FLOTATION OF SULPHIDE MINERALS Contents Xanthate adsorption Flotation





- Slides: 45

FLOTATION OF SULPHIDE MINERALS

Contents • • Xanthate adsorption Flotation of Galena Flotation of Chalcocite Flotation of Pyrite Flotation of Chalcopyrite Flotation of Sphalerite Selective & bulk flotation

Xanthate Adsorption (1) • Sulphide minerals are floated by sulphydryl collectors (thio-componds) • Adsorption of sulphydryl collectors may occur by – chemisorption at metal ion sites – by electrochemical mechanisms – also physisorption may occur by van der Waals bonding between hydrocarbon chains.

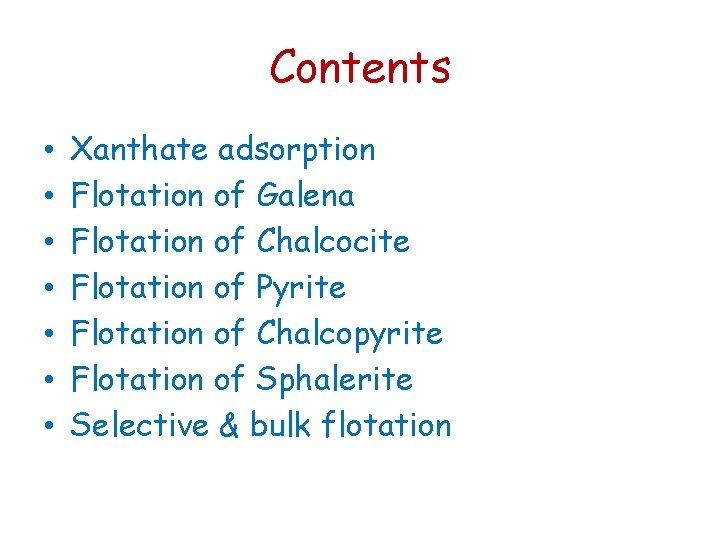
Chemical theory of xanthate adsorption (1) Consider first the following reaction: Pb. S(s) + 2 O 2(g) Pb. SO 4(s) K = 10124 With the following equilibrium expression (a. Pb. SO 4) / ((a. Pb. S). (p. O 2)2) = 10124

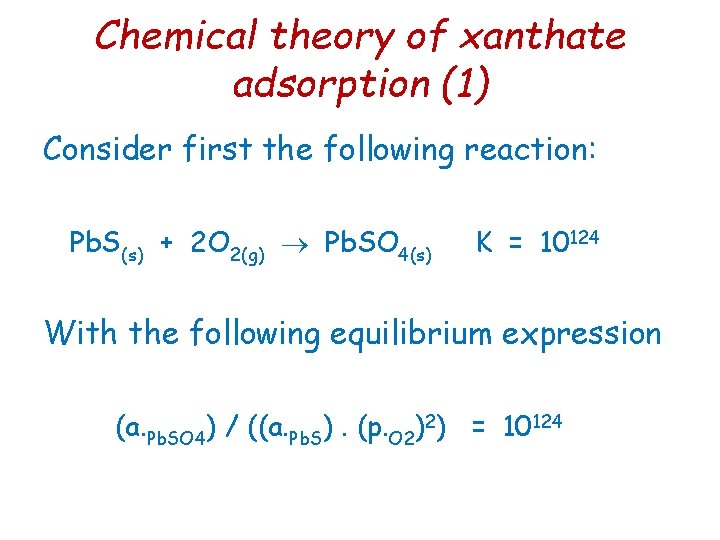
Chemical theory of xanthate adsorption (2) With a. Pb. SO 4 = 1 & a. Pb. S = 1 p. O 2 = 10 -62 atmosphere The partial pressure of oxygen in the air is 0. 2 atm >> 10 -62 atm thus oxidation of galena occurs to thiosulphate or sulphate.

