Flow Cytometry at Boston University Medical Campus Introduction

Flow Cytometry at Boston University Medical Campus Introduction to some methods that we offer Yan Deng (X 4 -5225), ydeng@bu. edu Gerald Denis (X 4 -1371), gdenis@bu. edu
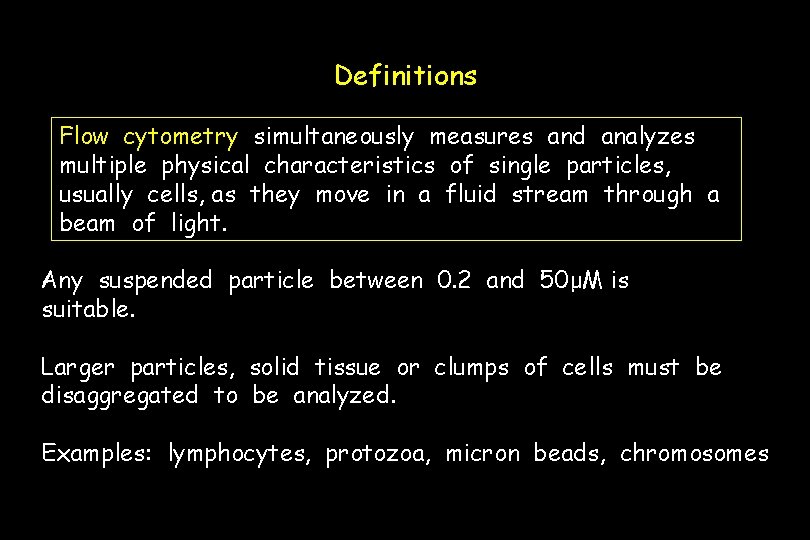
Definitions Flow cytometry simultaneously measures and analyzes multiple physical characteristics of single particles, usually cells, as they move in a fluid stream through a beam of light. Any suspended particle between 0. 2 and 50μM is suitable. Larger particles, solid tissue or clumps of cells must be disaggregated to be analyzed. Examples: lymphocytes, protozoa, micron beads, chromosomes

Definitions The particles in the fluid stream scatter incident light, which reveals internal properties, size and granularity. The particles also fluoresce; they emit laser light at the interrogation point; this light is picked up by detectors arrayed at a different angle to detectors of scattered light.
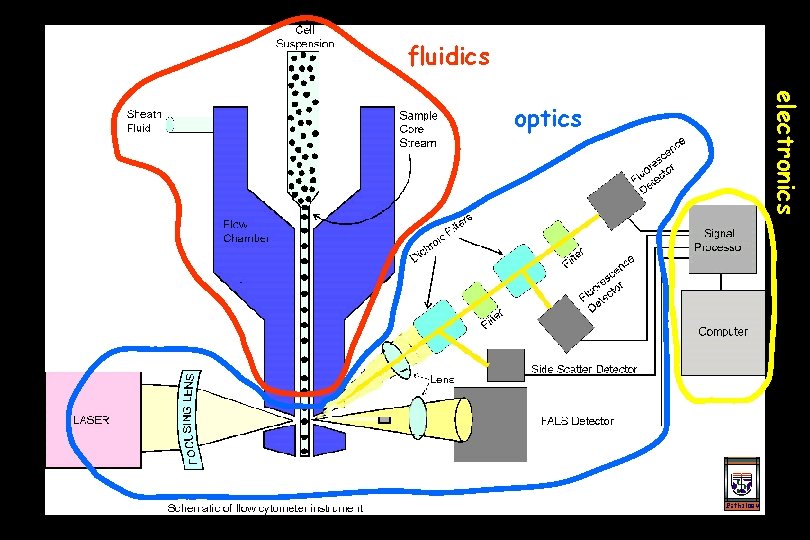
fluidics electronics optics
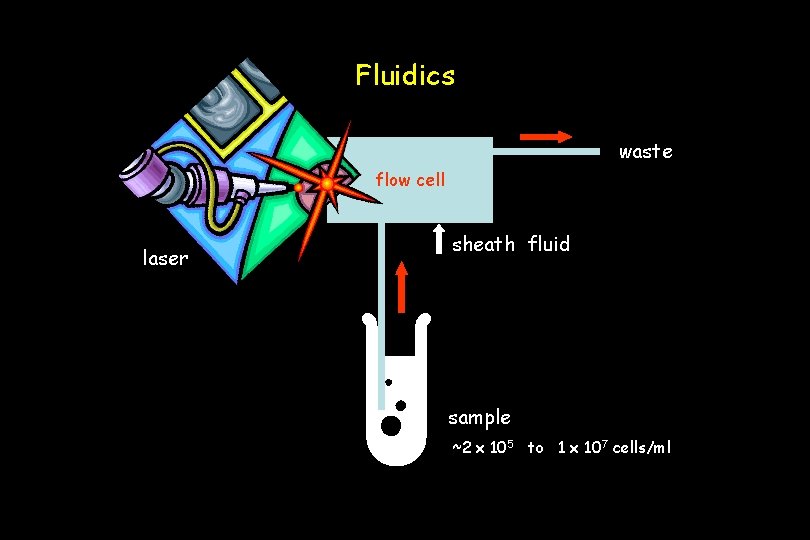
