Fluid and Electrolyte Balance Muse Lecture 10 32311

Fluid and Electrolyte Balance Muse Lecture #10 3/23/11 Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
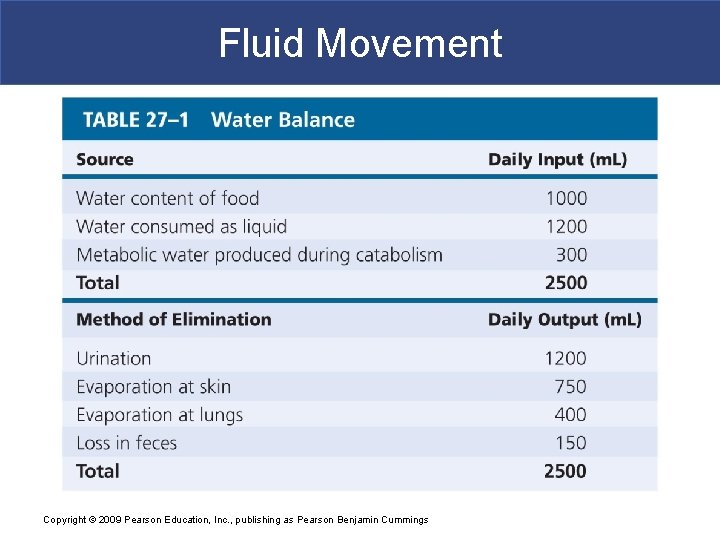
Fluid, Electrolyte, and Acid–Base Balance § Fluid Balance § Is a daily balance between § Amount of water gained § Amount of water lost to environment § Involves regulating content and distribution of body water in ECF and ICF Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid, Electrolyte, and Acid–Base Balance § The Digestive System § Is the primary source of water gains § Plus a small amount from metabolic activity § The Urinary System § Is the primary route of water loss Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid, Electrolyte, and Acid–Base Balance § Electrolytes § Are ions released through dissociation of inorganic compounds (Na+ , K+, Cl-, CO 3 - ) § Can conduct electrical current in solution § Electrolyte balance § When the gains and losses of all electrolytes are equal § Primarily involves balancing rates of absorption across digestive tract with rates of loss at kidneys and sweat glands Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid, Electrolyte, and Acid–Base Balance § Precisely balances production and loss of hydrogen ions (p. H) § The body generates acids during normal metabolism § Tends to reduce p. H Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid, Electrolyte, and Acid–Base Balance § The Kidneys § Secrete hydrogen ions into urine § Generate buffers that enter bloodstream § In distal segments of distal convoluted tubule (DCT) and collecting system § The Lungs § Affect p. H balance through elimination of carbon dioxide Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Compartments § Water Exchange § Water exchange between ICF and ECF occurs across plasma membranes by § Osmosis § Diffusion § Carrier-mediated transport Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Body Fluid Compartments § In lean adults, body fluids constitute 55% of female and 60% of male total body mass § Intracellular fluid (ICF) inside cells § About 2/3 of body fluid § Extracellular fluid (ECF) outside cells § Interstitial fluid between cell is 80% of ECF § Plasma in blood is 20% of ECF Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings § Also includes lymph, cerebrospinal fluid, synovial