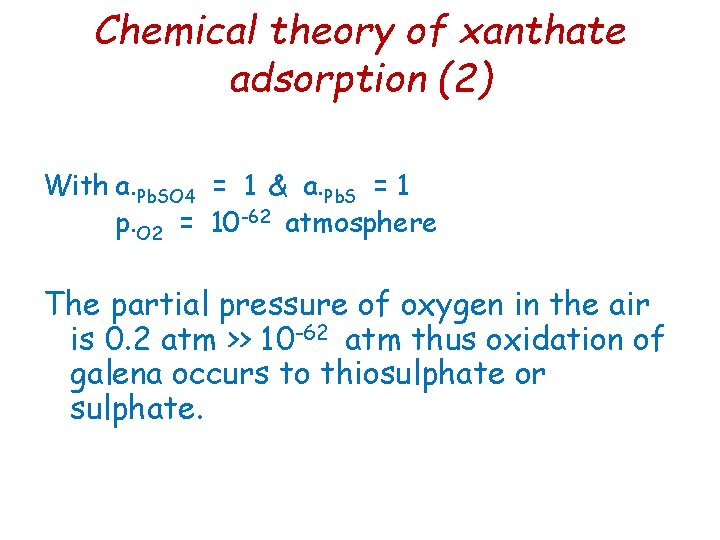
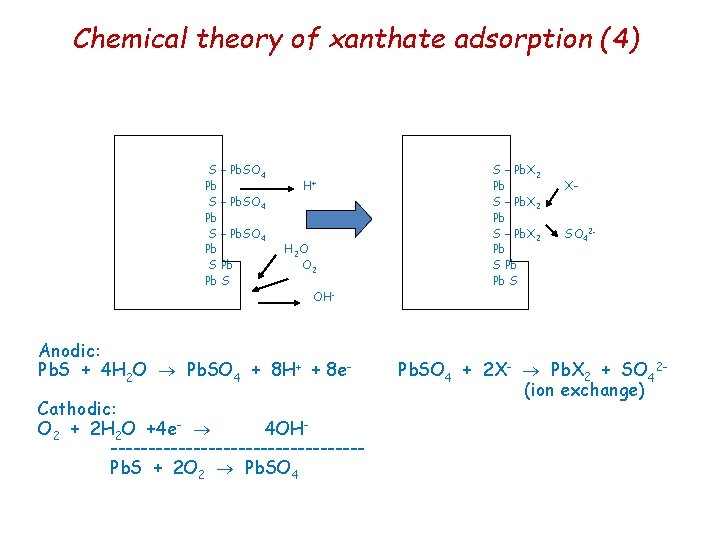
Chemical theory of xanthate adsorption (3) With systems open to air there will also be carbon dioxide present, and the galena surface will form lead carbonate at the expense of sulphate. Pb. SO 4(s) + CO 32 - Pb. CO 3(s) + SO 42 At usual flotation p. H of 8 or 9 lead xanthate is more stable than Pb. CO 3 or Pb. SO 4, and Pb. X 2 forms by replacement of these lead salts.

Chemical theory of xanthate adsorption (4) S – Pb. SO 4 Pb S Pb Pb S H+ H 2 O O 2 OH - Anodic: Pb. S + 4 H 2 O Pb. SO 4 + 8 H+ + 8 e. Cathodic: O 2 + 2 H 2 O +4 e- 4 OH-----------------Pb. S + 2 O 2 Pb. SO 4 S – Pb. X 2 Pb S Pb Pb S X- SO 42 - Pb. SO 4 + 2 X- Pb. X 2 + SO 42(ion exchange)

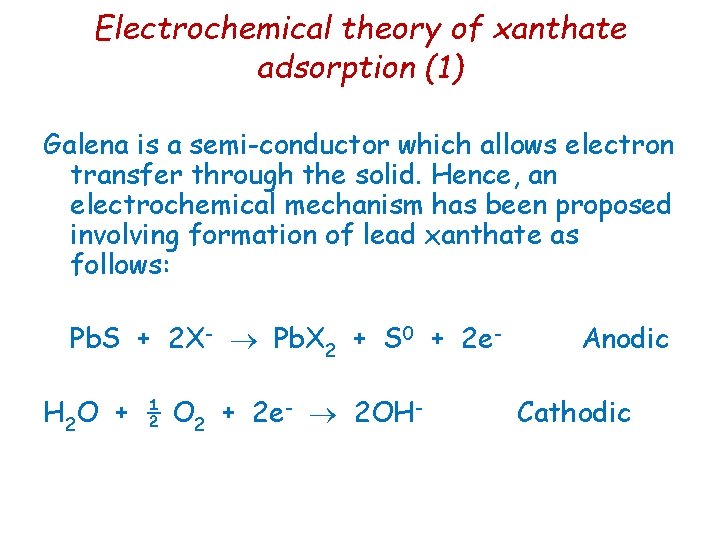
Electrochemical theory of xanthate adsorption (1) Galena is a semi-conductor which allows electron transfer through the solid. Hence, an electrochemical mechanism has been proposed involving formation of lead xanthate as follows: Pb. S + 2 X- Pb. X 2 + S 0 + 2 e. H 2 O + ½ O 2 + 2 e- 2 OH- Anodic Cathodic