Fluidics waste flow cell laser sheath fluid sample ~2 x 105 to 1 x 107 cells/ml
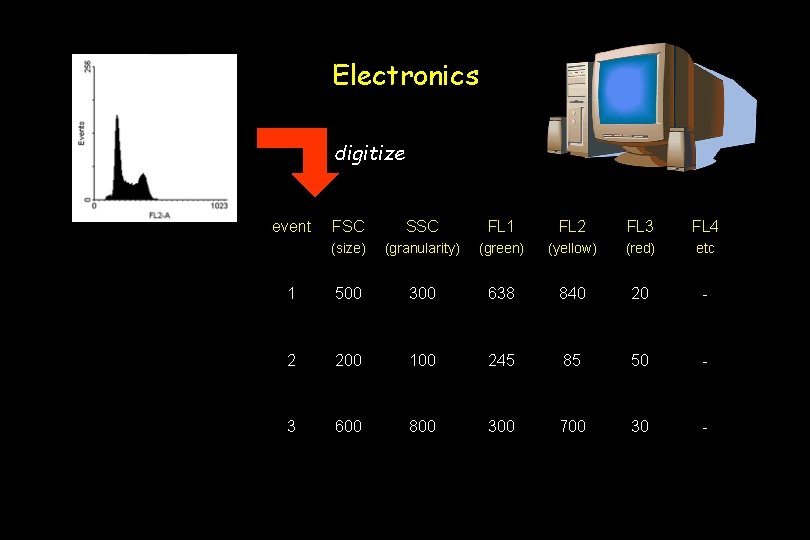
Electronics digitize event FSC SSC FL 1 FL 2 FL 3 FL 4 (size) (granularity) (green) (yellow) (red) etc 1 500 300 638 840 20 - 2 200 100 245 85 50 - 3 600 800 300 700 30 -
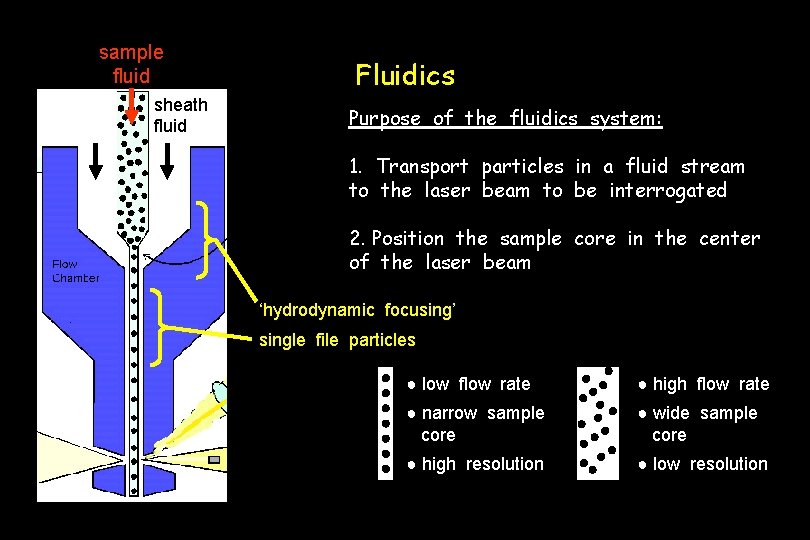
sample fluid sheath fluid Fluidics Purpose of the fluidics system: 1. Transport particles in a fluid stream to the laser beam to be interrogated 2. Position the sample core in the center of the laser beam ‘hydrodynamic focusing’ single file particles ● low flow rate ● high flow rate ● narrow sample core ● wide sample core ● high resolution ● low resolution

Fluidics Concerns 1. Shear rates for cells: check after you complete a run to ensure that the cells are intact. 2. Larger tips are needed for cell sorting.

Optics The laser beam is focused on the sample core; lasers must be fixed in place. Excitation is accomplished by lasers that emit light at specified wavelengths. The atomic properties of the excitation media define this wavelength for a particular laser. Argon blue lasers emit light at 488 nm. This is the most common and versatile wavelength for excitation of fluorochromes.
