Fluid Electrolyte Balance Part 4 Regulation Maintenance Fluid

Fluid & Electrolyte Balance Part 4: Regulation & Maintenance

Fluid n In a lean adult…. . ¨ Females have 55% body mass as fluids ¨ Males have 60% body mass as fluids. n That’s a lot of fluid!

Fluid Compartments n Two fluid compartments where fluids are present! ¨ Intracellular Fluid Compartment: Contains the fluid within the cells – composes 2/3 of the body fluid! n ¨ Intracellular fluid (ICF) aka Cytosol: The bodily fluid actually located inside cells. Extracellular Fluid Compartment: Contains the fluid not inside body cells – the other 1/3 of body fluid! n n n Interstitial Compartment: Contains the fluid between cells. Intravascular Compartments: Contains the fluid within the blood vessels. Extracellular Fluid (ECF): The fluid outside of body cells, including interstitial fluid, plasma, etc. ¨ ¨ Interstitial Fluid: Occupies the microscopic spaces between tissue – 80% of ECF. Intravascular Fluid aka Plasma: The liquid portion of the blood – 20% of the ECF.

Fluid Compartments n Barriers exist between the intracellular fluid, interstitial fluid, & blood plasma. ¨ Plasma Membranes of the Body Cells ¨ Blood Vessel Walls (except for capillaries with thin walls designed to allow leakage)
Fluid Balance n Fluid Balance: The presence of the required amount of water & solutes, in correct proportions, in the all compartments. ¨ Water: 45 -75% of body mass, dependent on age & gender. ¨ Electrolytes: The solid inorganic compounds within the solute that dissociate into ions. n Fluid Balance is maintained by the kidneys via filtration, reabsorption, diffusion, & osmosis.
Water Gain n Preformed Water: Water ingested via food & liquids – roughly 2300 m. L. Metabolic Water: Water produced as a by-product of cellular respiration, depending on the level of aerobic cellular respiration. The more ATP used, the more water is produced – roughly 200 m. L per day. Thirst Center: The area in the hypothalamus that controls the urge to drink – triggered by an increase in osmolarity, Angiotensin II, and a drop in blood pressure. ¨ Thirst also triggered by neurons in the mouth detecting dryness due to decreased salivary production, or baroreceptors detecting low blood pressure in the heart and blood vessels.

Water Loss n Water Loss is controlled via variations in urine volume. ¨ Sodium reabsorption is adjusted proportionately to the amount of water excreted. ¨ ADH is used to minimize water loss by stimulating the collecting tubules to retain more water, causing the sodium levels to become imbalanced. ¨ ADH is inhibited if the blood volume & pressure are too high or osmolarity is too low to encourage water output.
Water Loss n Dehydration: Occurs when the rate of water loss is greater than the rate of water gain. ¨ Causes reduced blood volume, lowered blood pressure, and increased osmolarity of bodily fluids. ¨ Can be triggered by thirst not occurring quickly enough, fluid access being restricted, excessive heat, etc. ¨ The body compensates by decreasing saliva production, decreasing the amount of water excreted by the kidneys, and the triggering of ADH to control water output.
Water Loss n n Total body fluid volume is determined mainly by the extent of urinary salt excretion. Extracellular fluid contains two main ions – Sodium ions (Na+) and Chloride ions (Cl-). ¨ n Excretion rates must be varied to maintain homeostasis. Fluid osmolarity is regulated via water loss. Increases in Na. Cl would lead to increase in plasma Na+ & Clwhich increases osmolarity. ¨ Water moves from intercellular fluid to interstitial fluid then into blood plasma to increase blood volume. ¨ n Renal sodiom & chloride reabsorption and loss via urine is regulated via Angiotensin II, Aldosterone, & Atrial Natriuetic Peptide (ANP).
