Flow Cytometry Publications using the keyword flow cytometry

Flow Cytometry

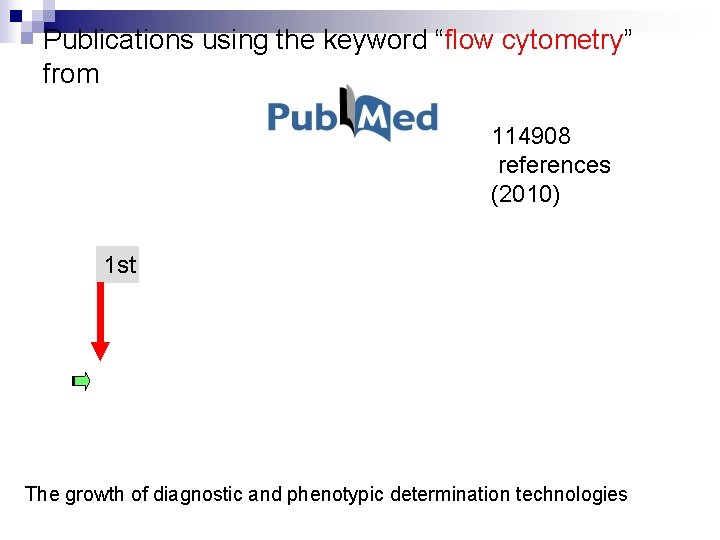
Publications using the keyword “flow cytometry” from 114908 references (2010) 1 st The growth of diagnostic and phenotypic determination technologies
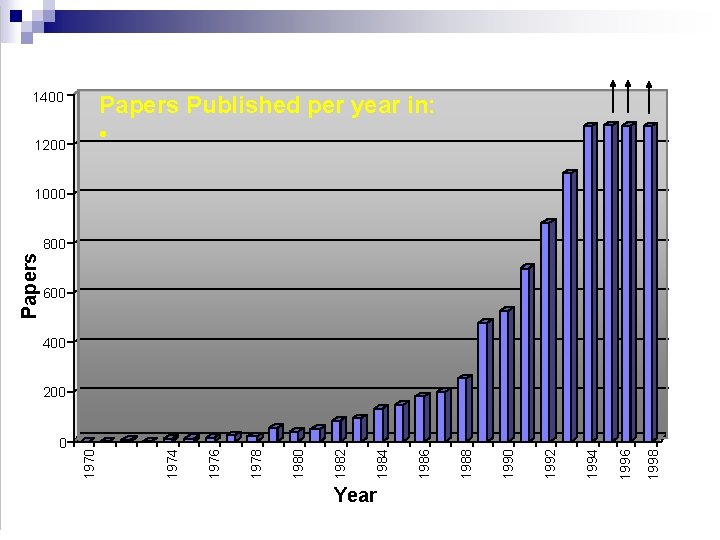
0 Year 1998 1996 1994 1992 1990 1988 1986 1984 1982 1980 1978 1976 1200 1974 1970 Papers 1400 Papers Published per year in: • 1000 800 600 400 200
Flow Cytometry n FACS – Fluorescence Activated Cell Sorter is the generic term used for flow cytometry (even without sorting) n Simultaneous analysis of different physical parameters in a single cell n Can analyze up to several thousands of cells per second n Versatile, sensitive
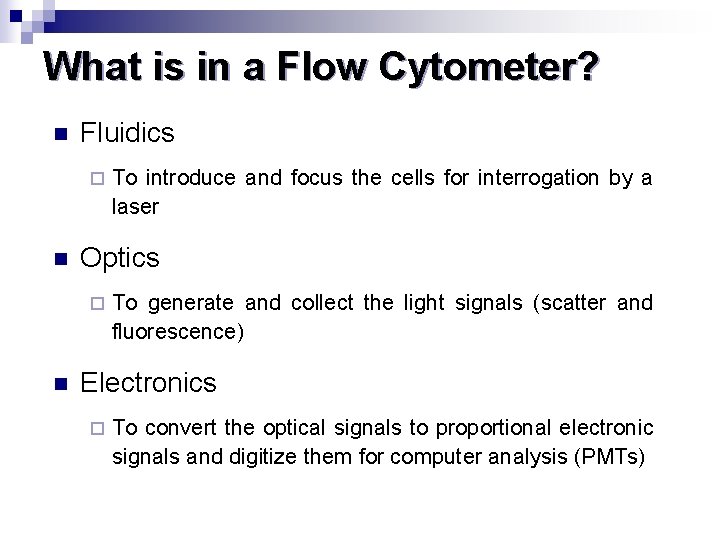