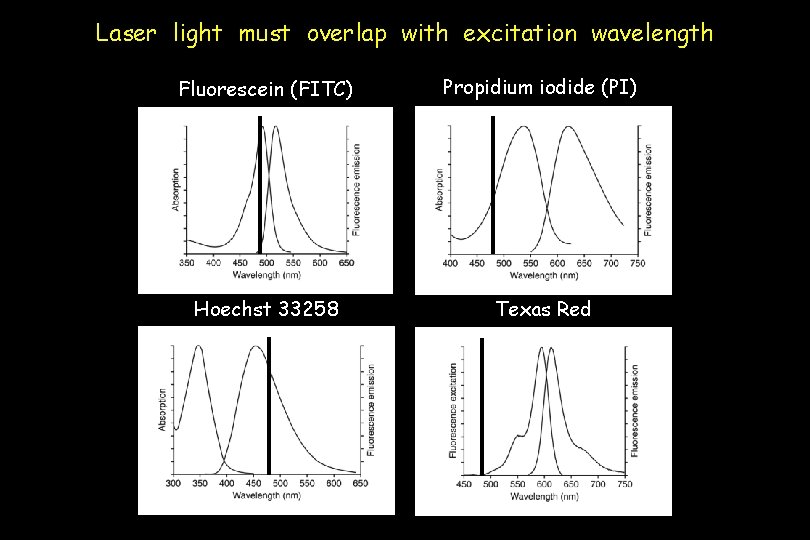
Laser light must overlap with excitation wavelength Fluorescein (FITC) Propidium iodide (PI) Hoechst 33258 Texas Red

Optics Filters resolve overlapping wavelengths of emitted light Longpass filter: transmits light of longer than or equal to a specific wavelength Shortpass filter: transmits light of shorter than or equal to a specific wavelength Bandpass filter: transmits light only within a narrow range of wavelengths
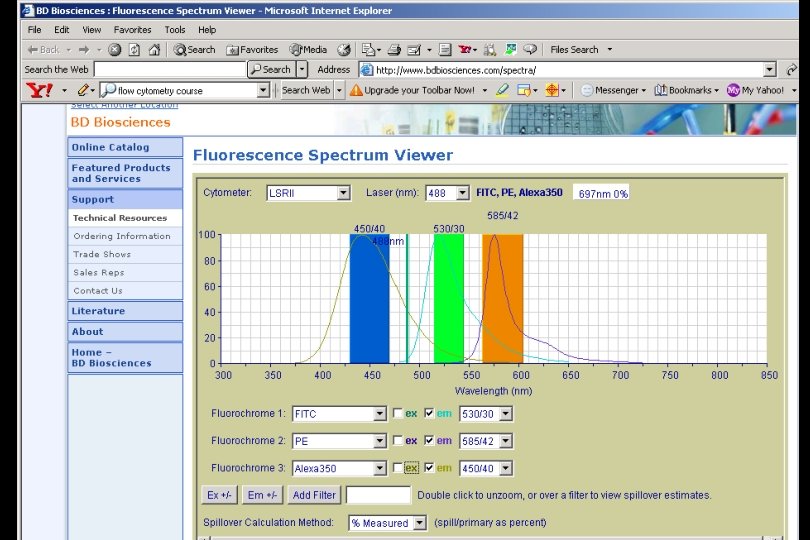
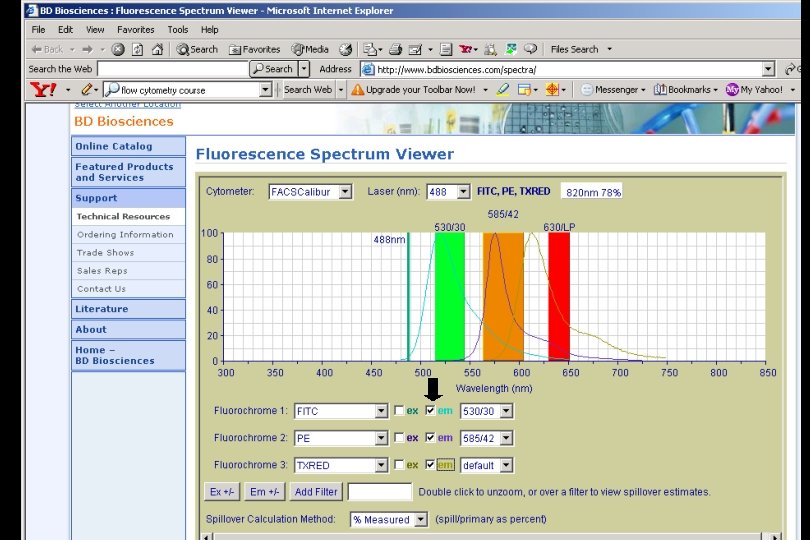
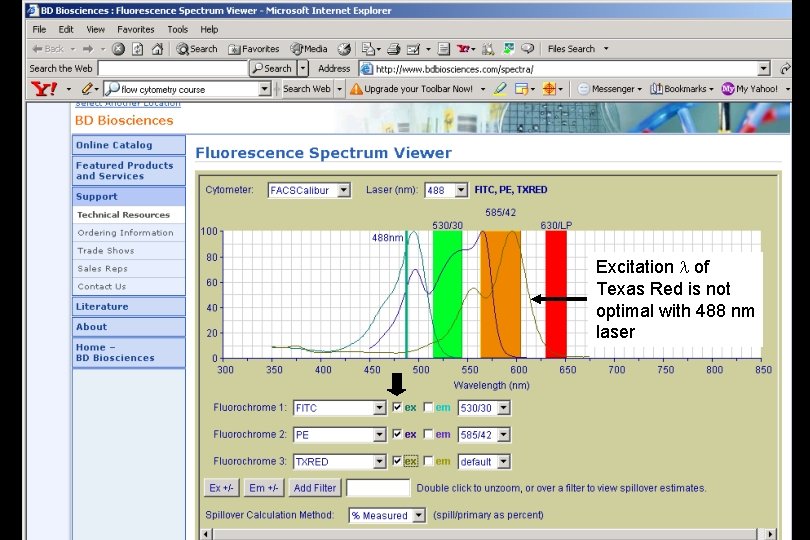
Excitation l of Texas Red is not optimal with 488 nm laser

For more information http: //probes. invitrogen. com/resources/spectraviewer/ http: //www. bdbiosciences. com/spectra/
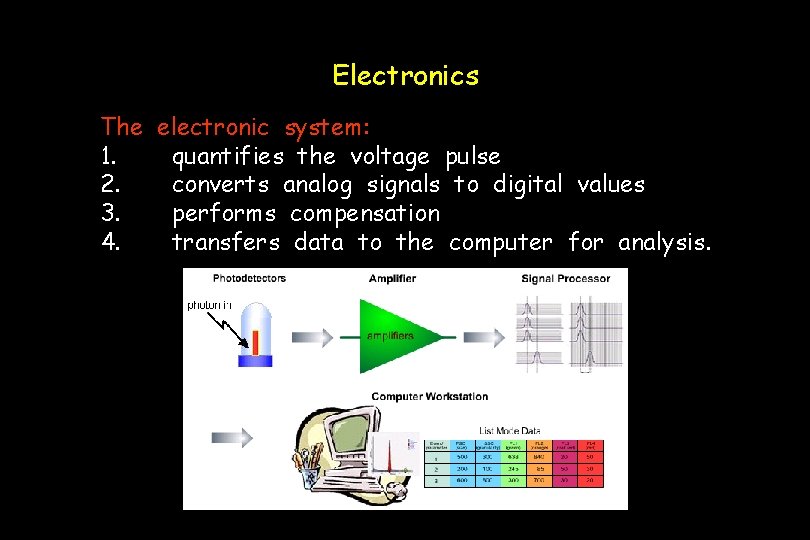
Electronics The electronic system: 1. quantifies the voltage pulse 2. converts analog signals to digital values 3. performs compensation 4. transfers data to the computer for analysis.