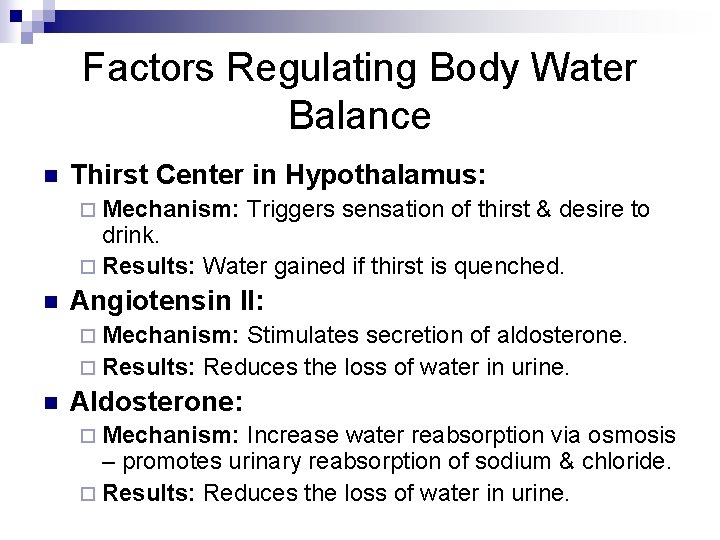
Factors Regulating Body Water Balance n Thirst Center in Hypothalamus: ¨ Mechanism: Triggers sensation of thirst & desire to drink. ¨ Results: Water gained if thirst is quenched. n Angiotensin II: ¨ Mechanism: Stimulates secretion of aldosterone. ¨ Results: Reduces the loss of water in urine. n Aldosterone: ¨ Mechanism: Increase water reabsorption via osmosis – promotes urinary reabsorption of sodium & chloride. ¨ Results: Reduces the loss of water in urine.

Factors Regulating Body Water Balance n Atrial Natriuretic Peptide: ¨ Mechanism: Promotes natriuresis (elevated urinary excretion of sodium, chloride, & water). ¨ Results: Increases the loss of water in urine. n Antidiuretic Hormone: ¨ Mechanism: Promotes reabsorption of water in collecting ducts of kidneys. ¨ Results: Reduces the loss of water in urine.
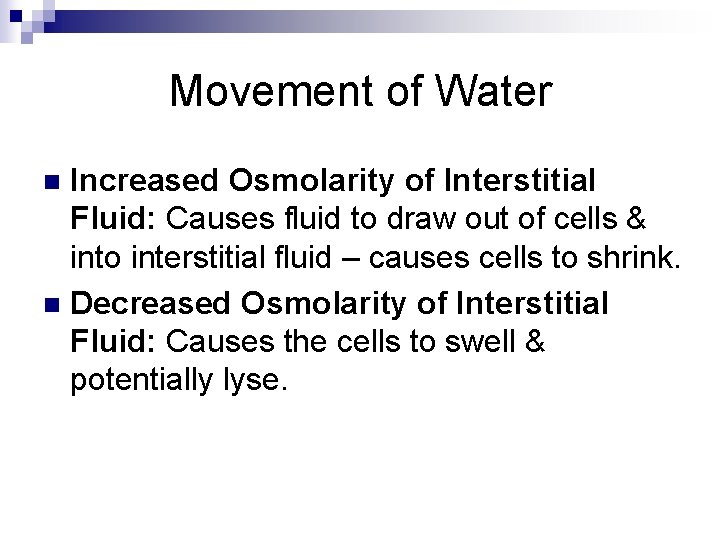
Movement of Water Increased Osmolarity of Interstitial Fluid: Causes fluid to draw out of cells & into interstitial fluid – causes cells to shrink. n Decreased Osmolarity of Interstitial Fluid: Causes the cells to swell & potentially lyse. n

Movement of Water n Water Intoxication: Occurs when the person consumes water faster than the kidneys can excrete it. ¨ Causes excess levels of body water, which causes the cells to swell to dangerous sizes. ¨ Can cause cell lysing and tissue death.