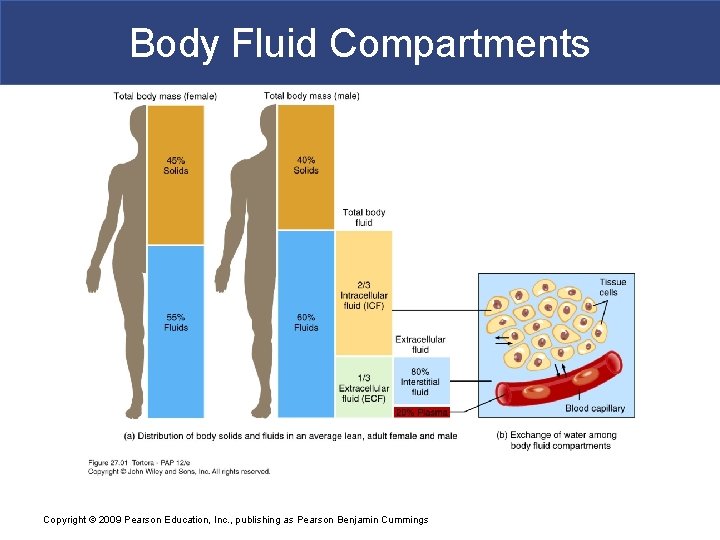
Body Fluid Compartments Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
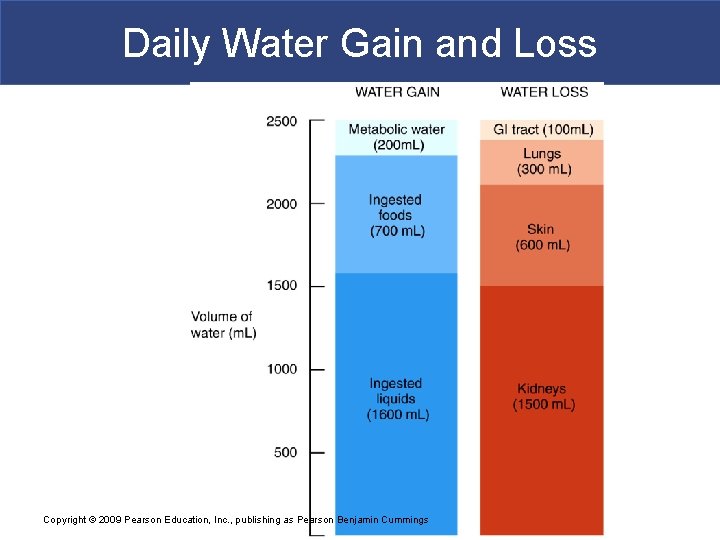
Daily Water Gain and Loss Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fluid Compartments § Major Subdivisions of ECF § Interstitial fluid of peripheral tissues § Plasma of circulating blood Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Compartments § Minor Subdivisions of ECF § Lymph, perilymph, and endolymph § Cerebrospinal fluid (CSF) § Synovial fluid § Serous fluids (pleural, pericardial, and peritoneal) § Aqueous humor Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
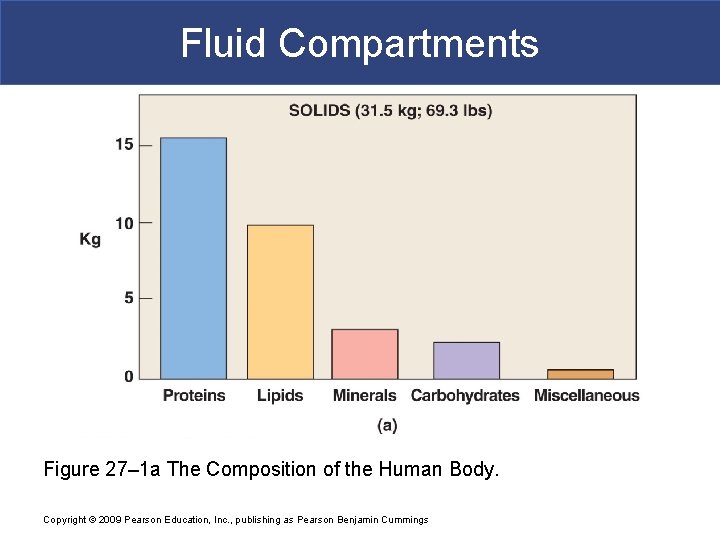
Fluid Compartments Figure 27– 1 a The Composition of the Human Body. Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
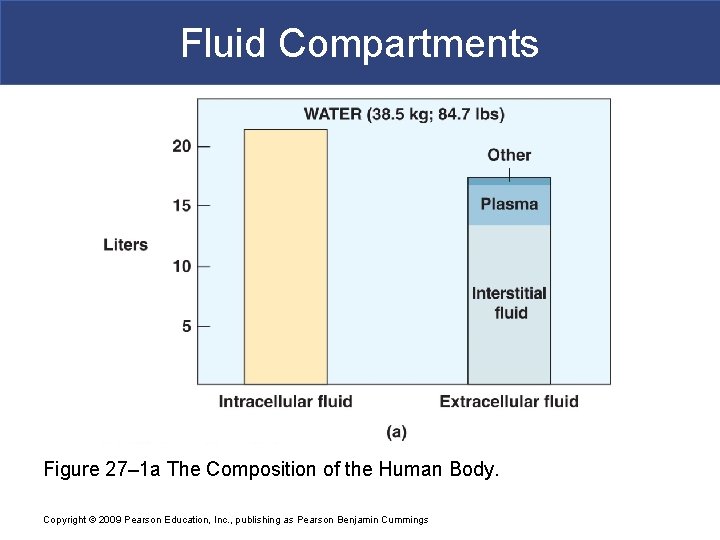
Fluid Compartments Figure 27– 1 a The Composition of the Human Body. Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Movement § Edema § The movement of abnormal amounts of water from plasma into interstitial fluid Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
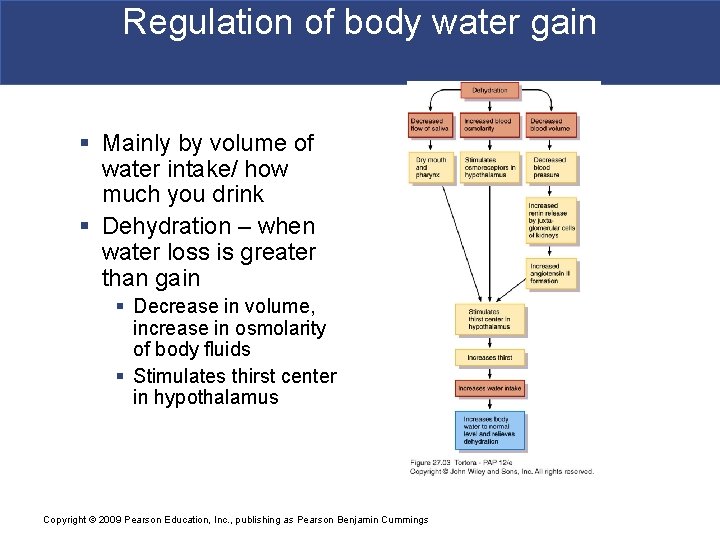
Regulation of body water gain § Mainly by volume of water intake/ how much you drink § Dehydration – when water loss is greater than gain § Decrease in volume, increase in osmolarity of body fluids § Stimulates thirst center in hypothalamus Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Regulation of water and solute loss § Elimination of excess body water through urine § Extent of urinary salt (Na. Cl) loss is the main factor that determines body fluid volume § Main factor that determines body fluid osmolarity is extent of urinary water loss § 3 hormones regulate renal Na+ and Cl- reabsorption (or not) § Angiotensin II and aldosterone promote urinary Na+ and Cl- reabsorption of (and water by osmosis) when dehydrated Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Compartments § An Overview of the Primary Regulatory Hormones § Affecting fluid and electrolyte balance: – Antidiuretic hormone – Aldosterone – Natriuretic peptides Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Compartments § ADH Production § Osmoreceptors in hypothalamus § Monitor osmotic concentration of ECF § Change in osmotic concentration § Alters osmoreceptor activity § Osmoreceptor neurons secrete ADH Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Major hormone regulating water loss is antidiuretic hormone (ADH) § Also known as vasopressin § Produced by hypothalamus, released from posterior pituitary § Promotes insertion of aquaporin-2 into principal cells of collecting duct § Permeability to water increases § Produces concentrated urine Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
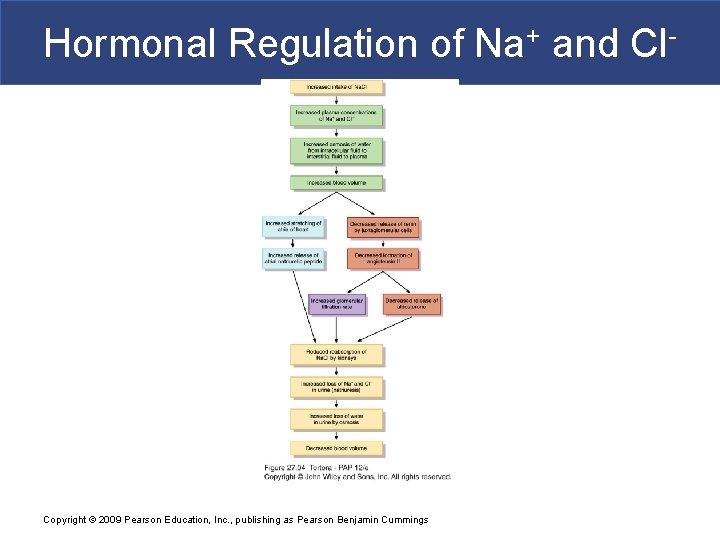
Hormonal Regulation of Na+ and Cl- Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Compartments § Aldosterone § Is secreted by suprarenal cortex in response to § Rising K+ or falling Na+ levels in blood § Activation of renin–angiotensin system § Determines rate of Na+ absorption and K+ loss along DCT and collecting system Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Compartments § “Water Follows Salt” salt pumps in PCT and ALOH § High aldosterone plasma concentration § Causes kidneys to conserve salt § Conservation of Na+ by aldosterone § Also stimulates water retention Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Concentrations in body fluids § Concentration of ions typically expressed in milliequivalents per liter (m. Eq/liter) § Na+ or Cl- number of m. Eq/liter = mmol/liter § Ca 2+ or HPO 42 - number of m. Eq/liter = 2 x mmol/liter § Chief difference between 2 ECF compartments (plasma and interstitial fluid) is plasma contains many more protein anions § Largely responsible for blood colloid osmotic pressure Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Fluid Movement Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