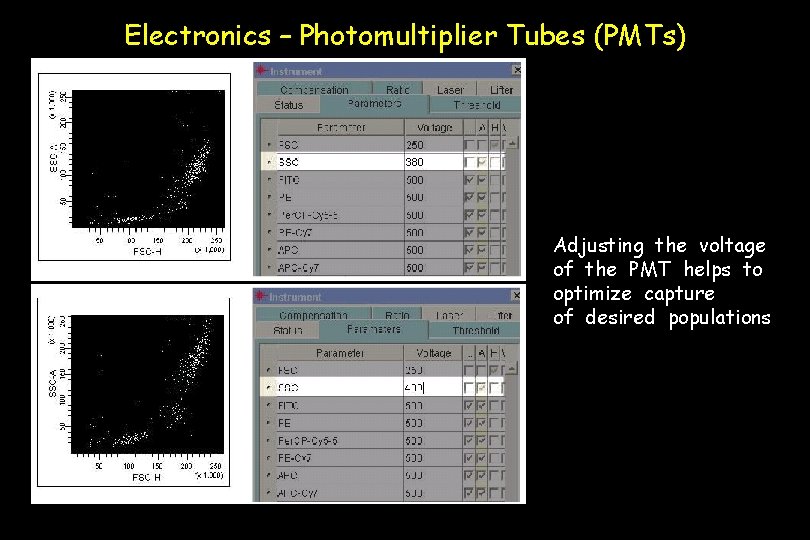
Electronics – Photomultiplier Tubes (PMTs) Adjusting the voltage of the PMT helps to optimize capture of desired populations
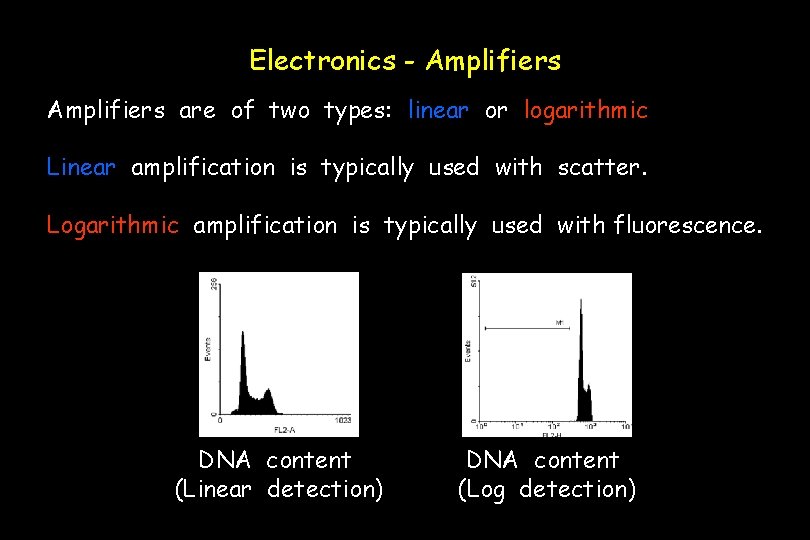
Electronics - Amplifiers are of two types: linear or logarithmic Linear amplification is typically used with scatter. Logarithmic amplification is typically used with fluorescence. DNA content (Linear detection) DNA content (Log detection)
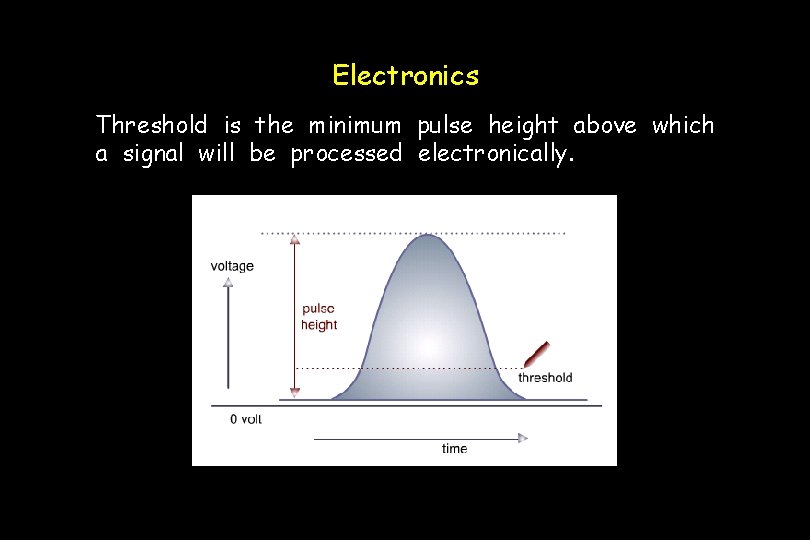
Electronics Threshold is the minimum pulse height above which a signal will be processed electronically.
