Fluid electrolyte acid base balance Fluid intake derived

Fluid, electrolyte & acid – base balance Fluid intake : derived from 2 sources 1. Exogenous water ; from drink & solid food, average 23 liters / 24 h Children needs more fluid requirement due to: • Larger surface area • Greater metabolic activity • Immature kidneys (poor concentrating ability)

2. Endogenous water: Released during oxidation of ingested food ( during starvation from breakdown of body tissues) Normally constitutes < 500 ml / 24 h
Fluid output Water is lost from the body by 4 routes : By the lungs: about 400 ml/24 h lost in expired air, it increased in : dry atmosphere tachypnea tracheostomy By the skin: average loss 600 -1000 ml/24 h In a temperate climate as invisible perspiration

Faeces : 60 – 150 ml of water s daily lost, in diarrhea the amount is greatly multiplied Urine : average 1500 ml/24 h minimum 400 ml/24 h is required to excrete end-product of protein metabolism However, urine output is under control of multiple influences like blood volume, hormonal & nervous factors among which ADH plays a major role in controlling a tonicity of body fluids, by stimulating reabsorption of water from renal tubules, thus varying the amount excreted after the requirement of first three routes have been met.

water depletion 1. Diminished intake : due to Lack of availability Difficult swallowing e. g. any painful conditions of mouth, pharynx Inability of swallowing e. g. esophageal obstruction, paresis of pharyngeal muscle 2. Increased loss : e. g. after tracheostomy

Clinical features : Weakness & intense thirst (the main symptoms) diminished U. OP + increased specific gravity Intracellular dehydration. this delays onset of overt compensated hypovolemia

Water intoxication 1. 2. Excessive water, low sodium intake by any route, excessive hypotonic solutions e. g. overprescribing of dextrose 5% to postoperative pt. , also may occur in colorectal washout with plain water or during TURP SIADH ; occur in association with lung condition (pneumonia, empyema), oat cell carcinoma of bronchus, head injury
C/F : Drowsiness, weakness, sometimes convulsion & coma Nausea & vomiting of clear fluid In SIADH, large amount of dilute urine Lab. Test; low level of PCV, serum Na & other electrolytes concentrations Tx : Water restriction, if failed ICU admission & controlled manipulation of fluid & electrolytes

Electrolyte balance 2 kinds : 1. Cations, electropositive & include sodium, potassium, calcium, magnesium 2. Anions, electronegative & include Chloride, phosphate, bicarbonate & sulphate Described in mmol/litre Their distribution within fluid compartment of body controls passage of water through the cell walls & maintains acid –base equilibrium

Sodium balance It is the principle cation of extracellular fluid Total body amount = 5000 mmol-44% ECF -9% ICF -47%bone daily intake of Nacl =1 mmol/kg =500 ml of isotonic 0. 9% saline Equivalent amount is excreted daily mainly in urine, some in feces & sweating

Control by adrenal corticoids: The most powerful conservator of Na being Aldosterone (in the renal tubules & sweat gland) Following trauma there is shutdown of Na excretion due to increased adrenal activity, hence it is inadvisable to give large amount of isotonic saline 0. 9% after operation

Sodium depletion Causes : Small bowel obstruction High intestinal external fistula Severe diarrhea ((hyponatremia+ acidosis) Adrenocortical insufficiency (hyponatremia+ hyperkalemia) SIADH Postoperative dilutional hyponatremia. . ? Na robbing by NGT…. ?
Clinical features are due to extracellular dehydration & that : Sunken eye & drawn face Depressed anterior fontanelle (in infant) Coated, dry tongue Dry, wrinkled skin Collapsed vein, the S. C. tissue feels lax pt. Looks older than his age Thirst is not marked or evident…why? Scanty, dark urine Hemoconcentration (high PCV) Low serum Na, low urinary Na

Postoperative hyponatremia It is also called dilutional hyponatremia in which there is normal or increased extracellular fluid volume It occurs due to prolonged administration of sodium free solution = water intoxication

Sodium excess (hypernatremia) It arise when excessive amount of 0. 9% saline solution is given in early postoperative period resulting in overloading of circulation with salt & its accompanying water

C/F : Puffiness of face is the early sign Pitting edema especially in sacral region ( at least 4. 5 liters of excess fluid is accumulated in tissue spaces to give pitting edema) In infants who are very susceptible to overhydration, there are tense fontanelle, increased weight, increased frequency of urinations & edema