Movement of Water n Loss of Na+ and body water is dangerous! ¨ Can be caused by blood loss, excessive sweating, vomiting, or diarrhea leading to body water loss – if this is replaced by plain water it can cause problems! ¨ Hyponatremia: Na+ concentration in plasma & interstitial fluid to fall below normal, leading to osmolarity falling. Water moves from the interstitial fluid to the intercellular fluid to correct this, causing cells to swell & lyse. ¨ Can lead to convulsions, coma, & possibly death. ¨ Simply add electrolytes – even just a small amount of table salt. Electrolyte drinks are critical when these symptoms are present!
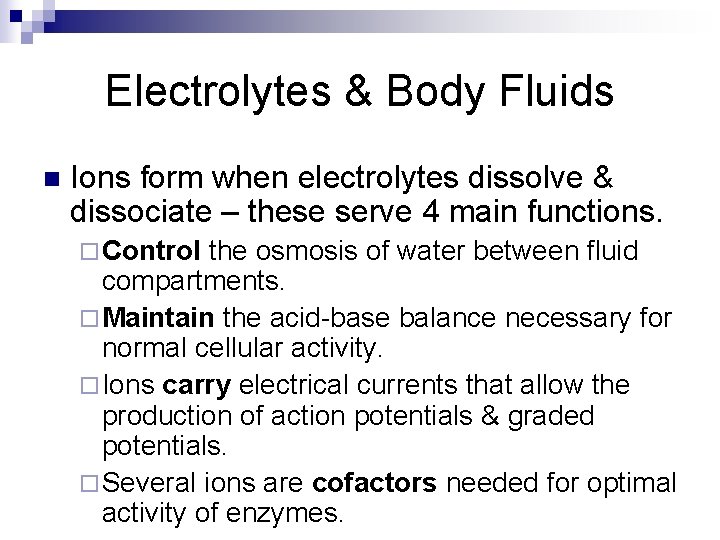
Electrolytes & Body Fluids n Ions form when electrolytes dissolve & dissociate – these serve 4 main functions. ¨ Control the osmosis of water between fluid compartments. ¨ Maintain the acid-base balance necessary for normal cellular activity. ¨ Ions carry electrical currents that allow the production of action potentials & graded potentials. ¨ Several ions are cofactors needed for optimal activity of enzymes.
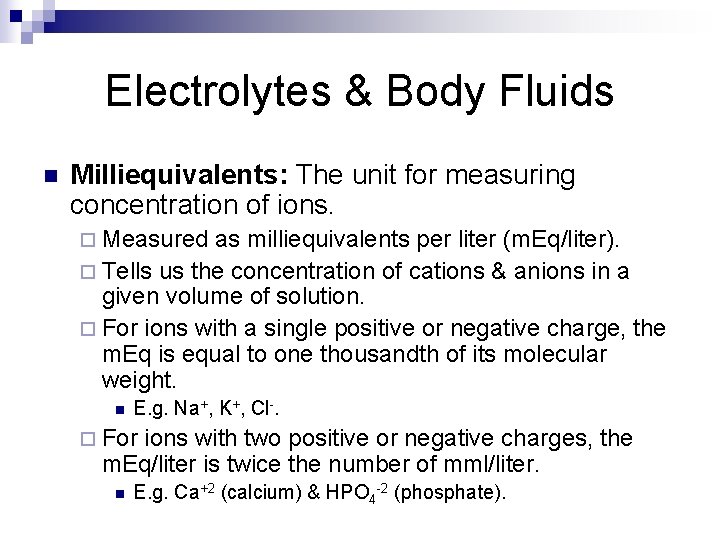
Electrolytes & Body Fluids n Milliequivalents: The unit for measuring concentration of ions. ¨ Measured as milliequivalents per liter (m. Eq/liter). ¨ Tells us the concentration of cations & anions in a given volume of solution. ¨ For ions with a single positive or negative charge, the m. Eq is equal to one thousandth of its molecular weight. n E. g. Na+, K+, Cl-. ¨ For ions with two positive or negative charges, the m. Eq/liter is twice the number of mml/liter. n E. g. Ca+2 (calcium) & HPO 4 -2 (phosphate).

