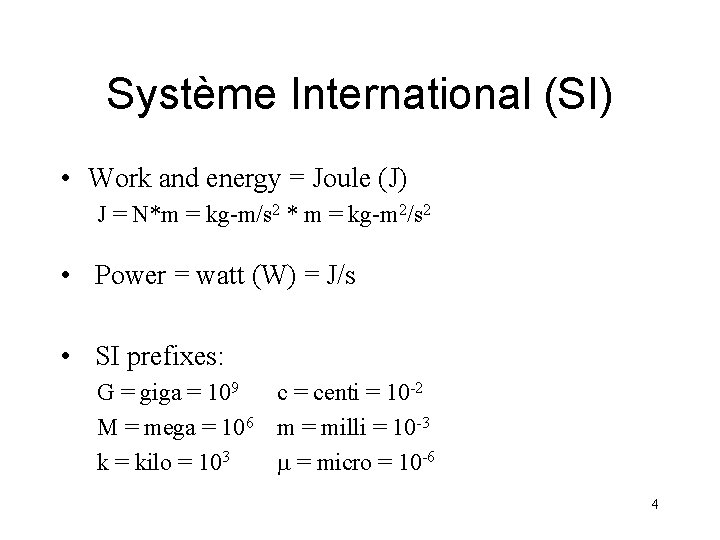
Système International (SI) • Work and energy = Joule (J) J = N*m = kg-m/s 2 * m = kg-m 2/s 2 • Power = watt (W) = J/s • SI prefixes: G = giga = 109 c = centi = 10 -2 M = mega = 106 m = milli = 10 -3 k = kilo = 103 m = micro = 10 -6 4

English (American) System • • Length = foot (ft) = 0. 3048 m Mass = slug or lbm (1 slug = 32. 2 lbm = 14. 59 kg) Time = second (s) Force = pound-force (lbf) – Force required to accelerate 1 slug @ 1 ft/s 2 • Temperature = (o. F or o. R) – o. Rankine = o. R = 460 + o. F • Work or energy = ft-lbf • Power = ft-lbf/s Banana Slug Mascot of UC Santa Cruz – 1 horsepower = 1 hp = 550 ft-lbf/s = 746 W 5

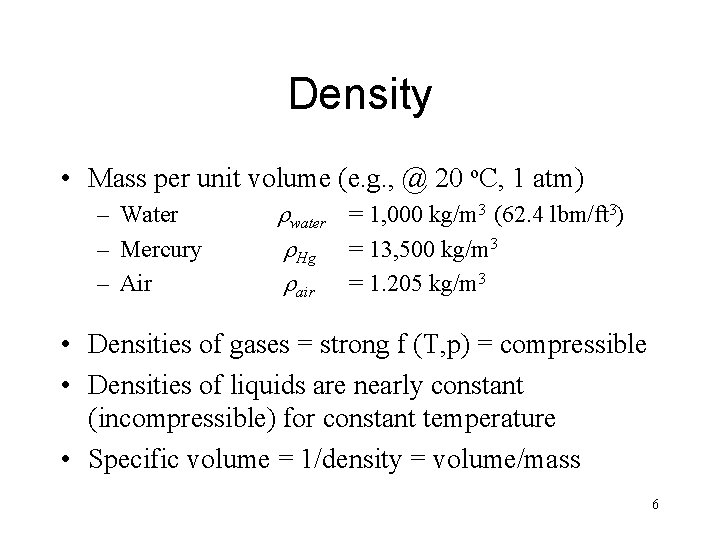
Density • Mass per unit volume (e. g. , @ 20 o. C, 1 atm) – Water – Mercury – Air rwater = 1, 000 kg/m 3 (62. 4 lbm/ft 3) r. Hg = 13, 500 kg/m 3 rair = 1. 205 kg/m 3 • Densities of gases = strong f (T, p) = compressible • Densities of liquids are nearly constant (incompressible) for constant temperature • Specific volume = 1/density = volume/mass 6

Example: Textbook Problem 2. 8 • Estimate the mass of 1 mi 3 of air in slugs and kgs. Assume rair = 0. 00237 slugs/ft 3, the value at sea level for standard conditions 7

Example • A 5 -L bottle of carbon tetrachloride is accidentally spilled onto a laboratory floor. What is the mass of carbon tetrachloride that was spilled in lbm? 8

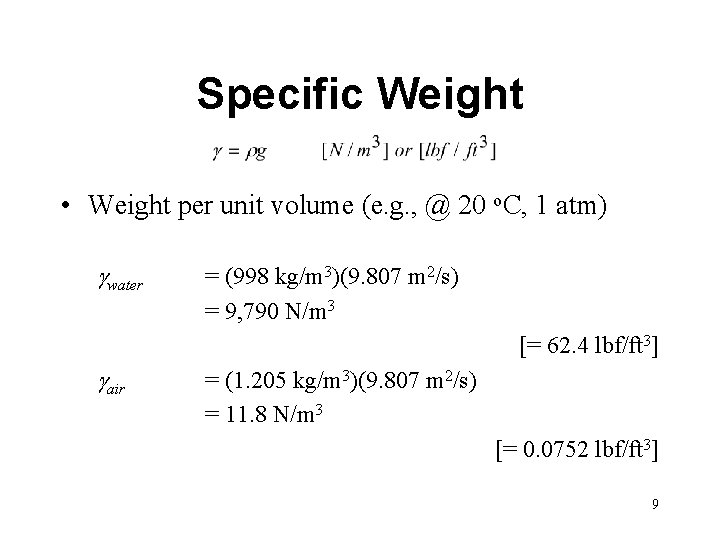
Specific Weight • Weight per unit volume (e. g. , @ 20 o. C, 1 atm) gwater = (998 kg/m 3)(9. 807 m 2/s) = 9, 790 N/m 3 [= 62. 4 lbf/ft 3] gair = (1. 205 kg/m 3)(9. 807 m 2/s) = 11. 8 N/m 3 [= 0. 0752 lbf/ft 3] 9