What is in a Flow Cytometer? n Fluidics ¨ n Optics ¨ n To introduce and focus the cells for interrogation by a laser To generate and collect the light signals (scatter and fluorescence) Electronics ¨ To convert the optical signals to proportional electronic signals and digitize them for computer analysis (PMTs)
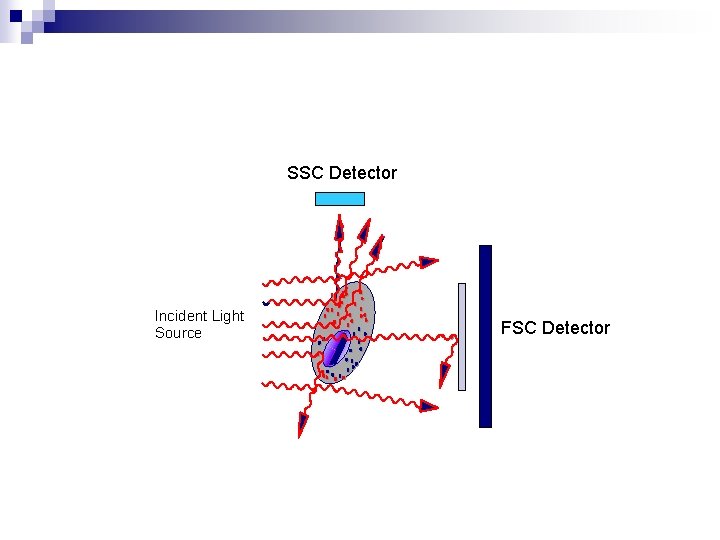
SSC Detector Incident Light Source FSC Detector
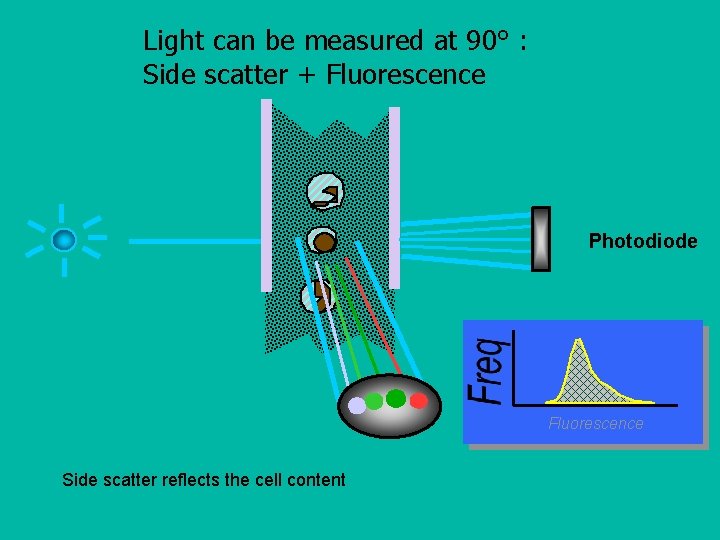
Light can be measured at 90° : Side scatter + Fluorescence Laser Photodiode Fluorescence Side scatter reflects the cell content
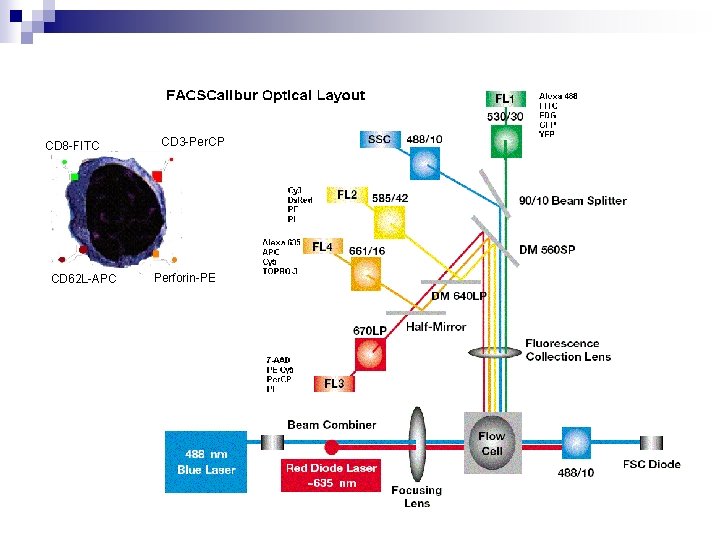
CD 8 -FITC CD 62 L-APC CD 3 -Per. CP Perforin-PE
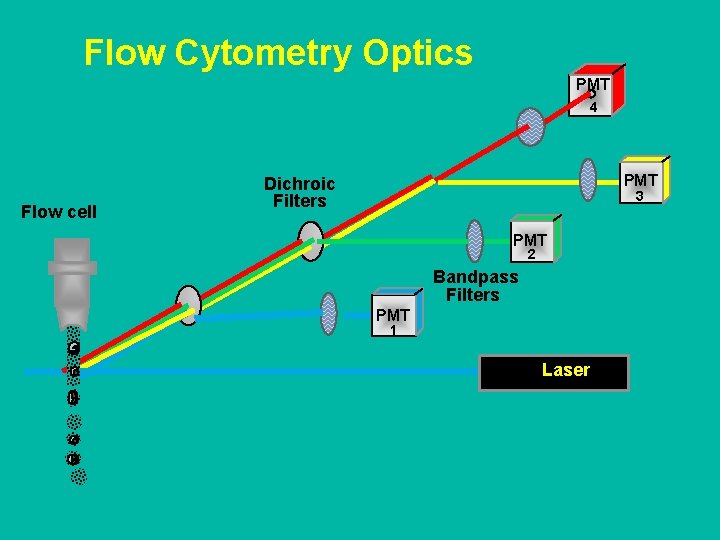
Flow Cytometry Optics PMT 4 Flow cell PMT Dichroic Filters 3 PMT 2 Bandpass Filters PMT 1 Laser
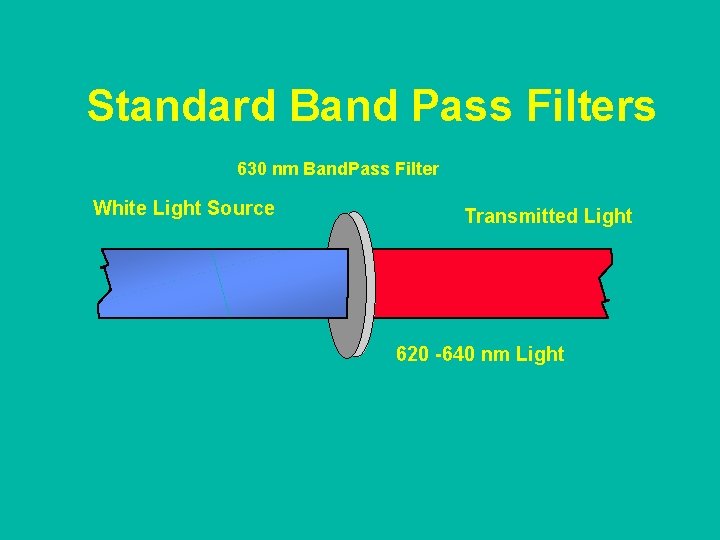
Standard Band Pass Filters 630 nm Band. Pass Filter White Light Source Transmitted Light 620 -640 nm Light