Potassium balance K is almost entirely intracellular (98%) & only 2% is extracellular 3/4 of total body K (3500 mmol) is found n skeletal muscles Normal daily requirement = 1 mmol/Kg After trauma there is augmented excretion of K especially in first 24 hour

Hypokalemia It can occur suddenly or gradually Sudden hypokalemia: occur most frequently in diabetic coma treated by insulin & prolonged infusion of saline solution Gradual hypokalemia: It is the type encountered in surgical practice
Causes : Preoperative chronic hypokalemia as result medications e. g. diuretics or cancer pt with cachexia, weight loss & K depletion Diarrhea from ulcerative colitis, villous tumors of rectum External fistula e. g. duodenal fistula, ileostomy Prolonged gastrodoudenal aspiration without replacement of K in IV fluid
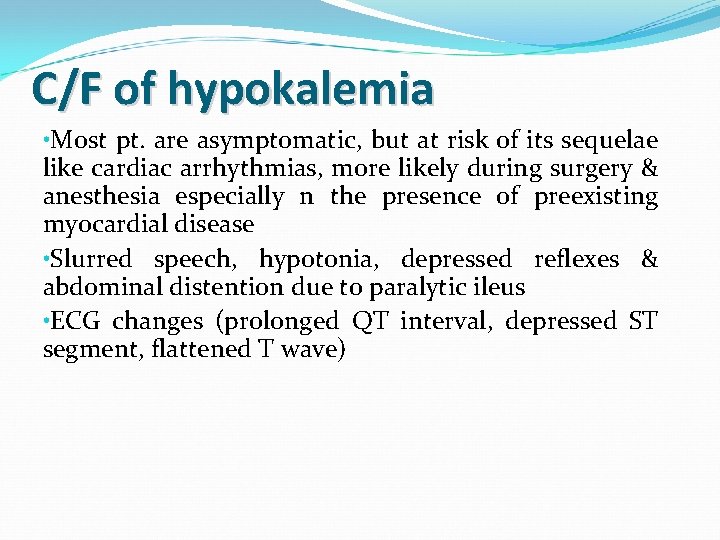
C/F of hypokalemia • Most pt. are asymptomatic, but at risk of its sequelae like cardiac arrhythmias, more likely during surgery & anesthesia especially n the presence of preexisting myocardial disease • Slurred speech, hypotonia, depressed reflexes & abdominal distention due to paralytic ileus • ECG changes (prolonged QT interval, depressed ST segment, flattened T wave)

Treatment Oral K : In form of milk, juices, honey, . . Effervesnt tablets of KCL 2 gm 6 hourly Intravenous K : Urine output should be adequate Administration should be properly controlled & level of K checked daily Given in form of infusion
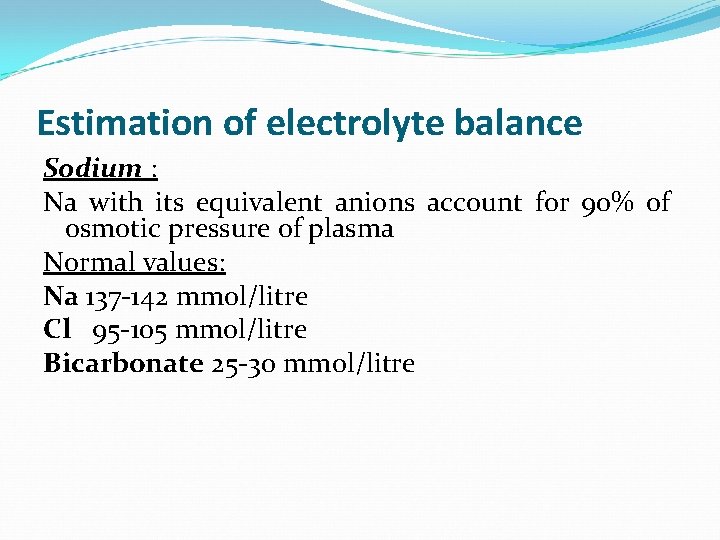
Estimation of electrolyte balance Sodium : Na with its equivalent anions account for 90% of osmotic pressure of plasma Normal values: Na 137 -142 mmol/litre Cl 95 -105 mmol/litre Bicarbonate 25 -30 mmol/litre

Potassium: Intracellular deficiency of K may be present in spite of normal plasma concentration Deficiency is to be expected if oral feeding is withheld for > 4 days Normally 3. 5 – 5 mmol/litre