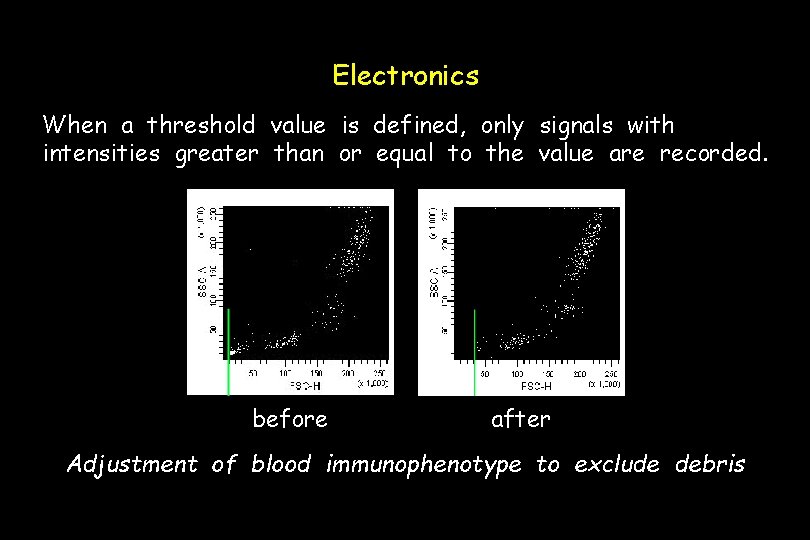
Electronics When a threshold value is defined, only signals with intensities greater than or equal to the value are recorded. before after Adjustment of blood immunophenotype to exclude debris
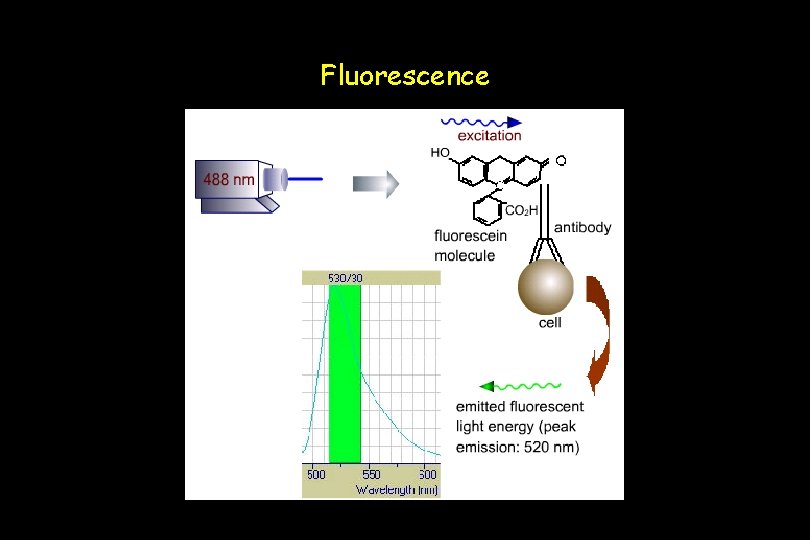
Fluorescence
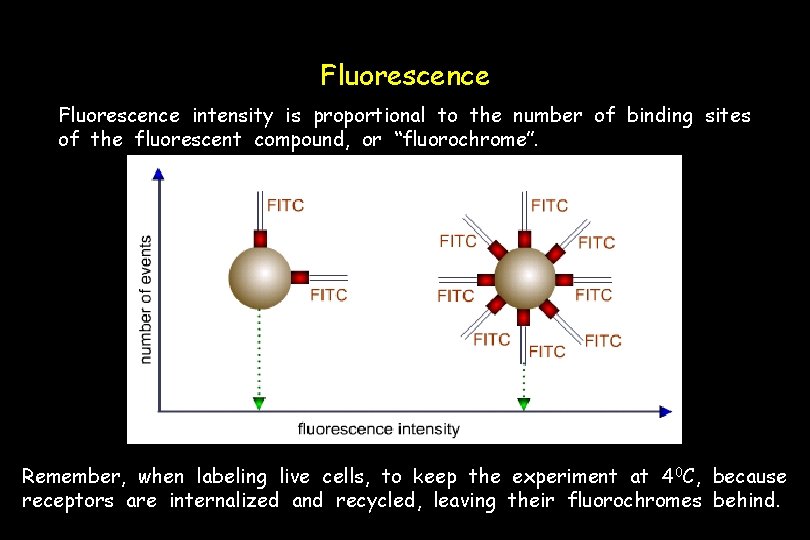
Fluorescence intensity is proportional to the number of binding sites of the fluorescent compound, or “fluorochrome”. Remember, when labeling live cells, to keep the experiment at 4 0 C, because receptors are internalized and recycled, leaving their fluorochromes behind.