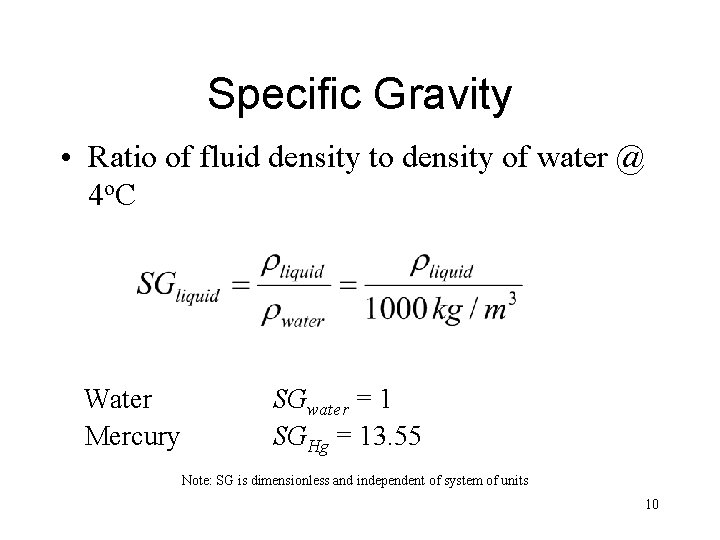
Specific Gravity • Ratio of fluid density to density of water @ 4 o. C Water Mercury SGwater = 1 SGHg = 13. 55 Note: SG is dimensionless and independent of system of units 10

Example • The specific gravity of a fresh gasoline is 0. 80. If the gasoline fills an 8 m 3 tank on a transport truck, what is the weight of the gasoline in the tank? 11

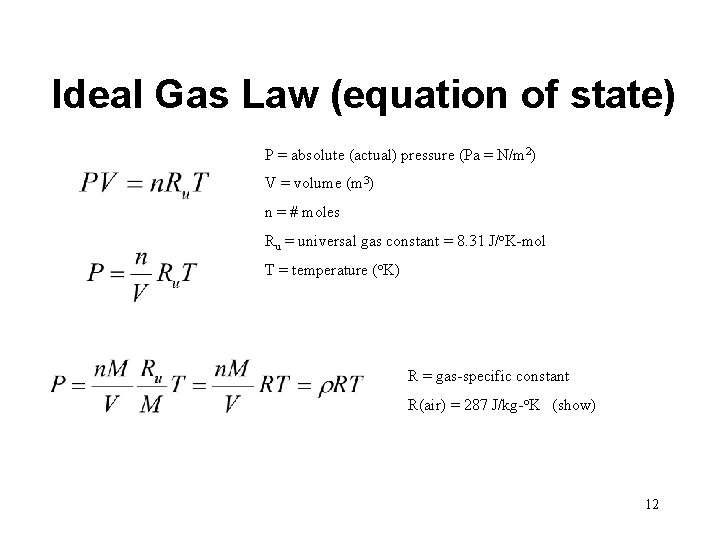
Ideal Gas Law (equation of state) P = absolute (actual) pressure (Pa = N/m 2) V = volume (m 3) n = # moles Ru = universal gas constant = 8. 31 J/o. K-mol T = temperature (o. K) R = gas-specific constant R(air) = 287 J/kg-o. K (show) 12

Example • Calculate the volume occupied by 1 mol of any ideal gas at a pressure of 1 atm (101, 000 Pa) and temperature of 20 o. C. 13

Example • The molecular weight of air is approximately 29 g/mol. Use this information to calculate the density of air near the earth’s surface (pressure = 1 atm = 101, 000 Pa) at 20 o. C. 14

Example: Textbook Problem 2. 4 • Given: Natural gas stored in a spherical tank – Time 1: T 1=10 o. C, p 1=100 k. Pa – Time 2: T 2=10 o. C, p 2=200 k. Pa • Find: Ratio of mass at time 2 to that at time 1 • Note: Ideal gas law (p is absolute pressure) 15

Viscosity 16

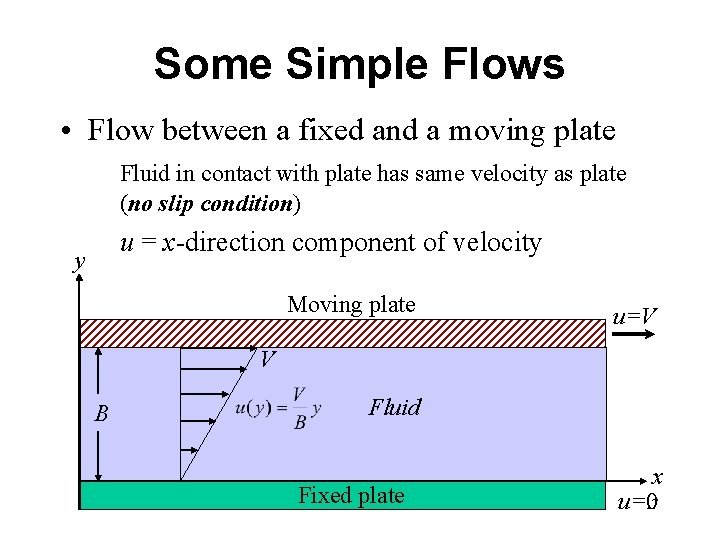
Some Simple Flows • Flow between a fixed and a moving plate Fluid in contact with plate has same velocity as plate (no slip condition) u = x-direction component of velocity y Moving plate u=V V B Fluid Fixed plate x 17 u=0