Electrolytes & Body Fluids Major Cations: Sodium, Potassium, Calcium, Magnesium. n Major Anions: Chloride, Bicarbonate, Phosphate. n Intracellular Fluid: K+ is the most abundant cation, while HPO 4 -2 is the most abundant anion. n Extracellular Fluid: Na+ is the most abundant cation, while Cl- is the most abundant anion. n
Electrolytes & Body Fluids n Why are electrolytes important? ¨ They are chemically active & participate in all metabolism. ¨ They determine the electrical potential across cell membranes. ¨ They strongly affect the osmolarity of body fluids & the body’s water content & distribution.
Ions n Sodium: The main ion responsible for resting membrane potential in cells – normal range for blood plasma concentration Is 136 -148 m. Eq/liter. Principle cation of the ECF. Important in determining total body water & its concentration among fluid compartments. ¨ Accounts for half the osmolarity of extracellular fluids. ¨ Primary homeostatic concern is adequate renal excretion of the excess. ¨ ¨ n Aldosterone increases renal reabsorption of sodium. Hyponatremia: Blood plasma sodium concentration below 135 m. Eq/liter – stops the release of ADH, permitting greater excretion of water in urine to restore the sodium in ECF. ¨ Hypernatremia: Blood plasma sodium concentration above 149 m. Eq/liter – triggers Atrial Natriuretic Peptide release to increase sodium excretion by the kidneys. ¨
Ions n Potassium: Greatest contributor to intracellular osmosis & cell volume – normal concentration is 3. 5 – 5. 0 m. Eq/liter. ¨ ¨ ¨ ¨ Most abundant cation in the ICF. Promotes resting membrane potentials & action potentials of nerve & muscle cells. Acts as a cofactor for protein synthesis & some other metabolic processes. Helps regulate p. H of body fluid by “exchanging” itself for hydrogen when moving through cells. 90% of potasssium ions are filtered by the glomerulus & reabsorbed by the PCT – the rest is secreted in urine. Aldosterone is used to control potassium levels in the blood plasma. Hyperkalemia: K+ concentrations in the blood plasma above normal – triggers aldosterone to be secreted, which causes increase excretion of K+ in the urine. Can cause death via ventricular fibrillation. Hypokalemia: K+ concentrations in the blood plasma below normal – prevents aldosterone secretion to minimize K+ excretion in the urine.
Ions n Chloride: Provide a major contribution to the osmolarity of the ECF – normal concentration is 95 – 105 m. Eq/liter. ¨ ¨ ¨ ¨ Most abundant anion in the ECF. Needed for the formation of stomach acid H+CL-. Involved in chloride shift mechanism that accompanies carbon dioxide loading & unloading in the red blood cells & plasma. Homeostasis achieved via sodium balance. Chloride secretion follows sodium’s excretion. Chloride ions easily move between the fluid compartments due to plasma membranes typically containing Cl- leakage channels & antiporters. Hyperchloremia: Blood plasma concentration of chloride above normal. Hypochloremia aka Hypochloraemia: Blood plasma concentration of chloride below normal ranges – rarely occurs as the only problem. Often due to vomiting if accompanied by metabolic alkalosis (decreased blood p. H).