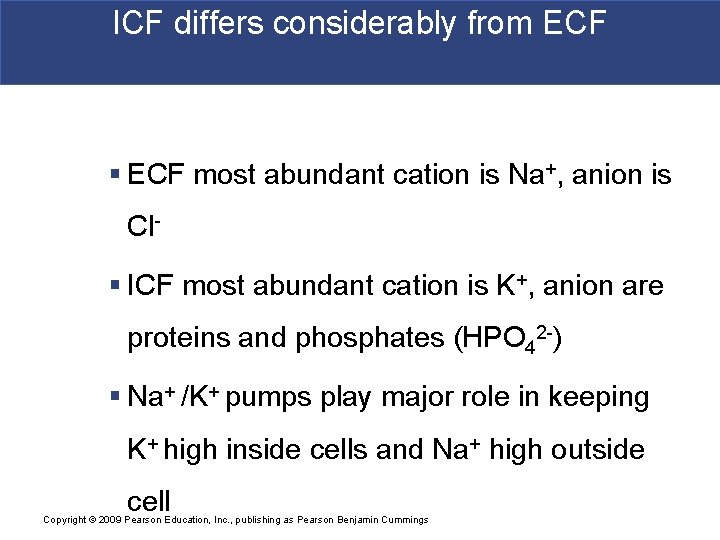
ICF differs considerably from ECF § ECF most abundant cation is Na+, anion is Cl- § ICF most abundant cation is K+, anion are proteins and phosphates (HPO 42 -) § Na+ /K+ pumps play major role in keeping K+ high inside cells and Na+ high outside cell Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
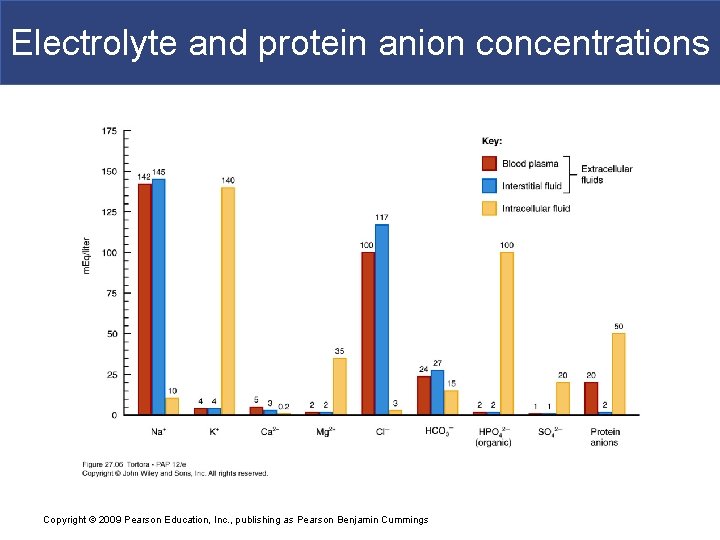
Electrolyte and protein anion concentrations Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Electrolytes in body fluids § Ions form when electrolytes dissolve ad dissociate § 4 general functions § Control osmosis of water between body fluid compartments § Help maintain the acid-base balance § Carry electrical current § Serve as cofactors Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Sodium Na+ § Most abundant ion in ECF § 90% of extracellular cations § Plays pivotal role in fluid and electrolyte balance because it account for almost half of the osmolarity of ECF § Level in blood controlled by § Aldosternone – increases renal reabsorption § ADH – if sodium too low, ADH release stops Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings § Atrial natriuretic peptide – increases renal excretion
Potassium K+ § Most abundant cations in ICF § Key role in establishing resting membrane potential in neurons and muscle fibers § Also helps maintain normal ICF fluid volume § Helps regulate p. H of body fluids when exchanged for H+ § Controlled by aldosterone – stimulates principal cells in renal collecting ducts to secrete excess K+ Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Chloride Cl§ Most prevalent anions in ECF § Moves relatively easily between ECF and ICF because most plasma membranes contain Clleakage channels and antiporters § Can help balance levels of anions in different fluids § Chloride shift in RBCs § Regulated by § ADH – governs extent of water loss in urine Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Bicarbonate HCO 3§ Second most prevalent extracellular anion § Concentration increases in blood passing through