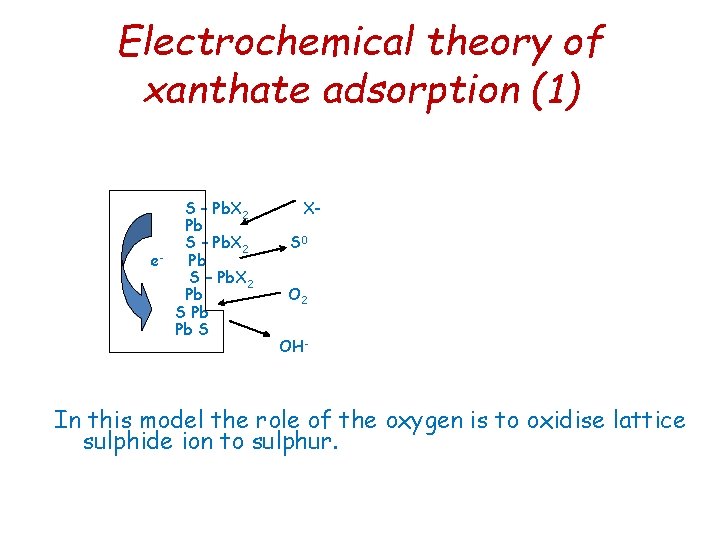
Electrochemical theory of xanthate adsorption (1) S – Pb. X 2 Pb S – Pb. X 2 e Pb S – Pb. X 2 Pb S Pb Pb S XS 0 O 2 OH - In this model the role of the oxygen is to oxidise lattice sulphide ion to sulphur.

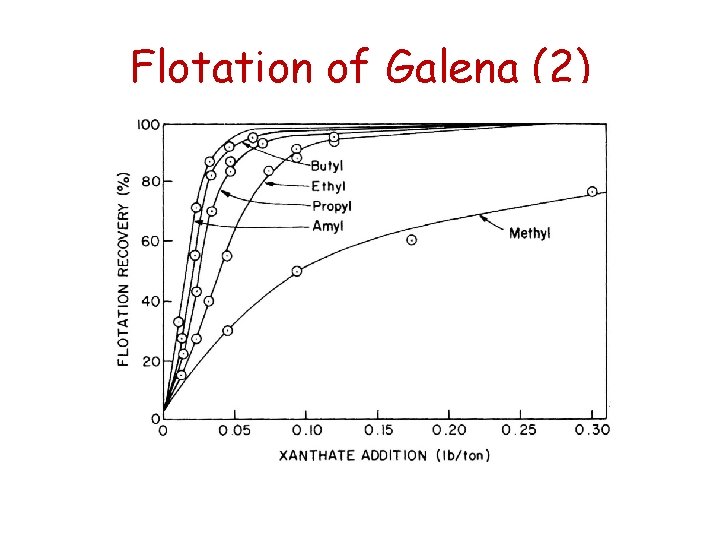
Flotation of Galena (1) • Complete flotation of galena is achieved from p. H 2 to p. H 10 using ethyl xanthate • The collector adsorption mechanisms were discussed above • The hydrophobic metal-collector species responsible for flotation is Pb. X 2 • Depending on the hydrocarbon chain length of the collector, the amount of collector required for flotation varies

Flotation of Galena (2)

Flotation of Galena (3) • For example, with the same concentration of Pb 2+, a smaller concentration of amyl xanthate (C 5) is required to form a precipitate of lead amyl xanthate than is required to form lead ethyl xanthate with ethyl xanthate. • The longer the hydrocarbon chain, the greater is the hydrophobicity imparted to the mineral surface.