Ions n Calcium: Plays countless roles in the body – normal blood plasma concentration is 4. 5 – 5. 5 m. Eq/liter. Most abundant mineral in the body. 98% of calcium in the body stored in bone. Combines with phosphates to form mineral salts. Mainly an extracellular cation. Makes bones & teeth hard. Plays important roles in blood clotting, neurotransmitter release, maintenance of muscle tone, & the excitability of nervous & muscle tissue. ¨ Calcium concentration in blood plasma regulated via parathyroid hormone (PTH) & calcitriol (vitamin D – to help absorb calcium from food). ¨ ¨ ¨ n If concentrations drop, PTH secretion is stimulated, allowing bone to be broken down and its calcium reabsorbed. Hypercalcemia: An elevated calcium level in the blood, causing increased urinary excretion – can be asymptomatic, but often is a sign of serious disease. ¨ Hypocalcemia: An abnormally low concentration of calcium in the blood plasma – typically triggers the kidneys to reabsorb calcium instead of excreting it. ¨
Ions n Phosphates: A critical component to several systems – normal blood plasma concentration is 1. 7 – 2. 5 m. Eq/liter. ¨ ¨ ¨ ¨ Needed to synthesize ATP, other nucleotide phosphates, nucleic acids, & phospholipids. 85% of phosphates in adults are calcium phosphate salts – a structural component of bones & teeth. 15% of phosphates are ionized. 3 Important Phosphate Ions: Dihydrogen phosphate (H 2 PO 4 -), orthophosphate (PO 4 -3 ), & monohydrogen phosphate (HPO 4 -2). HPO 4 -2 Is the most prevalent form – acts as an important buffer of H+ in body fluids & urine. Regulated by PTH - stimulates the release of phosphates & calcium into the blood stream – inhibits reabsorption of phosphate & stimulates the reabsorption of calcium in the kidneys to lower blood phosphate levels. Regulated by calcitriol (promotes the absorption of phosphates & calcium in the digestive tract).
Ions n Magnesium: One of those components needed for everything – normal blood plasma concentration is 1. 3 – 2. 1 m. Eq/liter. ¨ ¨ ¨ ¨ ¨ Second-most common intracellular cation. 54% of magnesium in adults is part of the bone matrix as magnesium salts. 46% if magnesium in adults is ions in the ECF & ICF. Cofactor for enzymes that metabolize proteins & carbohydrates & the sodium-potassium pumps. Needed for normal neuromuscular activity, synapse transmission & myocardial functioning. Controls the secretion of the parathyroid hormones. Blood plasma levels regulated by the rate at which it is excreted in the urine. Hypermagnesemia: An increase in magnesium concentration due to renal failure, increased in take of Mg+, increases in extracellular fluid volume, decreases Hypomagnesemia: A lowered amount of Mg+, typically due to either an inadequate intake or an excessive loss through urine or feces.
Ions n Bicarbonate: An important metabolic component – normal concentrations from 2. 2 – 2. 6 m. Eq/liter. ¨ Second most prevalent extracelular ion. ¨ HCO 3 - concentration increases as blood the systemic capillaries. n n n flows through CO 2 combines with H 2 O, forming carbonic acid. Carbonic acid dissociates into hydrogen & bicarbonate ions. Bicarbonate decreases as CO 2 is exhaled. ¨ Kidneys mainly responsible for regulating bicarbonate concentration. ¨ Intercalated cells in renal tubules form bicarbonate & release it into the blood stream when levels are low, or excrete the excess if levels are high.
Acid-Base Balance n Acid-Base Balance: The major homeostatic challenge responsible for keeping p. H (H+ concentration) of body fluids at the correct level. ¨ ¨ ¨ n n n This is vital for normal cellular function. Remember: Blood p. H is normally 7. 35 -7. 45! Any excess H+, which is a normal metabolic waste, must be removed from the body. Buffer Systems: Act quickly to temporarily bind H+ to raise p. H, but they don’t remove H+ from the body. Exhalation of CO 2: Increases the rate & depth of breathing to cause more CO 2 to be exhaled – this reduces the carbonic acid in the blood to raise p. H and reduce H+ levels. Kidney Excretion of H+: Urination eliminates acids other than carbonic acid – slowest mechanism for p. H change, but only method of removing acids.