Immunophenotyping negative stain positive stain
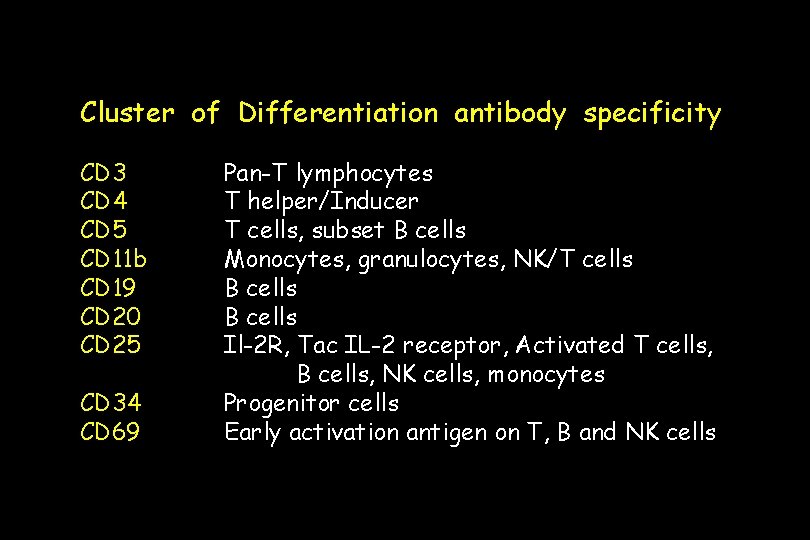
Cluster of Differentiation antibody specificity CD 3 CD 4 CD 5 CD 11 b CD 19 CD 20 CD 25 CD 34 CD 69 Pan-T lymphocytes T helper/Inducer T cells, subset B cells Monocytes, granulocytes, NK/T cells B cells Il-2 R, Tac IL-2 receptor, Activated T cells, B cells, NK cells, monocytes Progenitor cells Early activation antigen on T, B and NK cells
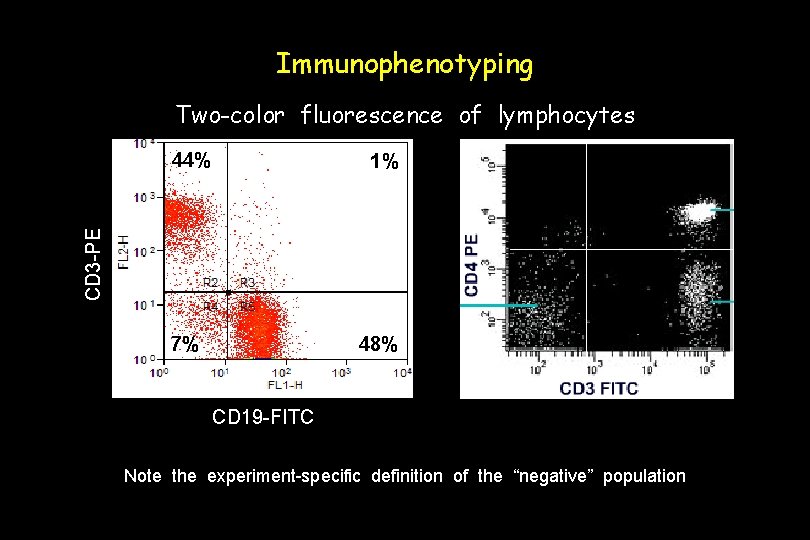