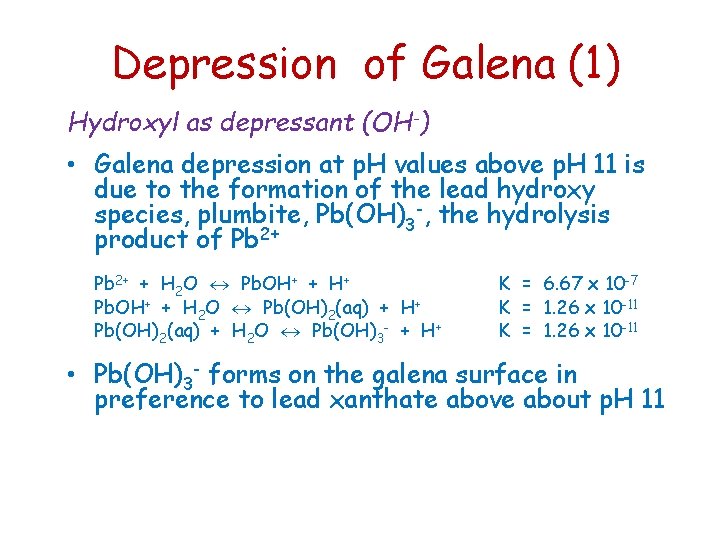
Depression of Galena (1) Hydroxyl as depressant (OH-) • Galena depression at p. H values above p. H 11 is due to the formation of the lead hydroxy species, plumbite, Pb(OH)3 -, the hydrolysis product of Pb 2+ + H 2 O Pb. OH+ + H+ Pb. OH+ + H 2 O Pb(OH)2(aq) + H+ Pb(OH)2(aq) + H 2 O Pb(OH)3 - + H+ K = 6. 67 x 10 -7 K = 1. 26 x 10 -11 • Pb(OH)3 - forms on the galena surface in preference to lead xanthate above about p. H 11

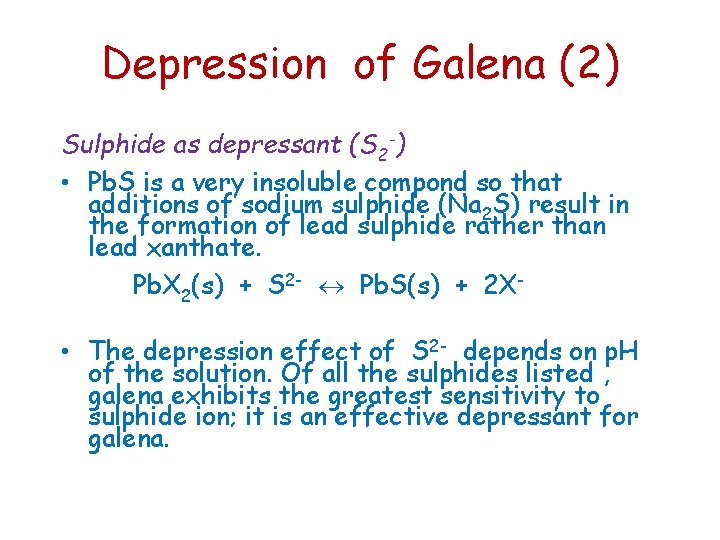
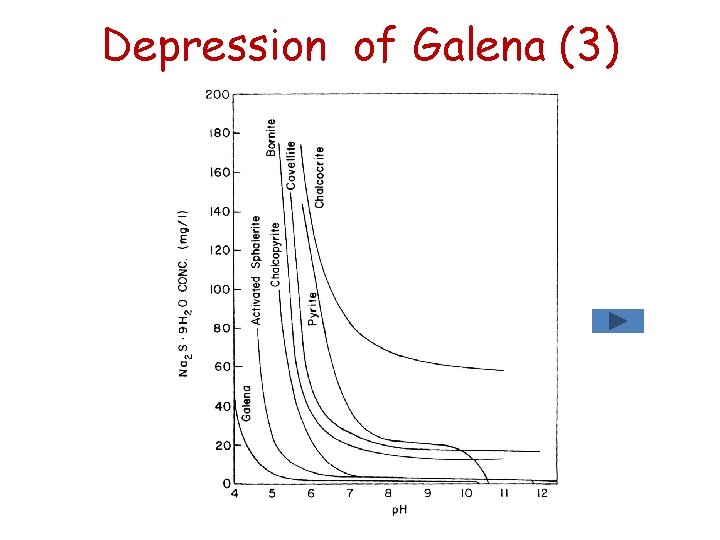
Depression of Galena (2) Sulphide as depressant (S 2 -) • Pb. S is a very insoluble compond so that additions of sodium sulphide (Na 2 S) result in the formation of lead sulphide rather than lead xanthate. Pb. X 2(s) + S 2 - Pb. S(s) + 2 X- • The depression effect of S 2 - depends on p. H of the solution. Of all the sulphides listed , galena exhibits the greatest sensitivity to sulphide ion; it is an effective depressant for galena.