Buffer Systems n n Buffer System: Consists of a weak acid & its salt (that functions as a weak base). Acid: A chemical that releases H+ into a solution. Base: Chemical that accepts H+. Buffer: Any mechanism that resists changes in p. H by rapidly converting a strong acid or base into a weak acid or base. Strong acids lower p. H more than weak acids due to their readily releasing H+. ¨ Strong bases raise p. H more than weak bases do. ¨ n 3 Main Buffer Systems: ¨ ¨ ¨ Protein Buffer System Carbonic Acid-Bicarbonate Bugger System Phosphate Buffer System
Protein Buffer Systems n Protein Buffer System: Most abundant buffer in intracellular fluid & blood plasma – can buffer acids or bases. ¨ Proteins are composed of amino acids with one carboxyl group (-COOH), which is a functional component of the buffer, & one amino group (NH 2). ¨ The free carboxyl group at one end of a protein acts like an acid by releasing H+ when p. H rises. ¨ When it dissociates, the H+ can react with excess OHin the solution to form water. ¨ NH 2 groups can act as a base by combining with H+ when p. H falls.

Carbonic Acid-Bicarbonate Buffer System n Carbonic Acid-Bicarbonate Buffer System: Includes bicarbonate ions acting as a weak base, and carbonic acid acting as a weak acid. ¨ Excessive H+ would cause bicarbonate ions to function as a weak base, removing excess H+. ¨ The carbonic acid dissociates into water & carbon dioxide, and the CO 2 is exhaled from the lungs. ¨ If there is a deficiency of H+ then carbonic acid can function as a weak base to relase more H+.
Phosphate Buffer System n Phosphate Buffer System: Works similarly to the carbonic acid-bicarbonate buffer system. ¨ ¨ ¨ Dihydrogen phosphate (H 2 PO 4 -) and monohydrogen phosphate (HPO 42 -) work as the major components. Phosphates are major anions in intracellular fluid & minor anions in extracellular fluid. Combining a strong base, such as OH- with a weak acid such as dihydrogen phosphate yields monohydrogen phosphate (a weak base). Monohydrogen phosphate ions can act as a weak base to buffer H+ released by strong acids (such as hydrochloric acid (HCL)). Dihydrogen phosphate forms in the presence of excess H+ in the kidney tubules that then combines with monohydrogen phosphate – the H+ then passes into the urine. The concentration of phosphates is higher in intracellular fluid, causing the buffer system to regulate within the cells.
Exhalation of Carbon Dioxide n n Breathing helps maintain the p. H of body fluid by exhaling CO 2. Exhaling CO 2 removes carbonic acid from the system, which raises the blood p. H. ¨ This is why carbonic acid is called a volatile acid (an acid produced from carbon dioxide). n n An increase in ventilation causes more CO 2 to be exhaled, resulting in H+ concentration falling & blood p. H raising. Decreases in ventilation causes less CO 2 to be exhaled, resulting in H+ concentration rising & blood p. H falling.
Renal Regulation n n Nonvolatile Acids: An acid produced from a source other than carbon dioxide, such as metabolic reactions. H+ secretion in the urine is the only way to get rid of these acids. Cells in the PTC & collecting ducts secrete H+ ions into the tubular fluid. ¨ Intercalated cells have apical membranes containing proton pumps (H+ ATPases) that secrete H+ into the tubular fluid. ¨ n n ¨ Bicarbonate ions inside the reneal intercalated cells cross the basolateral membrane by Cl-/HCO 3 - antiporters, then diffuse into peritubular capillaries. Some intercallated cells have proton pumps in the basolateral memrbanes and Cl-/HCO 3 - antiporters in the apical membranes – these secrete bicarbonate ions & reabsorb hydrogen. Intercallated cells regulate p. H by excreting excess bicarbonate ions when p. H is too high and H+ when p. H is too low.
Mechanisms Maintaining Fluid p. H n n n Buffer Systems: Mostly consist of a weak acid & that acid’s salt, which functions as a weak base – prevents drastic changes in p. H. Proteins: Most abundant buffers in body cells & blood – histidine & cysteine (amino acids) contribute most of the buttering along with hemoglobin. Carbonic Acid-Bicarbonate Phosphates: Important regulator of blood p. H – most abundant buffer in ECF – important ion intracellular fluid & urine. Exhalation of CO 2: Increase exhalation raises p. H while decreased exhalation lowers p. H. Kidneys: Renal tubules secrete H+ into the urine & reabsorb HCO 3 so it is not lost.