Immunophenotyping Two-color fluorescence of lymphocytes 44% CD 3 -PE 1% 48% 7% CD 19 -FITC Note the experiment-specific definition of the “negative” population
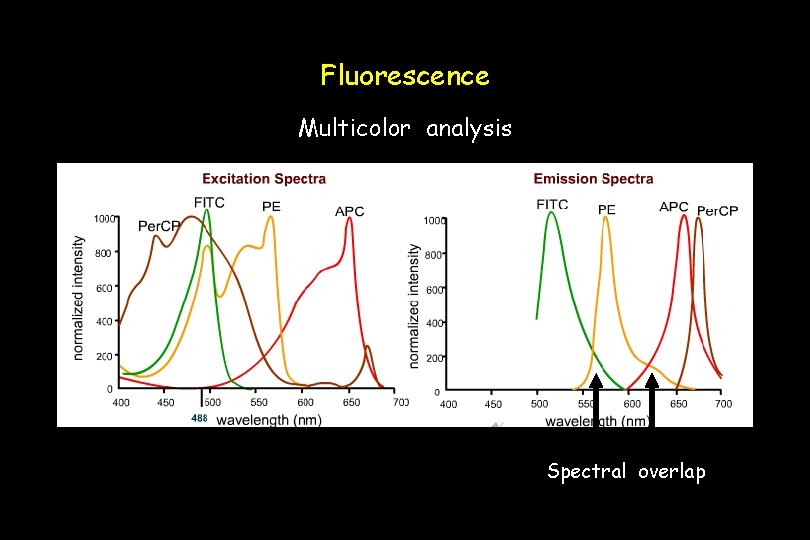
Fluorescence Multicolor analysis Spectral overlap
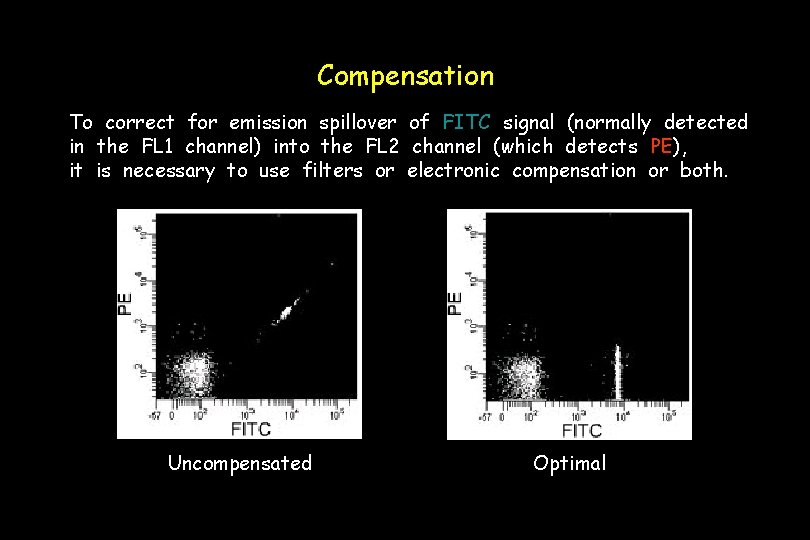
Compensation To correct for emission spillover of FITC signal (normally detected in the FL 1 channel) into the FL 2 channel (which detects PE), it is necessary to use filters or electronic compensation or both. Uncompensated Optimal
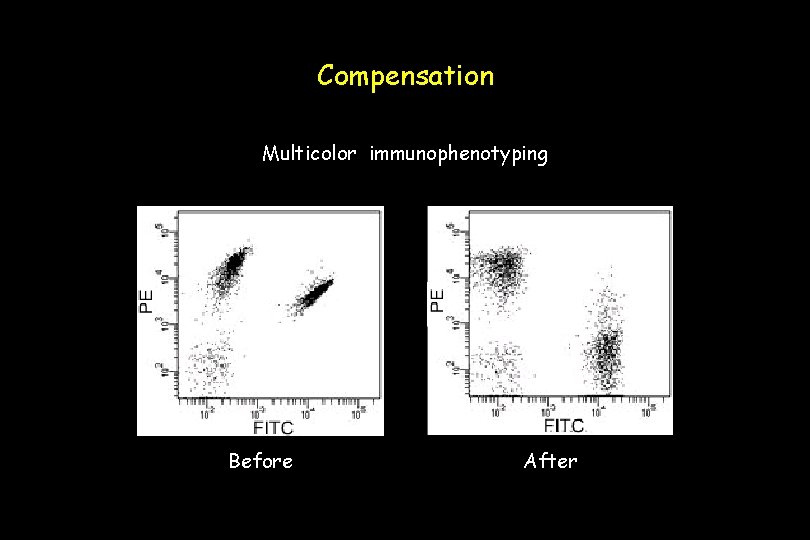