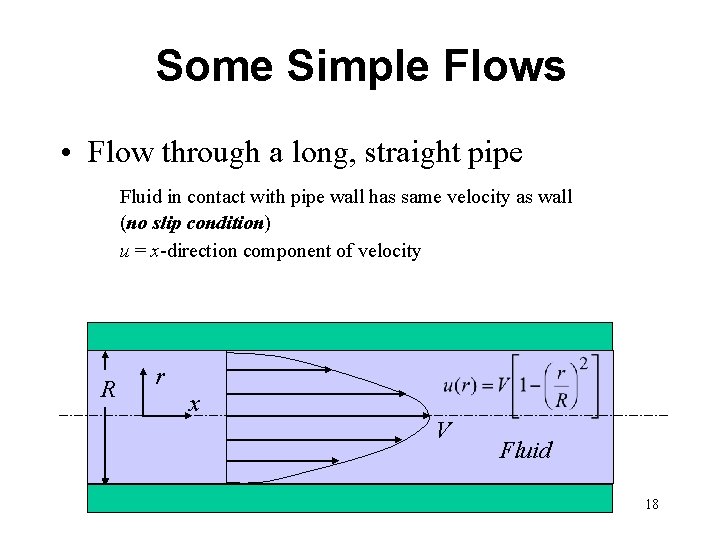
Some Simple Flows • Flow through a long, straight pipe Fluid in contact with pipe wall has same velocity as wall (no slip condition) u = x-direction component of velocity R r x V Fluid 18

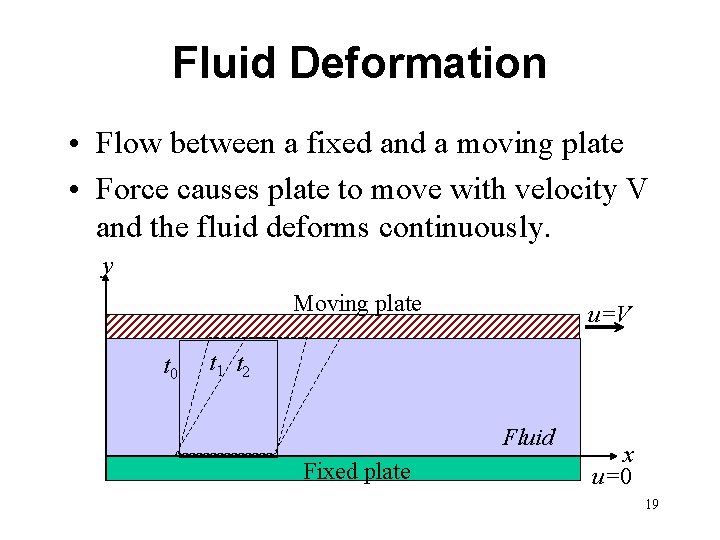
Fluid Deformation • Flow between a fixed and a moving plate • Force causes plate to move with velocity V and the fluid deforms continuously. y Moving plate t 0 u=V t 1 t 2 Fluid Fixed plate x u=0 19

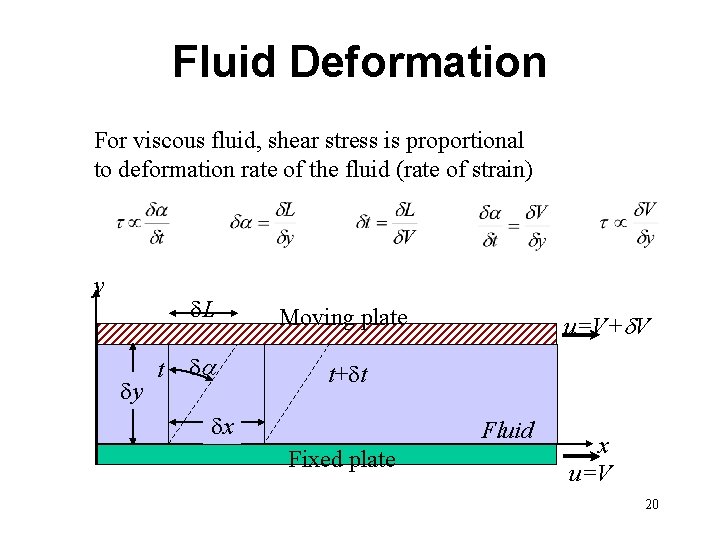
Fluid Deformation For viscous fluid, shear stress is proportional to deformation rate of the fluid (rate of strain) y dy t d. L Moving plate da t+dt dx u=V+d. V Fluid Fixed plate x u=V 20

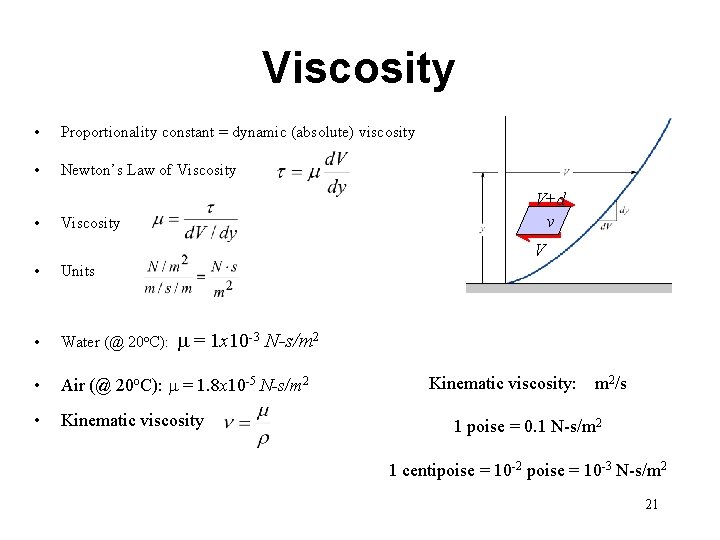
Viscosity • Proportionality constant = dynamic (absolute) viscosity • Newton’s Law of Viscosity • V+d v Viscosity V • Units • Water (@ 20 o. C): • Air (@ 20 o. C): m = 1. 8 x 10 -5 N-s/m 2 • Kinematic viscosity m = 1 x 10 -3 N-s/m 2 Kinematic viscosity: m 2/s 1 poise = 0. 1 N-s/m 2 1 centipoise = 10 -2 poise = 10 -3 N-s/m 2 21