systemic capillaries picking up carbon dioxide § Carbon dioxide combines with water to form carbonic acid which dissociates § Drops in pulmonary capillaries when carbon dioxide exhaled § Chloride shift helps maintain correct balance of anions in ECF and ICF § Kidneys are main regulators of blood HCO 3 - Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Calcium Ca 2+ § § § Most abundant mineral in body 98% of calcium in adults in skeleton and teeth In body fluids mainly an extracellular cation Contributes to hardness of teeth and bones Plays important roles in blood clotting, neurotransmitter release, muscle tone, and excitability of nervous and muscle tissue § Regulated by parathyroid hormone § Stimulates osteoclasts to release calcium from bone – resorption § Also enhances reabsorption from glomerular filtrate § Increases production of calcitrol to increase absorption for GI tract § Calcitonin lowers blood calcium levels Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Phosphate § About 85% in adults present as calcium phosphate salts in bone and teeth § Remaining 15% ionized – H 2 PO 4 -, HPO 42 -, and PO 43 are important intracellular anions § HPO 42 - important buffer of H+ in body fluids and urine § Same hormones governing calcium homeostasis also regulate HPO 42 - in blood § Parathyroid hormone – stimulates resorption of bone by osteoclasts releasing calcium and phosphate but inhibits reabsorption of phosphate Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Magnesium § In adults, about 54% of total body magnesium is part of bone as magnesium salts § Remaining 46% as Mg 2+ in ICF (45%) or ECF (1%) § Second most common intracellular cation § Cofactor for certain enzymes and sodiumpotassium pump § Essential for normal neuromuscular activity, synaptic transmission, and myocardial function Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid-base balance § Major homeostatic challenge is keeping H+ concentration (p. H) of body fluids at appropriate level § 3 D shape of proteins sensitive to p. H § Diets with large amounts of proteins produce more acids than bases which acidifies blood § Several mechanisms help maintain p. H of arterial blood between 7. 35 and 7. 45 § Buffer systems, exhalation of CO 2, and kidney excretion of H+ Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