Compensation Multicolor immunophenotyping Before After
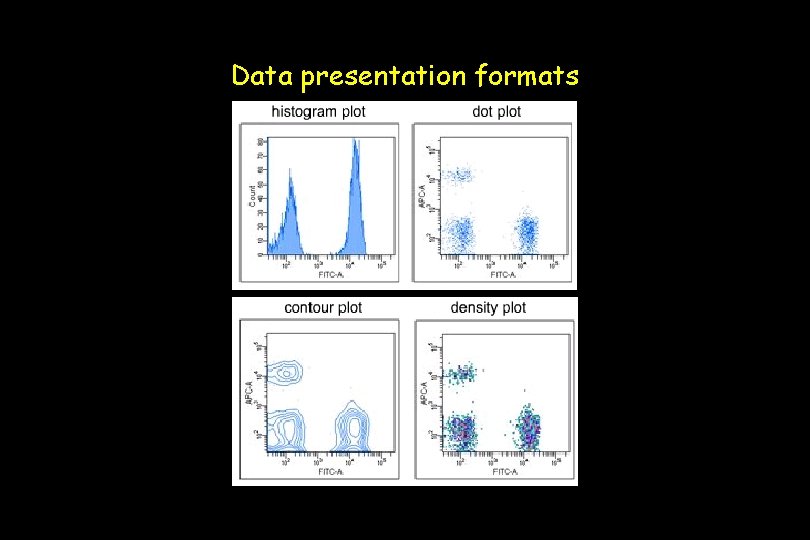
Data presentation formats
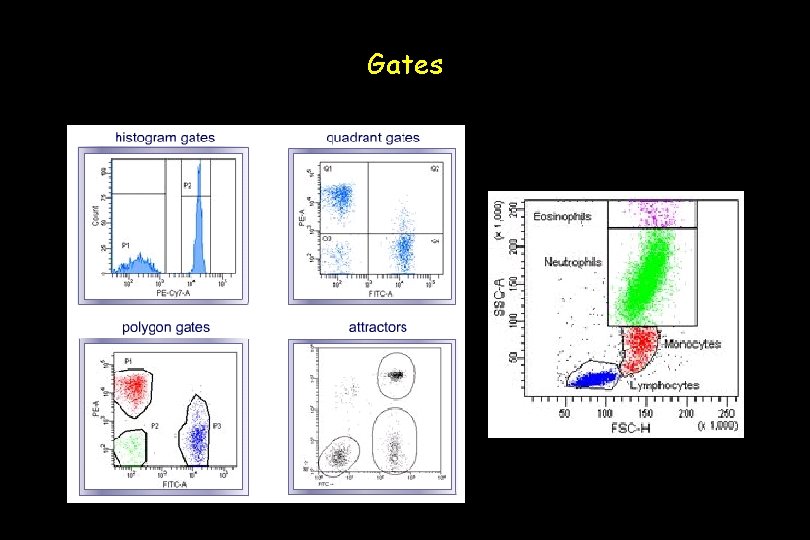
Gates
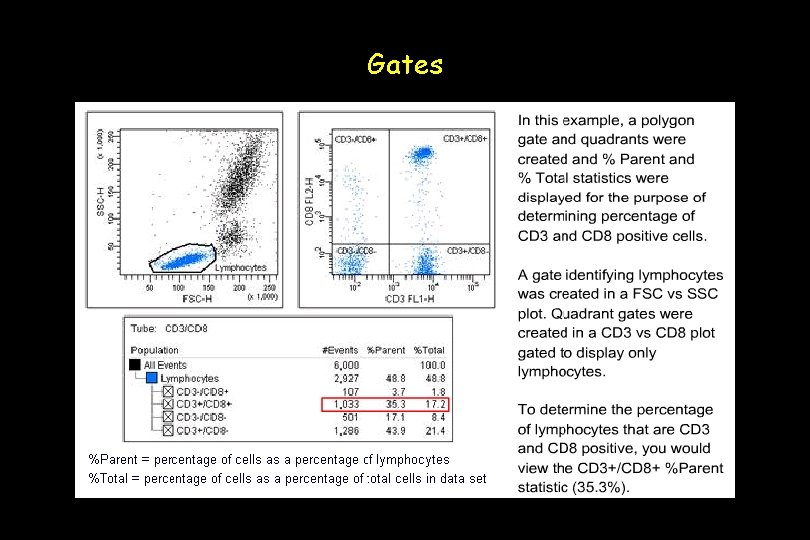
Gates
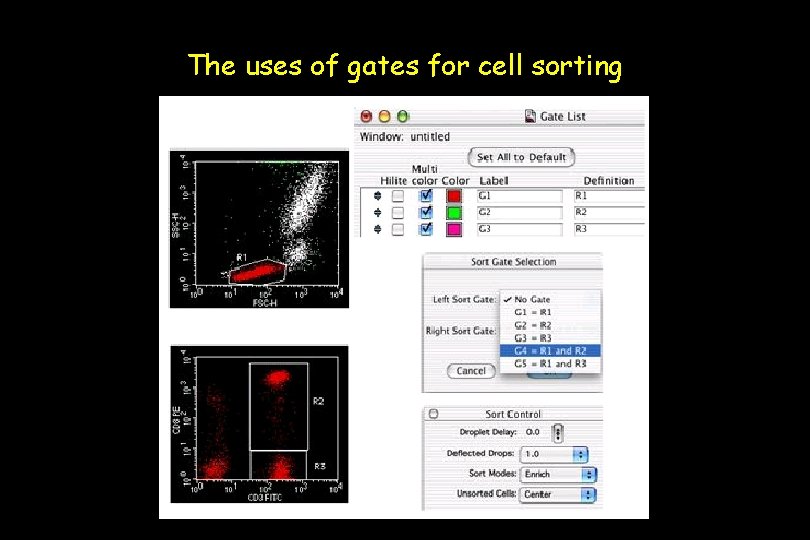
The uses of gates for cell sorting
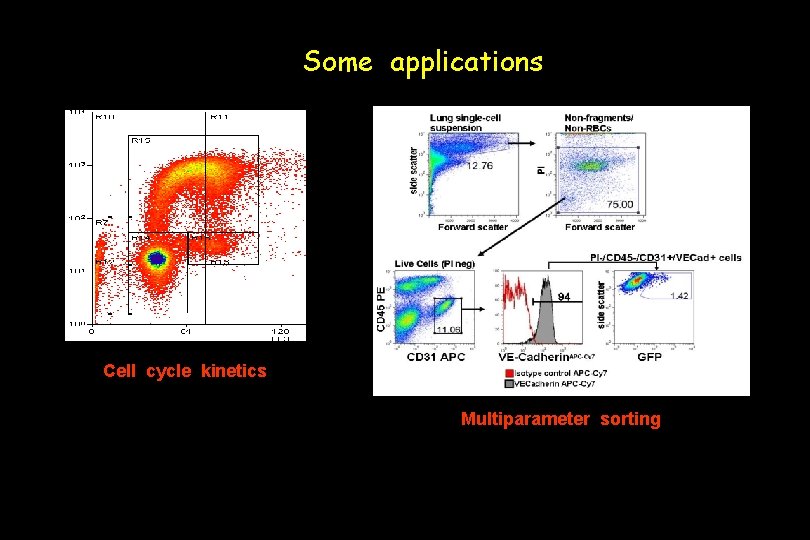
Some applications Cell cycle kinetics Multiparameter sorting
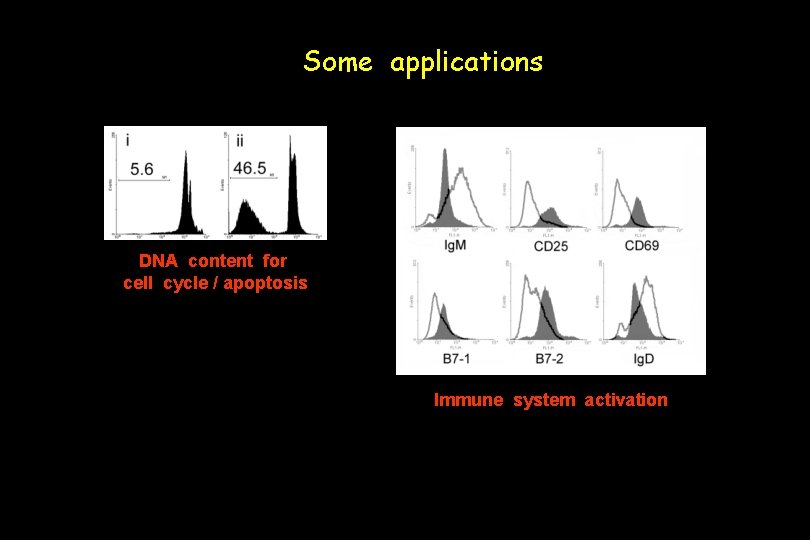
Some applications DNA content for cell cycle / apoptosis Immune system activation
- Slides: 34