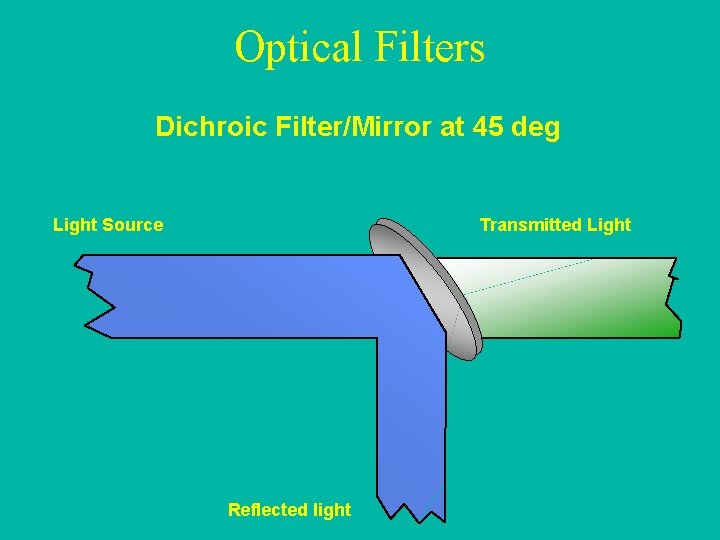
Optical Filters Dichroic Filter/Mirror at 45 deg Light Source Transmitted Light Reflected light

Standard Long Pass Filters 520 nm Long Pass Filter Light Source Transmitted Light >520 nm Light Standard Short Pass Filters 575 nm Short Pass Filter Light Source Transmitted Light <575 nm Light

Photomultiplier tubes (PMT’s) The PMTs in an Elite. 3 PMTs are shown, the other 2 have been removed to show their positions. A diode detector is used forward scatter and a PMT for side scatter. The Bio-Rad Bryte cytometer uses PMTs forward and wide angle light scatter as well as fluorescence
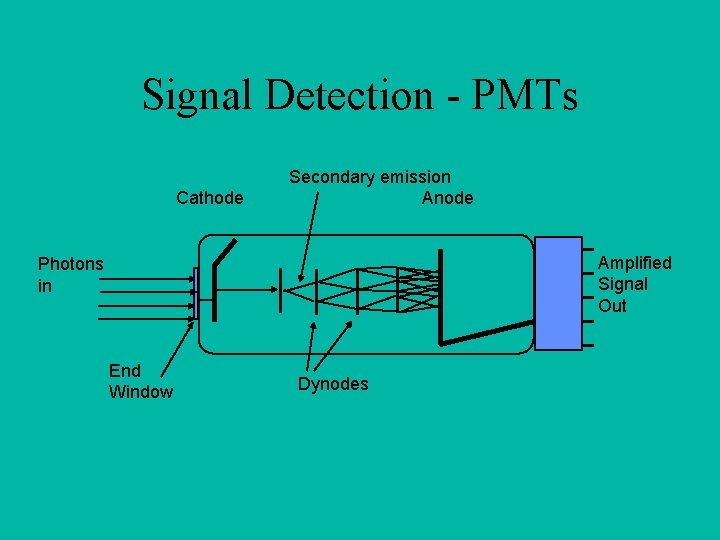
Signal Detection - PMTs Cathode Secondary emission Anode Amplified Signal Out Photons in End Window Dynodes
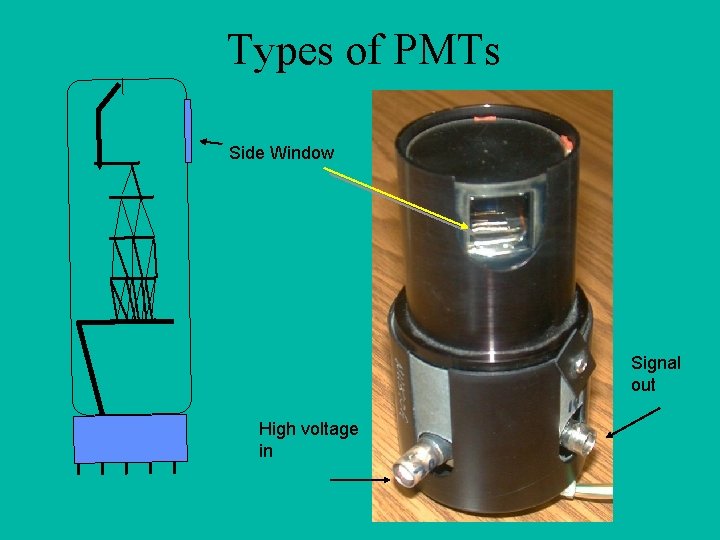