Acid-Base Imbalances n Acidosis (Acidemia): Occurs when the blood p. H falls below 7. 35, resulting in H+ diffusing into the cells and driving out potassium. Depresses the central nervous system by inhibiting synaptic transmission. ¨ Symptoms include confusion, disorientation, & coma (if p. H falls below 7). ¨ n Alkalosis (Alkalemia): Occurs when the blood p. H rises higher than 7. 45, resulting in H+ diffusing out of the cells while potassium diffuses in. Lowers the potassium concentration in the ECF. ¨ Over-excites the central & peripheral nervous systems, causing nervousness, muscle spasms, convulsions, and sometimes even death. ¨
Acid-Base Imbalances Respiratory Acidosis & Alkalosis: Result from changes in the partial pressure of CO 2 in the blood – compensated for by the kidneys. n Metabolic Acidosis & Alkalosis: Result from changes in the concentration of HCO 3 in the blood – compensated for by the lungs. n
Acid-Base Imbalances n Compensation: The response to an acid-base imbalance that seeks to normalize arterial blood p. H. ¨ Respiratory Compensation: Seeks to correct altered p. H dye to metabolic causes via hyperventillation & hypoventillation. n ¨ Renal Compensation: A change in the secretion of hydrogen & reabsorption of bicarbonate ions by the kidney tubules that counters altered p. H due to respiratory causes. n n Used to counter the effects of metabolic acidosis & metabolic alkalosis by elevating the bicarbonate concentration & p. H of the urine. Used to counter the effects of respiratory acidosis & respiratory alkalosis. Simple Version: If the lungs caused it, the kidneys try to fix it. If the kidneys caused it, the lungs try to fix it.
Acid-Base Imbalances n Respiratory Acidosis: Increased PCO 2 (above 45 mm. Hg) and decreased p. H (below 7. 35) if no compensation occurs. ¨ Common Causes: Hypoventilation due to emphysema, pulmonary edema, respiratory trauma, airway obstruction, or dysfunction of the respiratory muscles. ¨ Compensatory Mechanisms: Renal – kidneys increase the excretion of H+ & increase the reabsorption of HCO-3. n If compensation completes, p. H will be normal but PCO 2 will be high.
Acid-Base Imbalances n Respiratory Alkalosis: Decreased PCO 2 (below 35 mm. Hg) & increased p. H (above 7. 45) if no compensation occurs. ¨ Common Causes: Hyperventilation due to oxygen deficiency, pulmonary disease, cerebrovascular accident (CVA), or severe anxiety. ¨ Compensatory Mechanisms: Renal – kidneys decrease the excretion of H+ & decrease the reabsorption of HCO-3. n If compensation is complete, p. H will be normal but PCO 2 will be low.
Acid-Base Imbalances n Metabolic Acidosis: Decreased HCO 3 (below 22 m. Eq/liter) & decreased p. H (below 7. 35) if no compensation occurs. ¨ Common Causes: Loss of bicarbonate in the ions due to diarrhea, accumulation of acid (ketosis) or renal dysfunction. ¨ Compensatory Mechanisms: Respiratory – hyperventilation occurs to increase the loss of CO 2. n If compensation completes, p. H will be normal but HCO-3 will be low.
Acid-Base Imbalances n Metabolic Alkalosis: Increased HCO 3 (above 26 m. Eq/liter) & increased p. H (above 7. 45) if no compensation occurs. ¨ Common Causes: Loss of acid due to vomiting, gastric suctioning, use of certain diuretics or excessive intake of alkaline drugs. ¨ Compensatory Mechanisms: Respiratory – hypoventillation, which slows down the loss of CO 2. n If compensation completes, p. H will be normal but HCO-3 will be high.
- Slides: 40