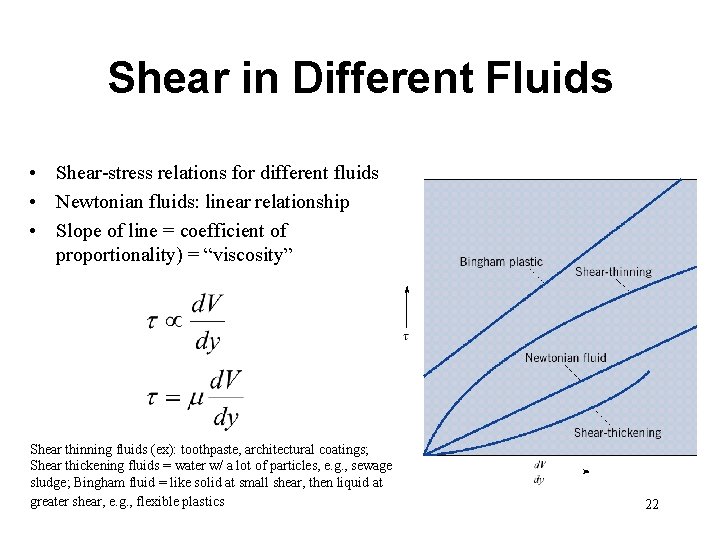
Shear in Different Fluids • Shear-stress relations for different fluids • Newtonian fluids: linear relationship • Slope of line = coefficient of proportionality) = “viscosity” Shear thinning fluids (ex): toothpaste, architectural coatings; Shear thickening fluids = water w/ a lot of particles, e. g. , sewage sludge; Bingham fluid = like solid at small shear, then liquid at greater shear, e. g. , flexible plastics 22

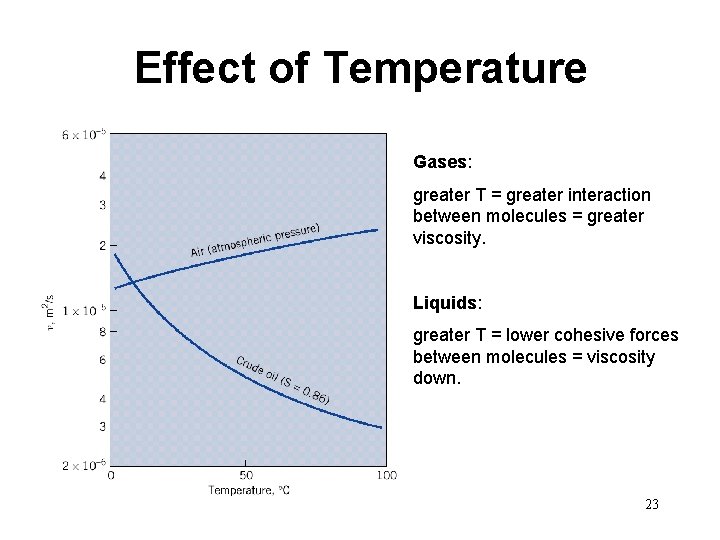
Effect of Temperature Gases: greater T = greater interaction between molecules = greater viscosity. Liquids: greater T = lower cohesive forces between molecules = viscosity down. 23

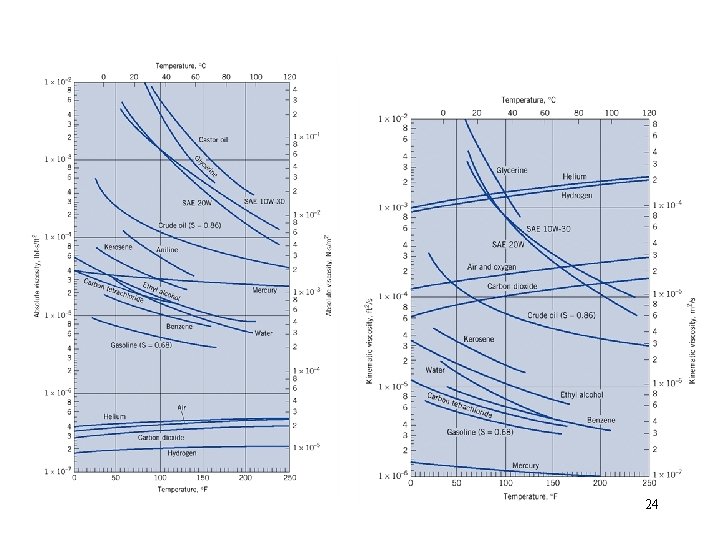
24

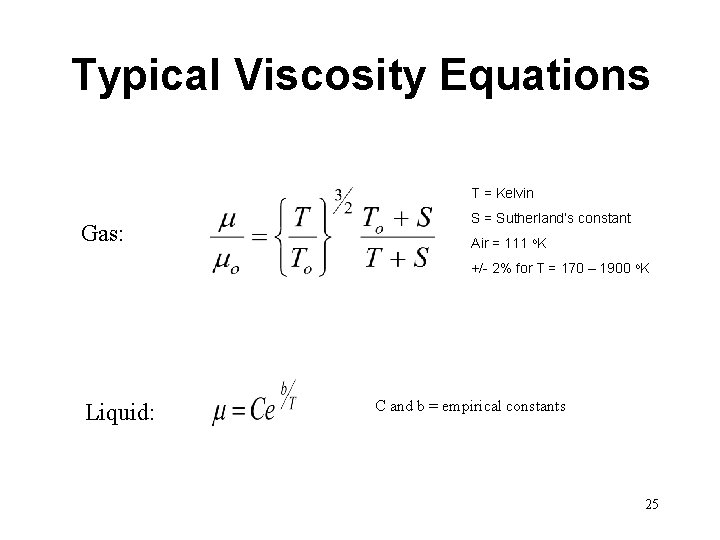
Typical Viscosity Equations T = Kelvin Gas: S = Sutherland’s constant Air = 111 o. K +/- 2% for T = 170 – 1900 o. K Liquid: C and b = empirical constants 25

