Fluid power Presenter Lonnie Wurst NDSCS References Fluid




























- Slides: 30

Fluid power Presenter: Lonnie Wurst NDSCS


References • Fluid Power Systems; Patrick J. Klette, American Technical Publishers, Inc. ; 2010. • Navy Fluid Power; NAVEDTRA 14105; Naval Education and Training Professional Development and Technology Center; 1999. • Various Manufacturer’s supplements

Topics to be Discussed • Properties of Gases • Absolute Pressure and Temperature • Gas laws • Pascal’s Law • Force, Work, and Power • Pneumatic Cylinder Operation • Pneumatic System Sizing

GAS PROPERTIES • No shape- form to the shape of their container • No attraction between gas molecules- only interact through collision • Can be compressed • Pressure affected by volume of container and temperature

Pressure • Measurement of the rate of collision between gas molecules • Units include: • • Pounds/Square Inch (PSI) Atmospheres (ATM) Bar (BAR) Pascals/Kilopascals (Pa/Kpa)

Absolute Pressure • Found by adding gauge pressure with atmospheric pressure • Ex: If a gauge is reading 100 PSI, what is the absolute pressure? • Absolute pressure = gauge pressure + 14. 7 • 100 + 14. 7 = 114. 7 psia Gas Law Formulas use Absolute Temp. Values in Calculation.

Absolute Temperature • Measurement of actual molecular motion • Used in Gas Law Formulas • Absolute Zero- hypothetical temperature at which all molecular motion ceases • Uses Rankine Scale • To convert from Fahrenheit to Rankine: • Rankine = 460 + Fahrenheit • Ex: if a thermometer reads 100 F, what is the temp. in Rankine? • 460 + 100 = 560 R

Boyle’s Law • When a system temperature remains constant, there is an inverse relationship between pressure and volume • When pressure increases, volume decreases • Equations: • V 1*p 1=v 2*p 2 • V 1/v 2=p 2/p 1 • V 1=(p 2*v 2)/p 1 Volume = cubic inches Pressure = psia

Boyle’s Law • Example: If a gas volume of 4 cubic inches at 100 psig is allowed to expand to 6 cubic inches, what will the new pressure be?

Boyle’s Law • Example: If a gas volume of 4 cubic inches at 100 psig is allowed to expand to 6 cubic inches, what will the new pressure be? • Solution: • • V 1*p 1=v 2*p 2 P 2=(v 1*p 1)/v 2 P 2=4(100+14. 7)/6 P 2=76. 47 psia or 61. 77 psig

Charles’s Law • The volume of a gas is proportional to absolute temperature if constant pressure is maintained. • Temperature increases- Volume increases • Formulas: • V 2=(v 1*t 2)/t 1 or • V 1/v 2=t 1/t 2

Gay-Lussac’s Law • When a volume is held constant, pressure and temperature are directly related. • If temperature increases, pressure increases • Formulas: • P 2= (p 1*t 2)/t 1

Gay-Lussac’s Law • Example: A cylinder with 1800 psig at 70 F is left out in the sun and heats up to 130 F. What is the new cylinder pressure?

Gay-Lussac’s Law • Example: A gas cylinder at 1800 psig and 70 F is left out in the sun and heats up to 130 F. What is the new cylinder pressure? • Solution: • • P 2=(p 1*t 2)/t 1 P 2=(1800+14. 7)*(130+460)/(70+460) P 2=(1814. 7*590)/530 P 2= 2020 psia or 2005. 3 psig

Combined Gas Law • Also known as the General Gas Law • Combines Boyle’s, Charles’s, and Gay-Lussac’s laws • Covers all relationships between pressure, temperature, and volume • Formulas: • P 1*v 1*t 2=p 2*v 2*t 1 or • (p 1*v 1)/t 1=(p 2*v 2)/t 2

Combined Gas law • Example: Two cubic feet of a gas at 75 psig and 80 F are compressed to 1 cubic foot and then heated to 300 F. What is the new gauge pressure?

Combined Gas Law • Example: Two cubic feet of a gas at 75 psig and 80 F are compressed to 1 cubic foot and then heated to 300 F. What is the new gauge pressure? • Solution: • • • P 1*v 1*t 2=p 2*v 2*t 1 P 2=(p 1*v 1*t 2)/(v 2*t 1) P 2=(75+14. 7)*2*(760)/(1*540) P 2=(179. 4)*(760)/540 P 2=252. 5 psia or 237. 8 psig


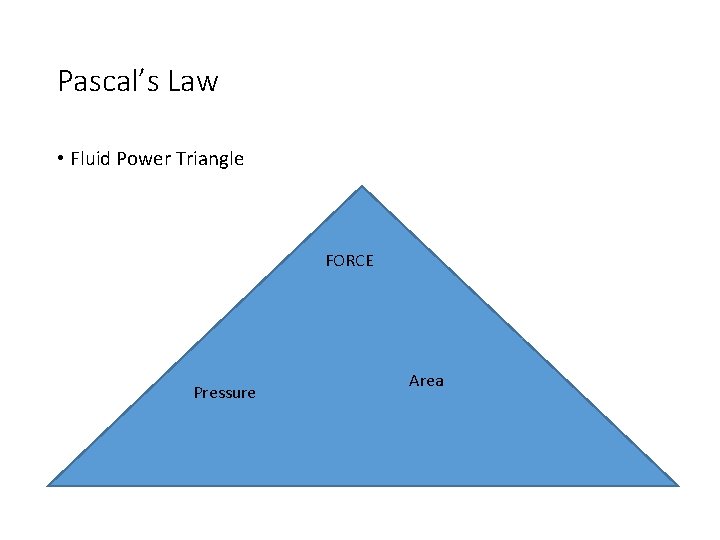
Pascal’s Law • When a force is applied to a fluid in a closed container, the force is equally applied throughout the fluid. • Basis of operation for fluid power

Pascal’s Law • Fluid Power Triangle FORCE Pressure Area

Cylinder Operation • Pneumatic cylinders and other equipment are controlled by three factors: • Pressure • Force • Surface area

Surface Area •

Force • Product of pressure applied to piston face and surface area of piston face in a cylinder • F = P*A • Example: If 100 psi of pressure is applied to a 6 inch diameter air cylinder with a 1 inch diameter rod, what is the force generated during extension? During Retraction?

Force • Solution: • • • Extension: Piston side of cylinder = 0. 7854 * 36 = 28. 27 sq. in. Rod side of cylinder = 0. 7854 * 35 = 27. 49 sq. in. Force = P*A Piston side: force = 28. 27 x 100 = 2827 pounds Rod side: force = 27. 49 x 100 = 2749 pounds

Cylinder Force • Ways to increase cylinder force • Increase operating pressure • Increase surface area of cylinder

Cylinder Sizing • Determine weight of load, including any brackets/fixtures that are attached • Include angle of movement in weight calculation • Add extra force to increase speed • Usually add 50 -100% depending on desired speed • See Bimba Catalog

Cylinder Speed • Exact speed is extremely difficult to calculate • Many factors involved: • • • Air line sizing Cylinder port sizing Length of air lines Flow rate of valves/solenoids System pressure And many more…. .

Coefficient of Velocity • Labeled Cv • Used to measure a component’s ability to allow or resist flow • Higher value- higher ability to pass flow

So…What does this mean? • As a technician: • Need to be aware that designs are not the same between replacement parts • Similar looking parts may have different flow characteristics