Types of PMTs Side Window Signal out High voltage in
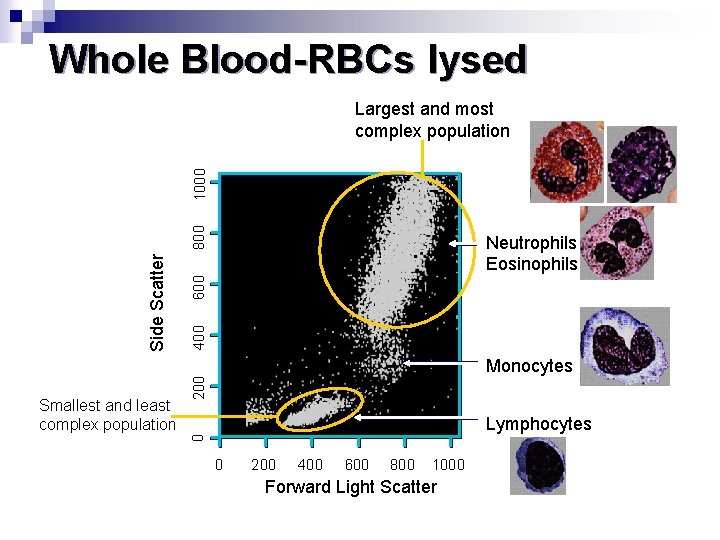
Whole Blood-RBCs lysed 600 400 Monocytes 200 Smallest and least complex population Neutrophils Eosinophils Lymphocytes 0 Side Scatter 800 1000 Largest and most complex population 0 200 400 600 800 1000 Forward Light Scatter
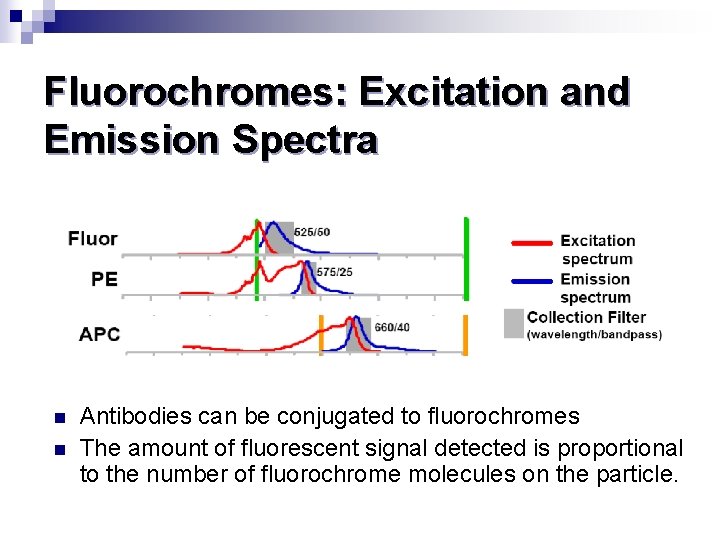
Fluorochromes: Excitation and Emission Spectra n n Antibodies can be conjugated to fluorochromes The amount of fluorescent signal detected is proportional to the number of fluorochrome molecules on the particle.
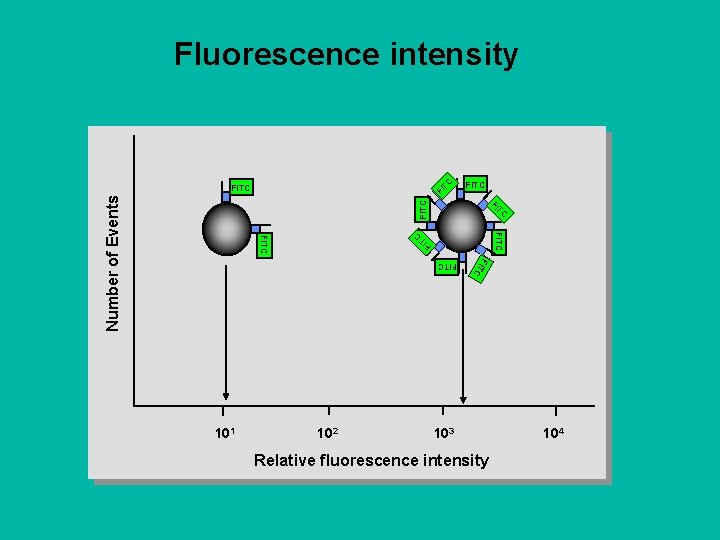
C Fluorescence intensity FI T FITC TC FI FITC Number of Events FITC TC FI FITC C FITC 102 FIT 101 103 Relative fluorescence intensity 104
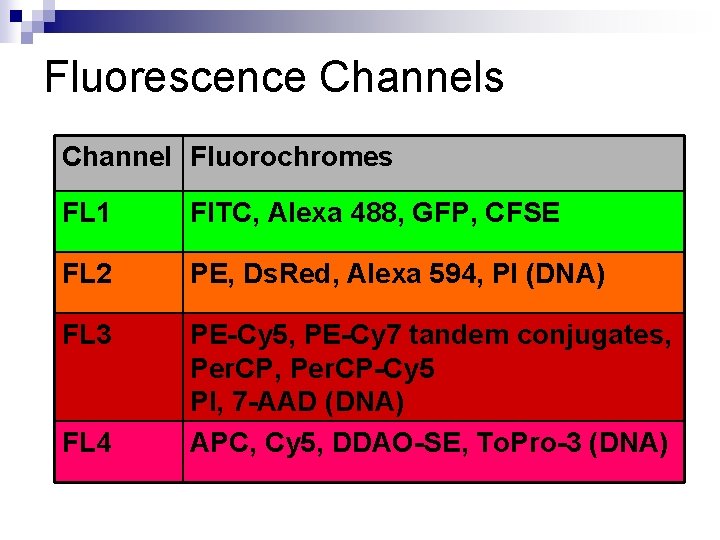