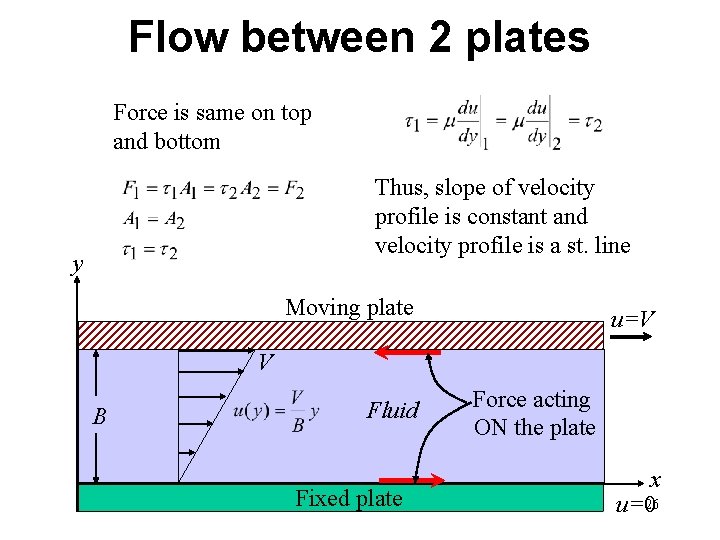
Flow between 2 plates Force is same on top and bottom Thus, slope of velocity profile is constant and velocity profile is a st. line y Moving plate u=V V B Fluid Fixed plate Force acting ON the plate x u=026

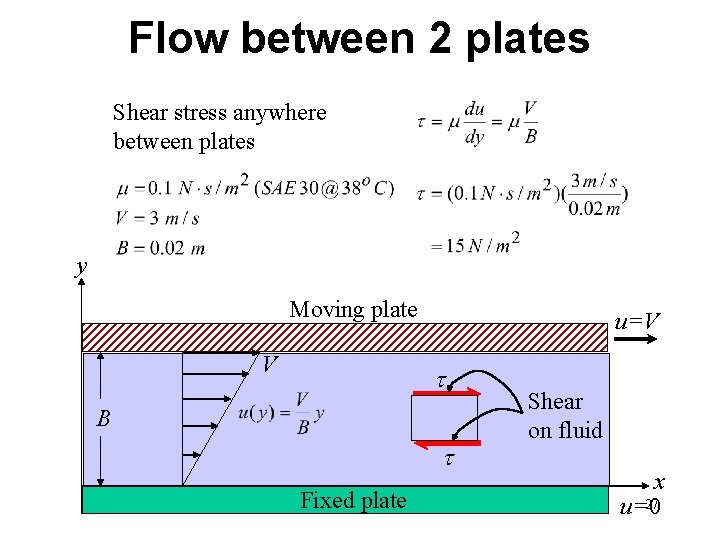
Flow between 2 plates Shear stress anywhere between plates y Moving plate V u=V t B t Fixed plate Shear on fluid x 27 u=0

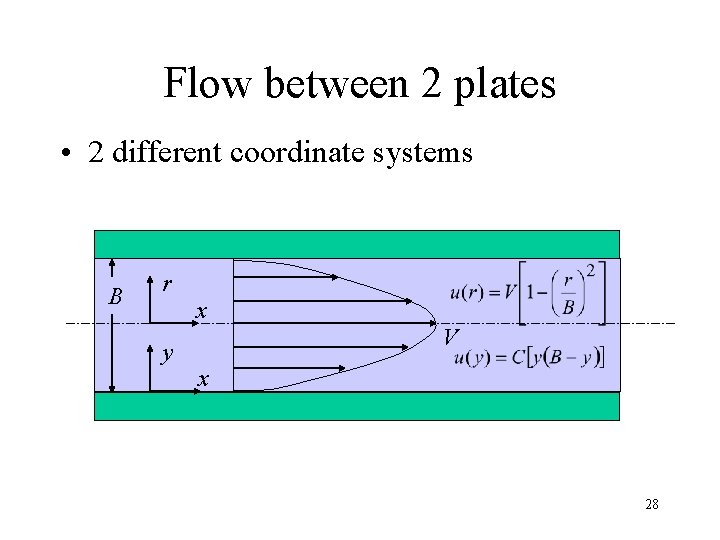
Flow between 2 plates • 2 different coordinate systems B r x y V x 28

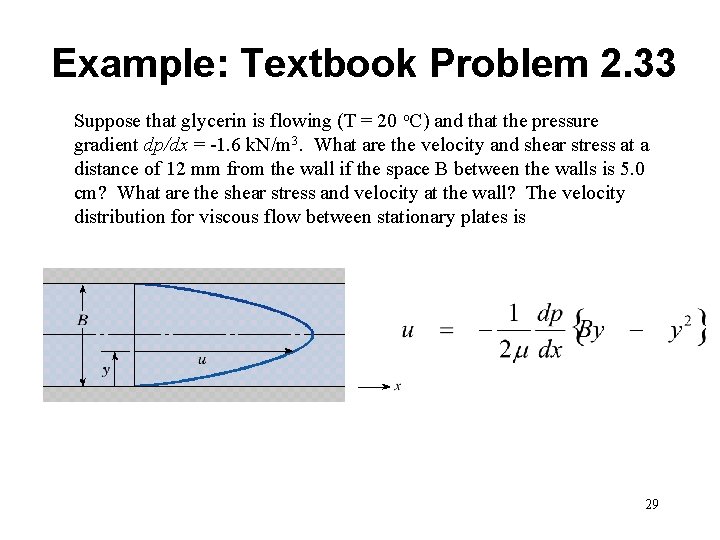
Example: Textbook Problem 2. 33 Suppose that glycerin is flowing (T = 20 o. C) and that the pressure gradient dp/dx = -1. 6 k. N/m 3. What are the velocity and shear stress at a distance of 12 mm from the wall if the space B between the walls is 5. 0 cm? What are the shear stress and velocity at the wall? The velocity distribution for viscous flow between stationary plates is 29