Depression of Galena (3)

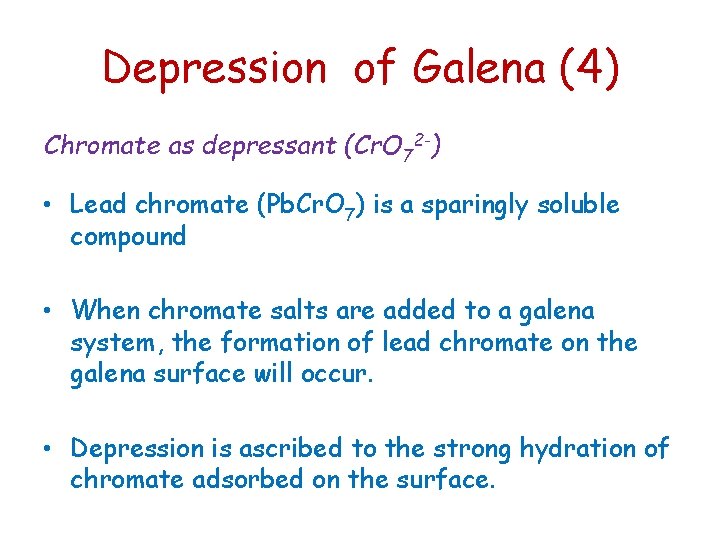
Depression of Galena (4) Chromate as depressant (Cr. O 72 -) • Lead chromate (Pb. Cr. O 7) is a sparingly soluble compound • When chromate salts are added to a galena system, the formation of lead chromate on the galena surface will occur. • Depression is ascribed to the strong hydration of chromate adsorbed on the surface.

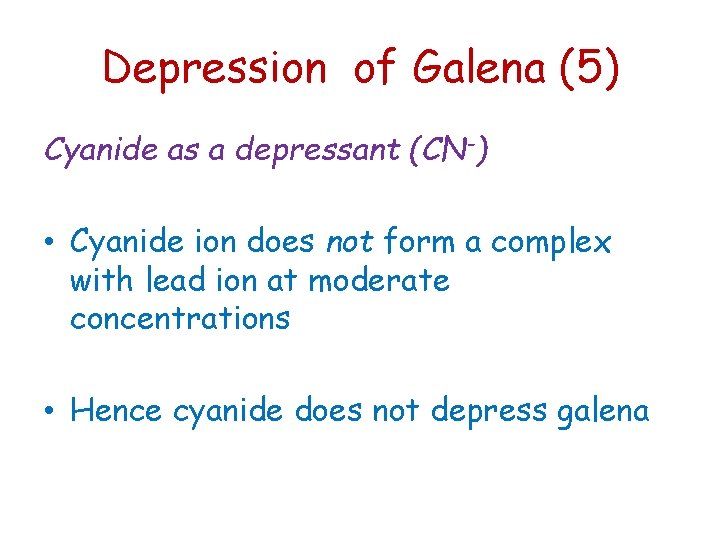
Depression of Galena (5) Cyanide as a depressant (CN-) • Cyanide ion does not form a complex with lead ion at moderate concentrations • Hence cyanide does not depress galena