Fluorescence Channels Channel Fluorochromes FL 1 FITC, Alexa 488, GFP, CFSE FL 2 PE, Ds. Red, Alexa 594, PI (DNA) FL 3 PE-Cy 5, PE-Cy 7 tandem conjugates, Per. CP-Cy 5 PI, 7 -AAD (DNA) APC, Cy 5, DDAO-SE, To. Pro-3 (DNA) FL 4
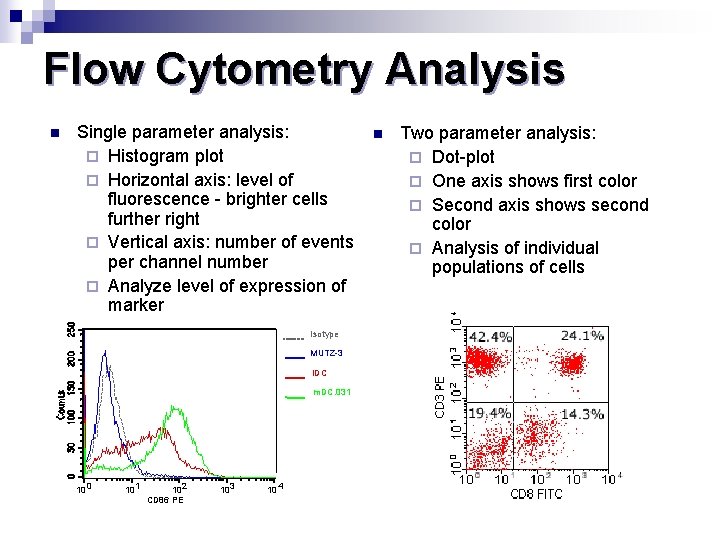
Flow Cytometry Analysis n Single parameter analysis: ¨ Histogram plot ¨ Horizontal axis: level of fluorescence - brighter cells further right ¨ Vertical axis: number of events per channel number ¨ Analyze level of expression of marker Isotype MUTZ-3 i. DC m. DC. 031 10 0 10 1 10 2 CD 86 PE 10 3 10 4 n Two parameter analysis: ¨ Dot-plot ¨ One axis shows first color ¨ Second axis shows second color ¨ Analysis of individual populations of cells
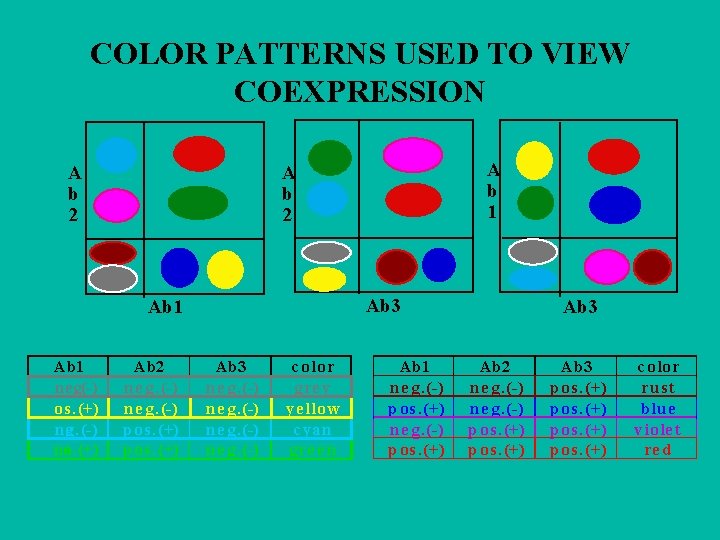
COLOR PATTERNS USED TO VIEW COEXPRESSION A b 2 Ab 3 Ab 1 neeg. (-) os. (+) neg. (-) nos. (+) A b 1 A b 2 Ab 2 n e g. (-) p os. (+) Ab 3 n e g. (-) c olor gre y ye llow c yan gre e n Ab 1 n e g. (-) p os. (+) Ab 3 Ab 2 n e g. (-) p os. (+) Ab 3 p os. (+) c olor ru s t blu e viole t re d