30

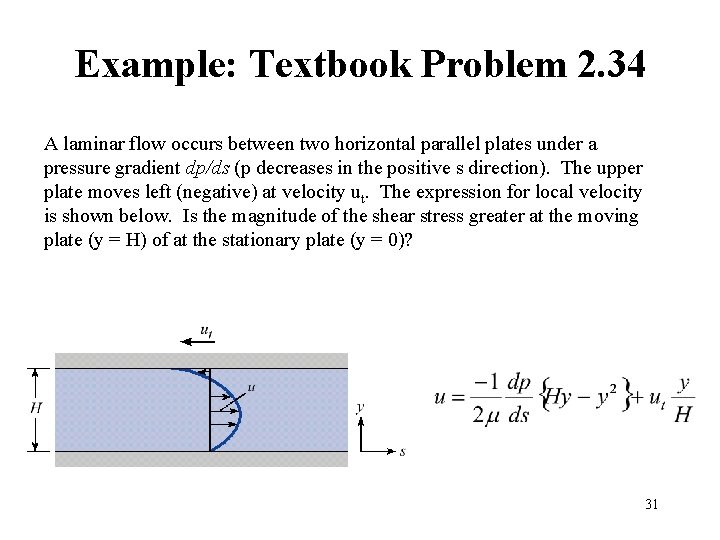
Example: Textbook Problem 2. 34 A laminar flow occurs between two horizontal parallel plates under a pressure gradient dp/ds (p decreases in the positive s direction). The upper plate moves left (negative) at velocity ut. The expression for local velocity is shown below. Is the magnitude of the shear stress greater at the moving plate (y = H) of at the stationary plate (y = 0)? 31

32

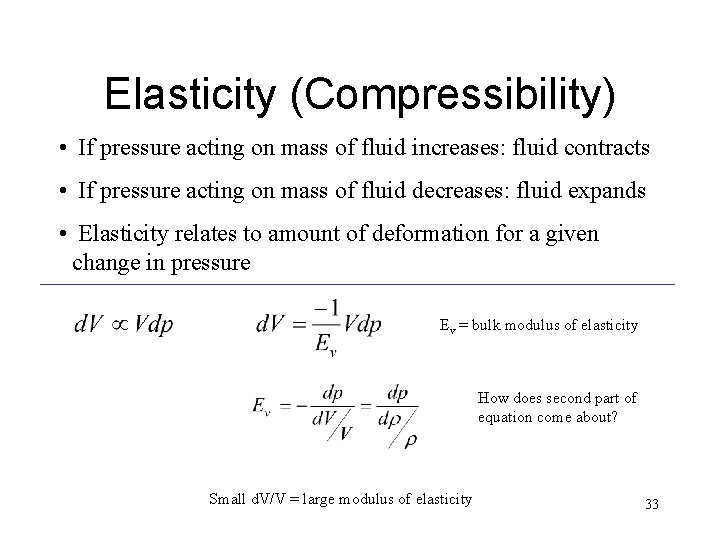
Elasticity (Compressibility) • If pressure acting on mass of fluid increases: fluid contracts • If pressure acting on mass of fluid decreases: fluid expands • Elasticity relates to amount of deformation for a given change in pressure Ev = bulk modulus of elasticity How does second part of equation come about? Small d. V/V = large modulus of elasticity 33

Example: Textbook Problem 2. 45 • Given: Pressure of 2 MPa is applied to a mass of water that initially filled 1000 -cm 3 (1 liter) volume. • • Find: Volume after the pressure is applied. • Ev = 2. 2 x 109 Pa (Table A. 5) 34

Example • Based on the definition of Ev and the equation of state, derive an equation for the modulus of elasticity of an ideal gas. 35

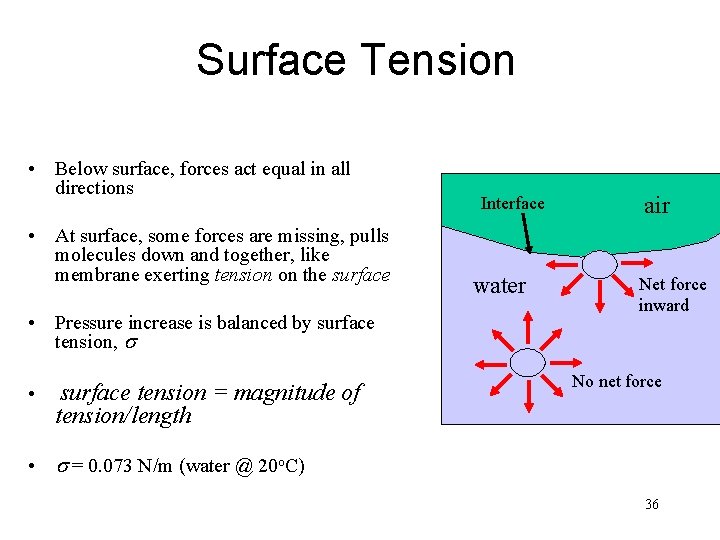
Surface Tension • Below surface, forces act equal in all directions • At surface, some forces are missing, pulls molecules down and together, like membrane exerting tension on the surface • Pressure increase is balanced by surface tension, s • surface tension = magnitude of tension/length Interface water air Net force inward No net force • s = 0. 073 N/m (water @ 20 o. C) 36

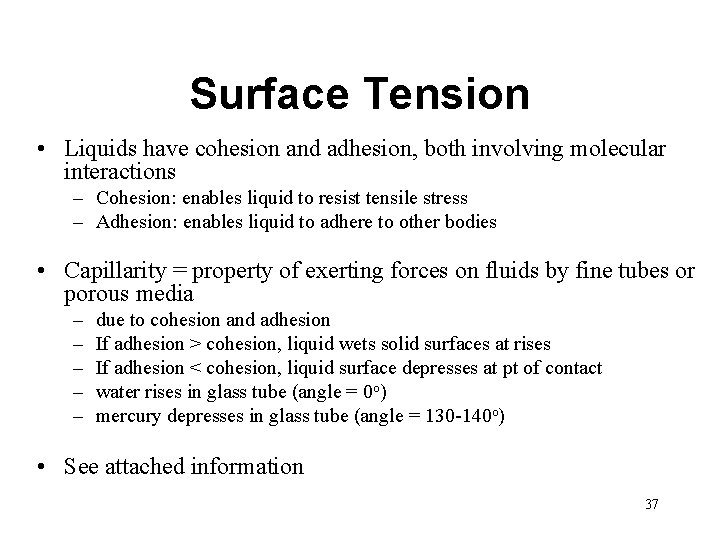
Surface Tension • Liquids have cohesion and adhesion, both involving molecular interactions – Cohesion: enables liquid to resist tensile stress – Adhesion: enables liquid to adhere to other bodies • Capillarity = property of exerting forces on fluids by fine tubes or porous media – – – due to cohesion and adhesion If adhesion > cohesion, liquid wets solid surfaces at rises If adhesion < cohesion, liquid surface depresses at pt of contact water rises in glass tube (angle = 0 o) mercury depresses in glass tube (angle = 130 -140 o) • See attached information 37