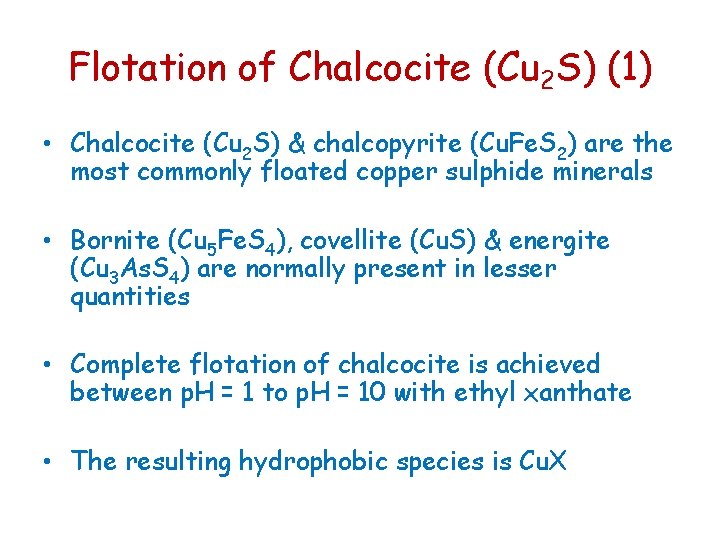
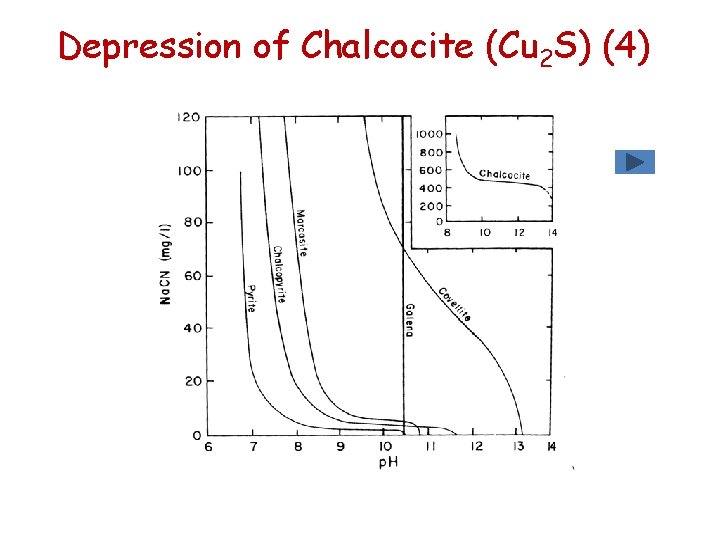
Flotation of Chalcocite (Cu 2 S) (1) • Chalcocite (Cu 2 S) & chalcopyrite (Cu. Fe. S 2) are the most commonly floated copper sulphide minerals • Bornite (Cu 5 Fe. S 4), covellite (Cu. S) & energite (Cu 3 As. S 4) are normally present in lesser quantities • Complete flotation of chalcocite is achieved between p. H = 1 to p. H = 10 with ethyl xanthate • The resulting hydrophobic species is Cu. X

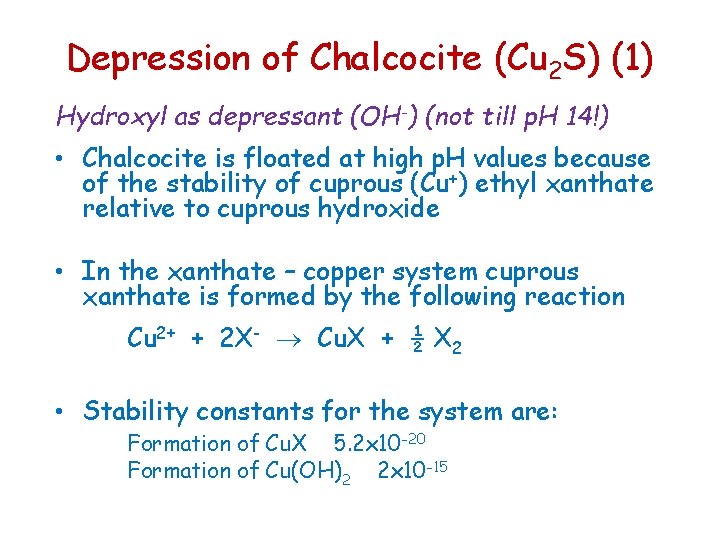
Depression of Chalcocite (Cu 2 S) (1) Hydroxyl as depressant (OH-) (not till p. H 14!) • Chalcocite is floated at high p. H values because of the stability of cuprous (Cu+) ethyl xanthate relative to cuprous hydroxide • In the xanthate – copper system cuprous xanthate is formed by the following reaction Cu 2+ + 2 X- Cu. X + ½ X 2 • Stability constants for the system are: Formation of Cu. X 5. 2 x 10 -20 Formation of Cu(OH)2 2 x 10 -15