Calcium : It is an extracelluar cation Plasma level= 2. 2 – 2. 5 mmol/litre It exist in three forms : 1. Protein bound 2. Free non-ionised 3. Free ionised, it is the active form (for blood coagulation, neuromuscular excitability) Ionised form falls with increasing PH, hence tetany may occur in respiratory alkalosis (as a result of hyperventilation)
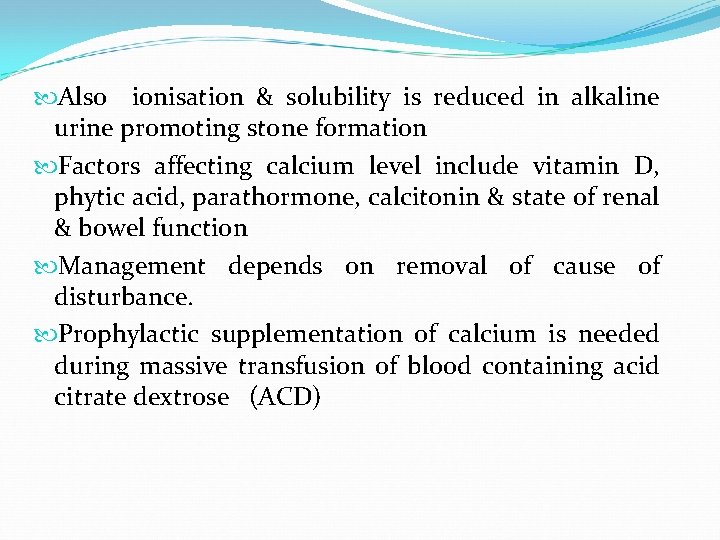
Also ionisation & solubility is reduced in alkaline urine promoting stone formation Factors affecting calcium level include vitamin D, phytic acid, parathormone, calcitonin & state of renal & bowel function Management depends on removal of cause of disturbance. Prophylactic supplementation of calcium is needed during massive transfusion of blood containing acid citrate dextrose (ACD)

Magnesium : It is an intracellular cation It shares some of properties of potassium & some of calcium Normal level= 0. 7 – 0. 9 mmol/litre Average daily intake 10 mmol/litre Its deficiency occur following prolonged loss of GIT secretions due to fistulae, ulcerative colitis, . . , also in liver cirrhosis, diseases of parathyroid
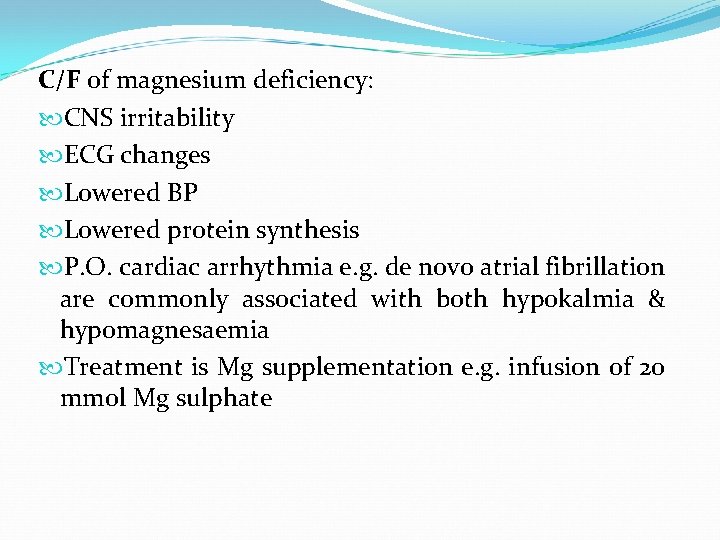
C/F of magnesium deficiency: CNS irritability ECG changes Lowered BP Lowered protein synthesis P. O. cardiac arrhythmia e. g. de novo atrial fibrillation are commonly associated with both hypokalmia & hypomagnesaemia Treatment is Mg supplementation e. g. infusion of 20 mmol Mg sulphate
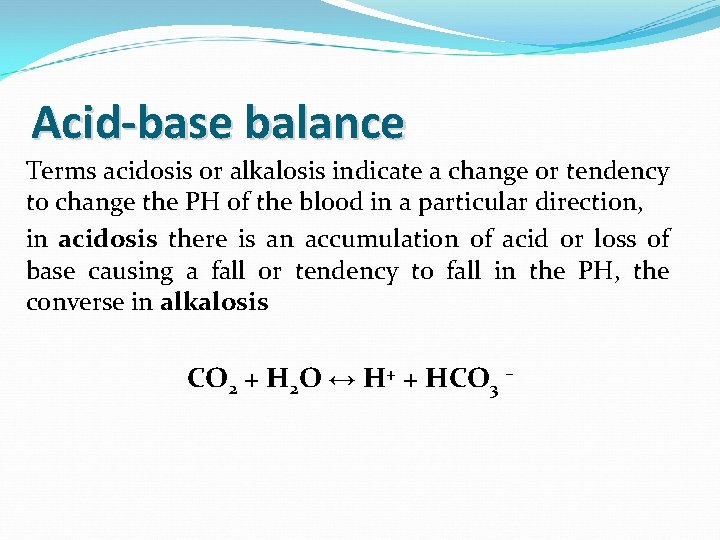
Acid-base balance Terms acidosis or alkalosis indicate a change or tendency to change the PH of the blood in a particular direction, in acidosis there is an accumulation of acid or loss of base causing a fall or tendency to fall in the PH, the converse in alkalosis CO 2 + H 2 O ↔ H+ + HCO 3 –