Example: Capillary Rise • Given: Water @ 20 o. C, d = 1. 6 mm • Find: Height of water W 38

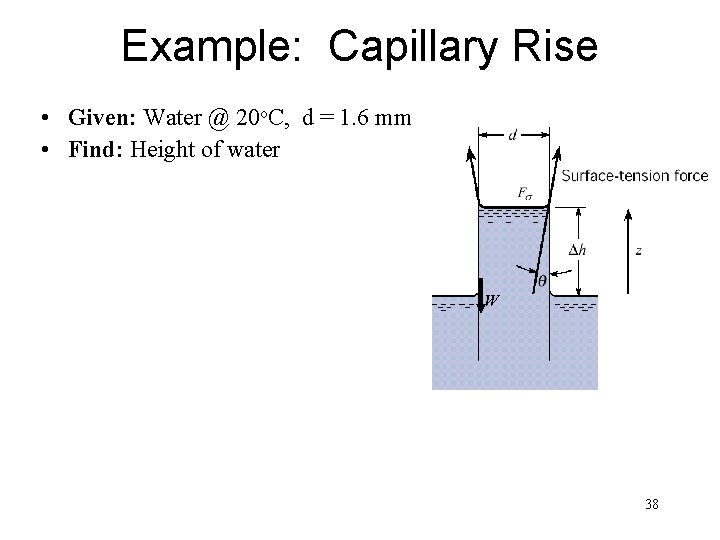
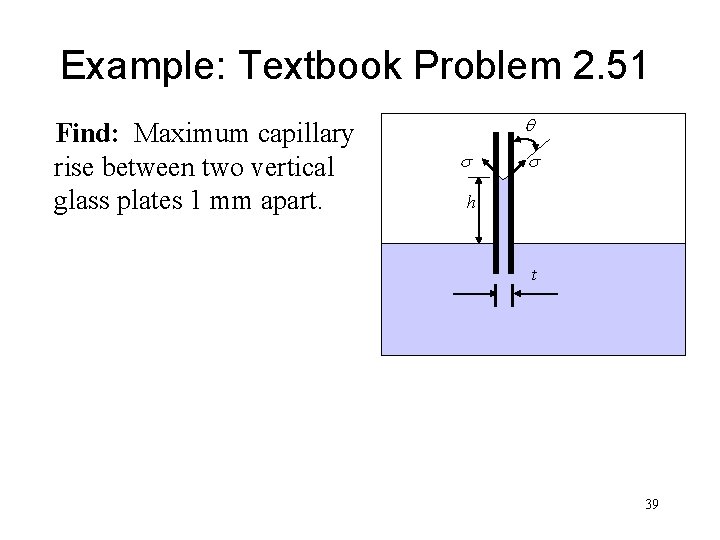
Example: Textbook Problem 2. 51 Find: Maximum capillary rise between two vertical glass plates 1 mm apart. q s s h t 39

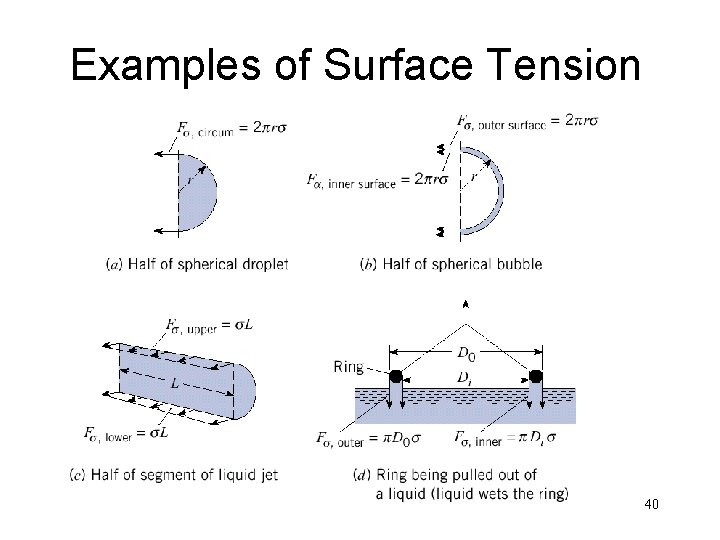
Examples of Surface Tension 40

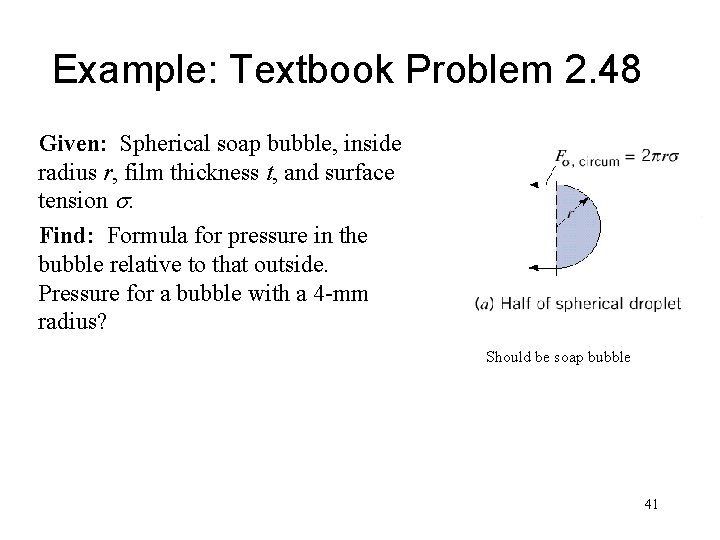
Example: Textbook Problem 2. 48 Given: Spherical soap bubble, inside radius r, film thickness t, and surface tension s. Find: Formula for pressure in the bubble relative to that outside. Pressure for a bubble with a 4 -mm radius? Should be soap bubble 41