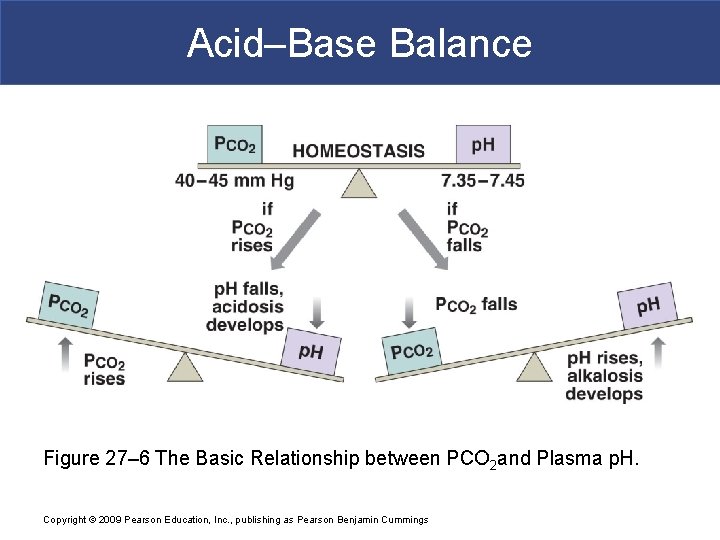
Acid–Base Balance Figure 27– 6 The Basic Relationship between PCO 2 and Plasma p. H. Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § Three Major Buffer Systems § § Protein buffer systems: § Help regulate p. H in ECF and ICF § Interact extensively with other buffer systems Carbonic acid–bicarbonate buffer system: § § Most important in ECF Phosphate buffer system: § Buffers p. H of ICF and urine Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
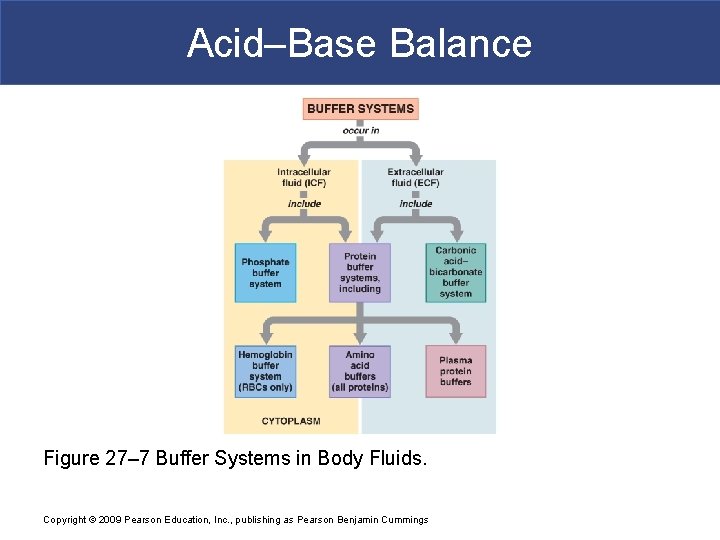
Acid–Base Balance Figure 27– 7 Buffer Systems in Body Fluids. Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
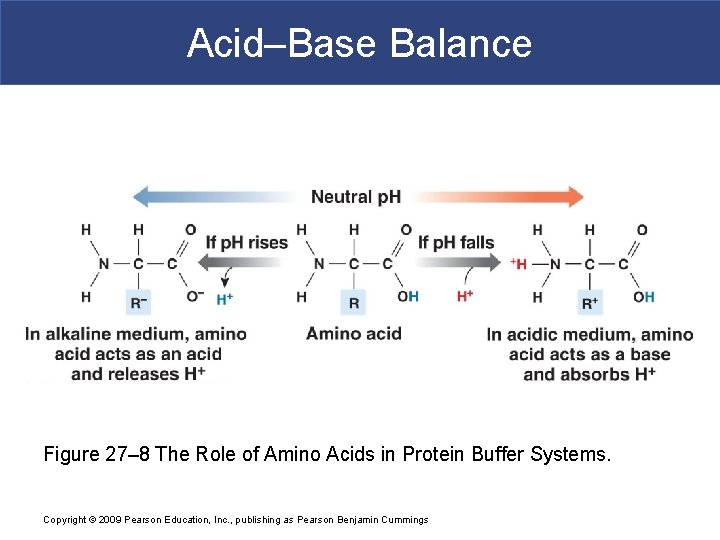
Acid–Base Balance Figure 27– 8 The Role of Amino Acids in Protein Buffer Systems. Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Buffer systems § Act to quickly temporarily bind H+ § Raise p. H but do not remove H+ § Most consist of weak acid and salt of that acid functioning as weak base § Protein buffer system § Most abundant buffer in ICF and blood plasma § Hemoglobin in RBCs § Albumin in blood plasma § Free carboxyl group acts like an acid by releasing H+ § Free amino group acts as a base to combine with H+ Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Buffer Systems § Carbonic acid- bicarbonate buffer system § Based on bicarbonate ion (HCO 3 -) acting as weak base and carbonic acid (H 2 CO 3) acting as weak acid § HCO 3 - is a significant anion in both ICF and ECF § Because CO 2 and H 2 O combine to form this buffer system cannot protect against p. H changes due to respiratory problems in which there is an excess or shortage of CO 2 § Phosphate buffer system § Dihydrogen phosphate (H 2 PO 4 -) and monohydrogen Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings phosphate (HPO 2 -)