Regulatory control By various buffering system : bicarbonate-carbonic acid ratio Lungs by removal of carbon dioxide Kidneys by excretion of both acids & base

Normal bicarbonate-carbonic acid ratio is 20: 1, a decrease in ratio leads to acidity & vice versa Bicarbonate level is altered by metabolic factors whereas carbonic acid altered by respiratory factors, alteration of one is followed automatically by a compensatory alteration in the other, so that the ratio & therefore PH of blood remains constant
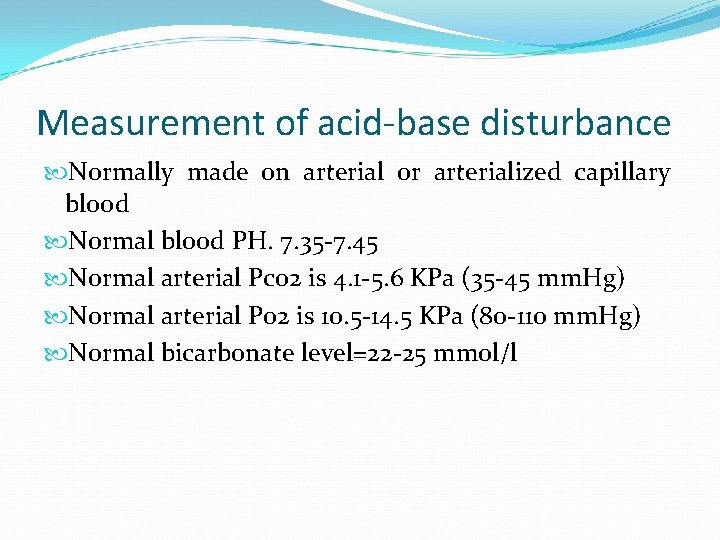
Measurement of acid-base disturbance Normally made on arterial or arterialized capillary blood Normal blood PH. 7. 35 -7. 45 Normal arterial Pco 2 is 4. 1 -5. 6 KPa (35 -45 mm. Hg) Normal arterial Po 2 is 10. 5 -14. 5 KPa (80 -110 mm. Hg) Normal bicarbonate level=22 -25 mmol/l

Metabolic alkalosis § p. H > 7. 45 § bicarbonate > 26 m. Eq/L Causes: Excessive ingestion of absorbable alkali e. g. antacids medication Loss of acid from the stomach as a result of repeated vomiting or aspiration Cortisone excess, due to overuse of corticoids medication or a result of Cushing syndrome
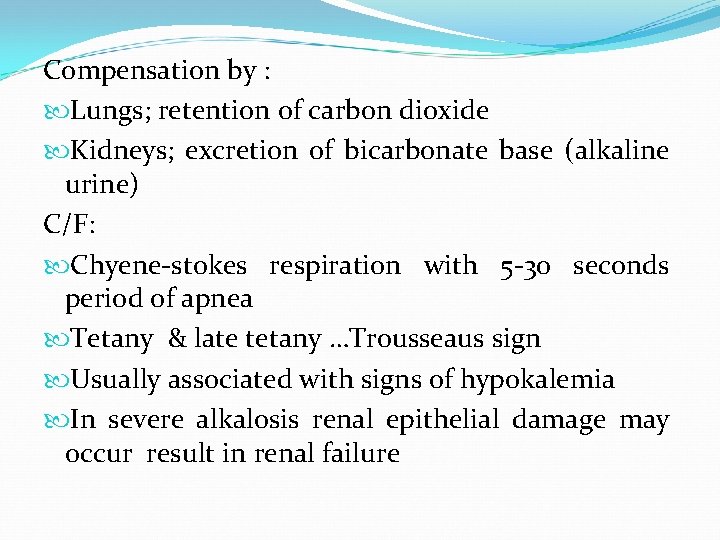
Compensation by : Lungs; retention of carbon dioxide Kidneys; excretion of bicarbonate base (alkaline urine) C/F: Chyene-stokes respiration with 5 -30 seconds period of apnea Tetany & late tetany …Trousseaus sign Usually associated with signs of hypokalemia In severe alkalosis renal epithelial damage may occur result in renal failure