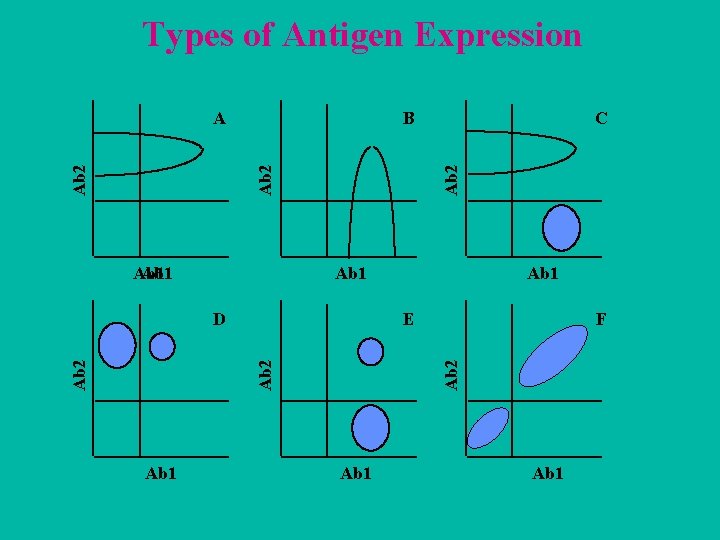
Types of Antigen Expression B Ab 1 F Ab 2 Ab 1 E Ab 2 D Ab 1 C Ab 2 A Ab 1
Common Apllications of Flow Cytometry in Immunology n n n n Phenotype of cell, surface molecules Intracellular cytokine staining Antigen specificity Cell proliferation (e. g. CFSE, Brd. U incorporation) Cell sorting Apoptosis analysis Cytotoxicity assays Phagocytosis assays Cell cycle analysis (DNA content analysis) Cell signalling molecules, Calcium flux assays Organelle-specific studies (e. g. lysosome) Cellular transport assays Transfection efficiencies
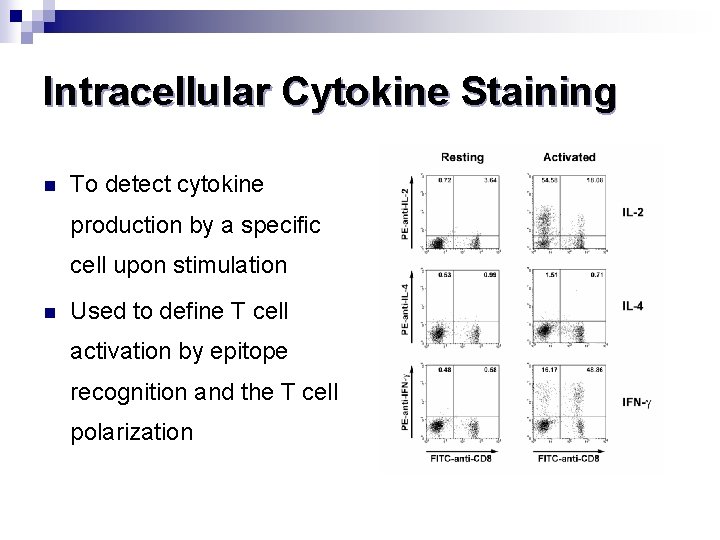
Intracellular Cytokine Staining n To detect cytokine production by a specific cell upon stimulation n Used to define T cell activation by epitope recognition and the T cell polarization