Acid–Base Balance § The Hemoglobin Buffer System § CO 2 diffuses across RBC membrane § No transport mechanism required § As carbonic acid dissociates § Bicarbonate ions diffuse into plasma § In exchange for chloride ions (chloride shift) § Hydrogen ions are buffered by hemoglobin molecules Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Exhalation of carbon dioxide § Increase in carbon dioxide in body fluids lowers p. H of body fluids § Because H 2 CO 3 can be eliminated by exhaling CO 2 it is called a volatile acid § Changes in the rate and depth of breathing can alter p. H of body fluids within minutes § Negative feedback loop Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § The Hemoglobin Buffer System § Is the only intracellular buffer system with an immediate effect on ECF p. H § Helps prevent major changes in p. H when plasma PCO is rising or falling 2 Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § Carbonic Acid–Bicarbonate Buffer System § Carbon Dioxide § Most body cells constantly generate carbon dioxide § Most carbon dioxide is converted to carbonic acid, which dissociates into H+ and a bicarbonate ion § Is formed by carbonic acid and its dissociation products § Prevents changes in p. H caused by organic acids and fixed acids in ECF Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
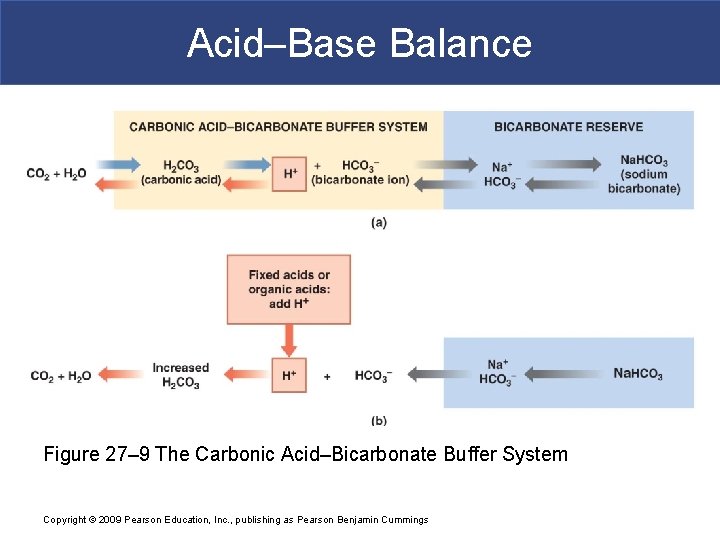
Acid–Base Balance Figure 27– 9 The Carbonic Acid–Bicarbonate Buffer System Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § Phosphate Buffer System § Consists of anion H 2 PO 4 - (a weak acid) § Works like the carbonic acid–bicarbonate buffer system § Is important in buffering p. H of ICF Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § Limitations of Buffer Systems § Provide only temporary solution to acid–base imbalance § Do not eliminate H+ ions § Supply of buffer molecules is limited Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § Renal Responses to Acidosis 1. Secretion of H+ 2. Activity of buffers in tubular fluid 3. Removal of CO 2 4. Reabsorption of Na. HCO 3 Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Kidney excretion of H+ § Metabolic reactions produce nonvolatile acids § One way to eliminate this huge load is to excrete H+ in urine § In the proximal convoluted tubule, Na+ /H+ antiporters secrete H+ as they reabsorb Na+ § Intercalated cells of collecting duct include proton pumps that secrete H+ into tubule fluid § Urine can be up to 1000 times more acidic than blood § 2 other buffers can combine with H+ in collecting duct § HPO 42 - and NH 3 Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
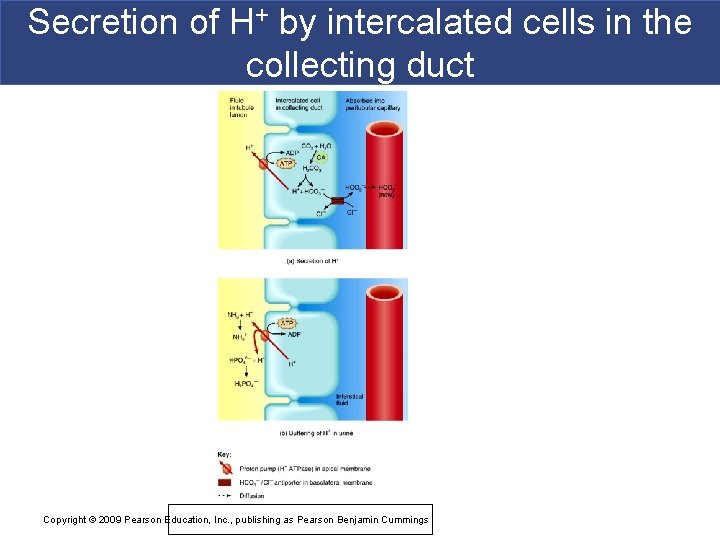
Secretion of H+ by intercalated cells in the collecting duct Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Metabolic alkalosis § Abnormally high HCO 3 - in systemic arterial blood § Nonrespiratory loss of acid - vomiting of acidic stomach contents, gastric suctioning § Excessive intake of alkaline drugs (antacids) § Use of certain diuretics § Severe dehydration § Hypoventilation can help § Give fluid solutions to correct Cl-, K+ and other Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings electrolyte deficiencies and correct cause of

Respiratory alkalosis § Abnormally low PCO 2 in systemic arterial blood § Cause is hyperventilation due to oxygen deficiency from high altitude or pulmonary disease, stroke or severe anxiety § Renal compensation can help § One simple treatment to breather into paper bag for short time Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
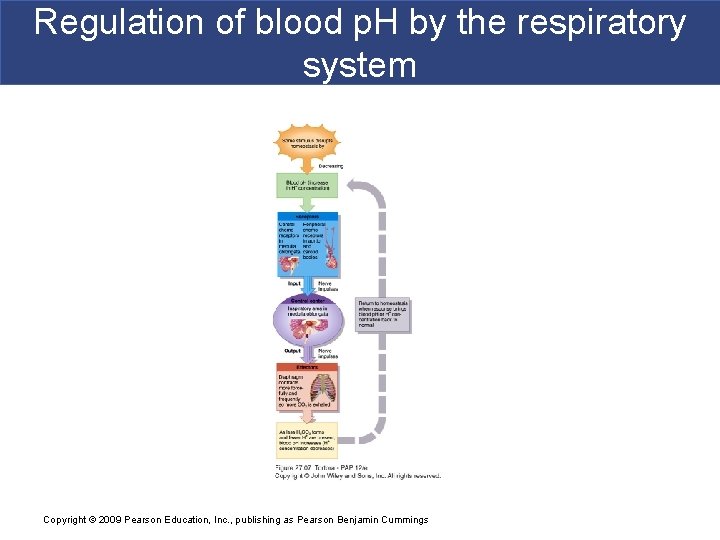
Regulation of blood p. H by the respiratory system Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
Acid–Base Balance § Renal Responses to Alkalosis 1. Rate of secretion of H+ at kidneys declines 2. Tubule cells do not reclaim bicarbonates in tubular fluid 3. Collecting system transports HCO 3 - into tubular fluid while releasing strong acid (HCl) into peritubular fluid Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
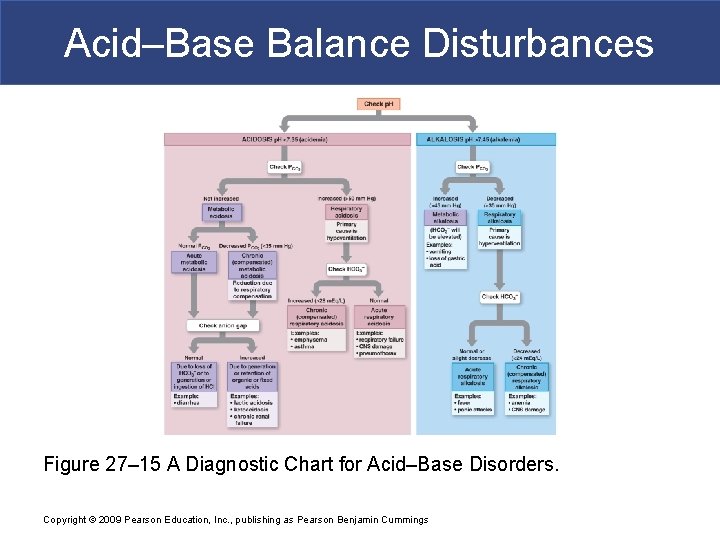
Acid–Base Balance Disturbances Figure 27– 15 A Diagnostic Chart for Acid–Base Disorders. Copyright © 2009 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
- Slides: 57