Treatment : Metabolic alkalosis without hypokalemia seldom requires treatment apart from removal the cause of alkalosis & ensuring high urine output

Metabolic acidosis § p. H < 7. 35 § bicarbonate - < 22 m. Eq/L It is a condition in which there is a deficit of base or an excess of any acid other than carbonic acid, occur as a result of : 1. Increase in fixed acids; due to formation Ketone bodies…. DM, starvation Metabolites…renal failure Lactic & pyruvic acid…release of aortic clamp or cardiac arrest ureterosigmoidostomy

2. Loss of base ; as in sustained diarrhea, ulcerative colitis, gastrocolic fistula, a high intestinal fistula or prolonged intestinal aspiration C/F : Rapid, deep noisy breathing ( due to overstimulation of respiratory centre) Urine is acidic except in renal acidosis Serum bicarbonate is reduced

Treatment Correction of underlying pathology Ensuring adequate tissue perfusion Bicarbonate solution is given in severe case of acidosis

Hyokalemic alkalosis Occur as a result of repeated vomiting e. g. pyloric stenosis Low serum K causes K to leaves the cell & replaced by Na & H leading to IC acidosis, kidneys conserve K & secrete H instead Tx : replacement with IV fluid & KCL infusion + ECG monitoring, the loss may reach to >1000 mmol

Respiratory alkalosis p. H > 7. 45 pa. CO 2 < 35 mm Hg Occur when CO 2 tension is below normal level as a result of hyperventilation Causes : Excessive pulmonary ventilation during anesthesia High altitudes, hyperpyrexia, a lesion of hypothalamus & hysteria

Compensation by renal excretion of bicarbonate During anesthesia, alkalosis is accompanied by pallor & hypotension & in severe cases respiratory arrest follows Tx : respiratory suppression due to alkalosis is corrected by insufflation of CO 2 + reverse underlying cause
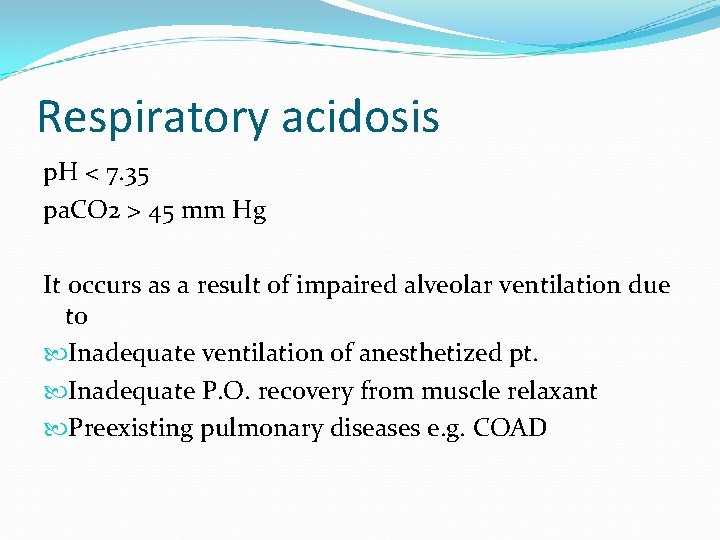
Respiratory acidosis p. H < 7. 35 pa. CO 2 > 45 mm Hg It occurs as a result of impaired alveolar ventilation due to Inadequate ventilation of anesthetized pt. Inadequate P. O. recovery from muscle relaxant Preexisting pulmonary diseases e. g. COAD

The anion gap It is a calculated estimation of the undetermined anions in the blood, it also used to establish the cause of metabolic acidosis Anion gap = (Na+K)-(HCO 3+Cl) The normal value is 10 -16 mmol

Metabolic acidosis + increased anion gap Ketoacidosis Lactic acidosis Salicylates poisoning Renal failure Metabolic acidosis + normal anion gap : RTA Loss of alkali due to diarrhea, intestinal obstruction or fistula Hyperchloremia of ureterocolic anastomosis
- Slides: 43