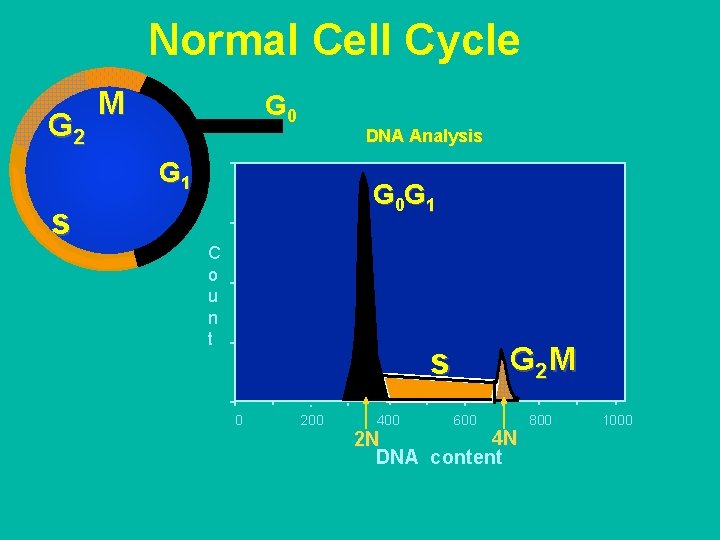
Normal Cell Cycle G 2 M G 0 DNA Analysis G 1 s G 0 G 1 C o u n t s 0 200 400 G 2 M 600 4 N 2 N DNA content 800 1000
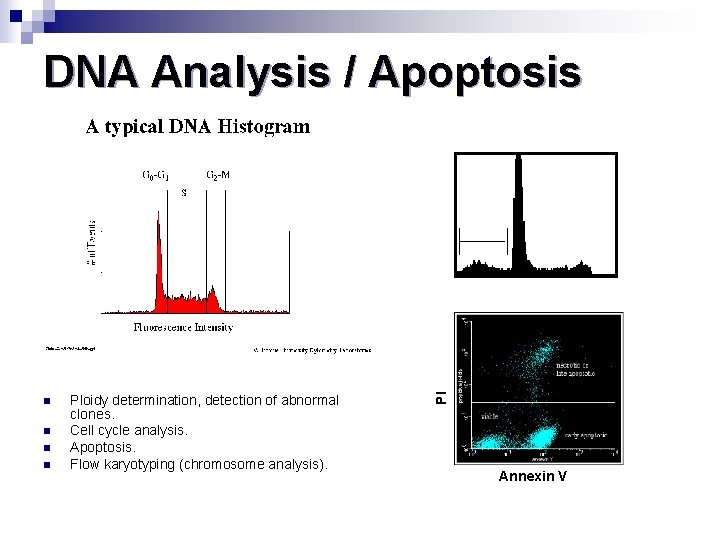
DNA Analysis / Apoptosis n n Ploidy determination, detection of abnormal clones. Cell cycle analysis. Apoptosis. Flow karyotyping (chromosome analysis). PI 12 Annexin V
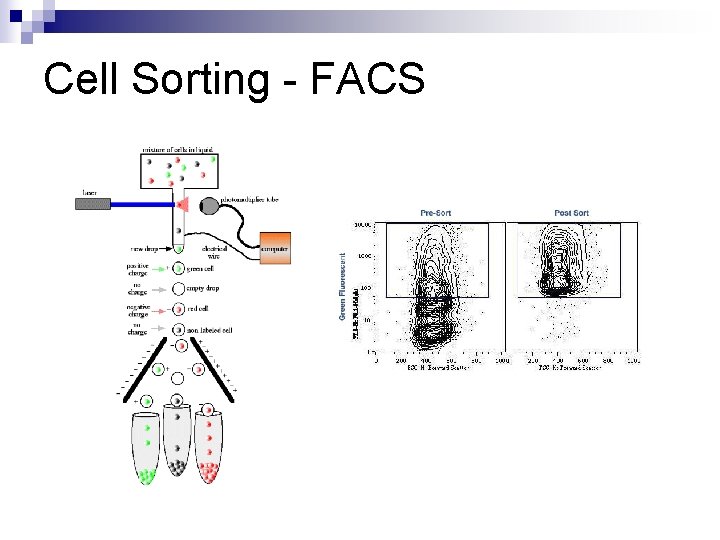
Cell Sorting - FACS


Sources of information n Flow Cytometry and Sorting, 2 nd ed. (M. R. Melamed, T. Lindmo, M. L. Mendelsohn, eds. ), Wiley-Liss, New York, 1990 referred to here as MLM n Flow Cytometry: Instrumentation and Data Analysis (M. A. Van Dilla, P. N. Dean, O. D. Laerum, M. R. Melamed, eds. ), Academic Press, London, 1985 – referred to as VDLM n Practical Flow Cytometry 3 nd edition (1994), H. Shapiro: Alan R. Liss, New York - referred to as PFC n Introduction to Flow Cytometry. J. Watson, Cambridge Press, 1991 referred to as IFC n Methods in Cell Biology: v. 40, 41, 63, 64 Darzynkiewicz, Robinson & Crissman, Academic Press, 1994, 2000 MCB n Data Analysis in Flow Cytometry: A Dynamic Approach-Book on CDROM M. Ormerod referred to as DAFC n Flow Cytometry: First Principles. (2 nd Ed) Alice Longobardi Givan, Wiley-Liss, 2001 referred to as AFCFP More information on flow cytometry books can be found on our website at: http: //www. cyto. purdue. edu/flowcyt/books/bookindx. htm Note: All of these books are in Prof. Robinson’s library in Hansen Hall, Room and ©J. Paul. B 50 Robinson, Purdue University BMS may be checked out for 24 hour periods with permission. Page 32 631 - LECTURE 1. PPT
- Slides: 32