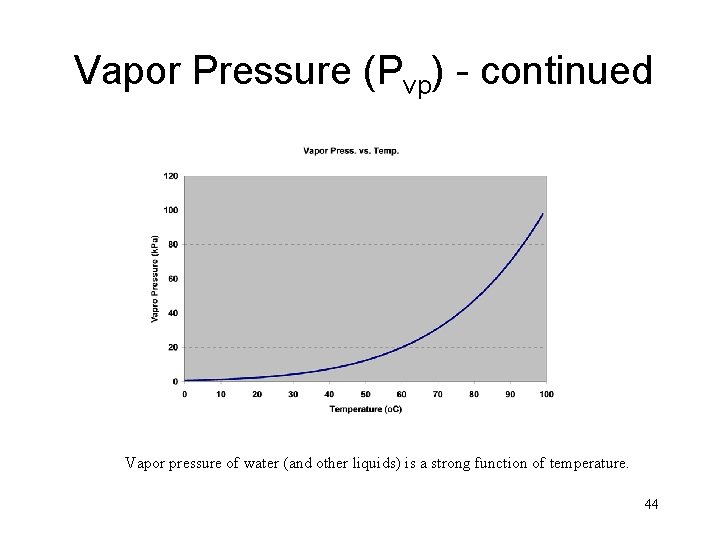
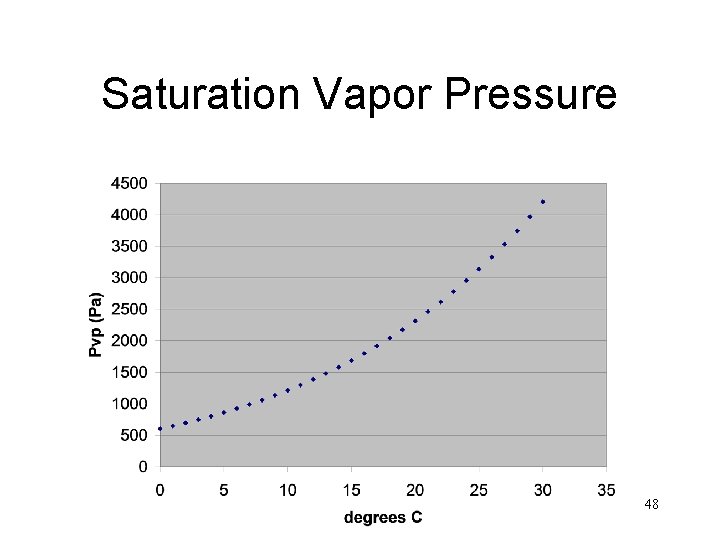
Vapor Pressure (Pvp) • Vapor pressure of a pure liquid = equilibrium partial pressure of the gas molecules of that species above a flat surface of the pure liquid – Concept on board – Very strong function of temperature (Pvp up as T up) – Very important parameter of liquids (highly variable – see attached page) • When vapor pressure exceeds total air pressure applied at surface, the liquid will boil. • Pressure at which a liquid will boil for a given temperature – At 10 o. C, vapor pressure of water = 0. 012 atm = 1200 Pa – If reduce pressure to this value can get boiling of water (can lead to “cavitation”) • If Pvp > 1 atm compound = gas • If Pvp < 1 atm compound = liquid or solid 42

Example • The vapor pressure of naphthalene at 25 o. C is 10. 6 Pa. What is the corresponding mass concentration of naphthalene in mg/m 3? (Hint: you can treat naphthalene vapor as an ideal gas). 43

Vapor Pressure (Pvp) - continued Vapor pressure of water (and other liquids) is a strong function of temperature. 44

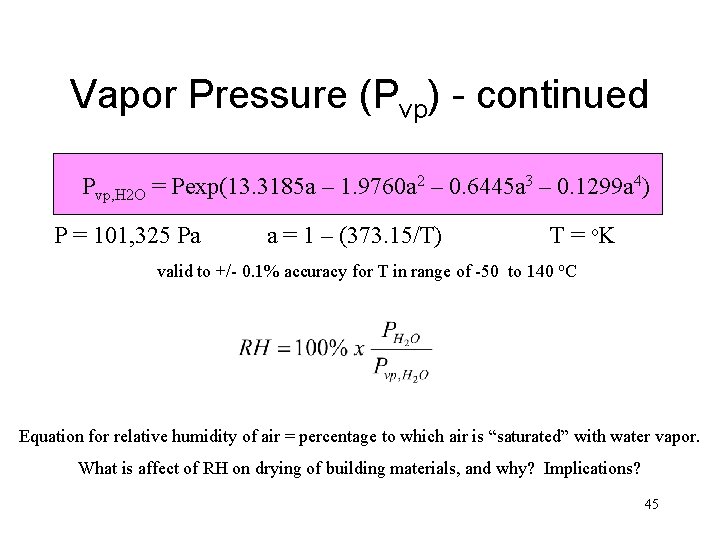
Vapor Pressure (Pvp) - continued Pvp, H 2 O = Pexp(13. 3185 a – 1. 9760 a 2 – 0. 6445 a 3 – 0. 1299 a 4) P = 101, 325 Pa a = 1 – (373. 15/T) T = o. K valid to +/- 0. 1% accuracy for T in range of -50 to 140 o. C Equation for relative humidity of air = percentage to which air is “saturated” with water vapor. What is affect of RH on drying of building materials, and why? Implications? 45

Example: Relative Humidity The relative humidity of air in a room is 80% at 25 o. C. (a) What is the concentration of water vapor in air on a volume percent basis? (b) If the air contacts a cold surface, water may condense (see effects on attached page). What temperature is required to cause water condensation? 46

47

Saturation Vapor Pressure 48

49