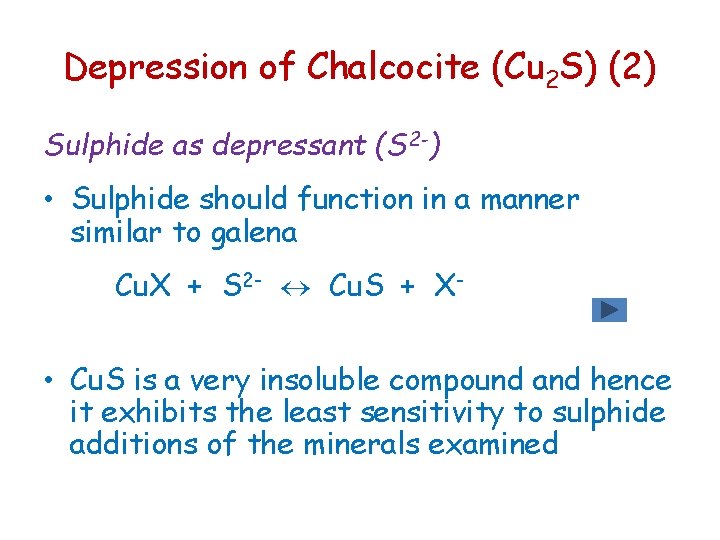
Depression of Chalcocite (Cu 2 S) (2) Sulphide as depressant (S 2 -) • Sulphide should function in a manner similar to galena Cu. X + S 2 - Cu. S + X- • Cu. S is a very insoluble compound and hence it exhibits the least sensitivity to sulphide additions of the minerals examined

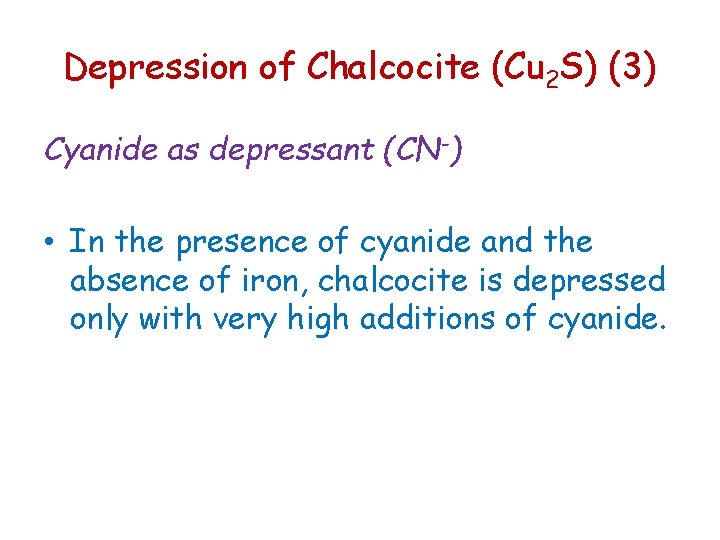
Depression of Chalcocite (Cu 2 S) (3) Cyanide as depressant (CN-) • In the presence of cyanide and the absence of iron, chalcocite is depressed only with very high additions of cyanide.

Depression of Chalcocite (Cu 2 S) (4)

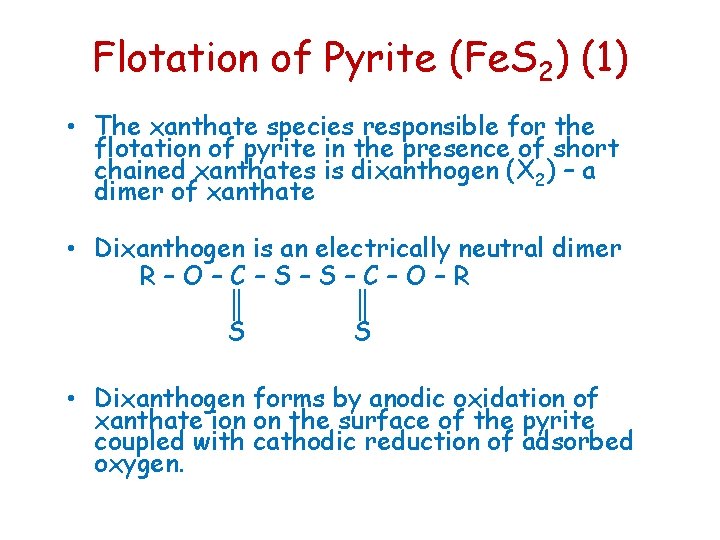
Flotation of Pyrite (Fe. S 2) (1) • The xanthate species responsible for the flotation of pyrite in the presence of short chained xanthates is dixanthogen (X 2) – a dimer of xanthate • Dixanthogen is an electrically neutral dimer R–O–C–S–S–C–O–R ║ ║ S S • Dixanthogen forms by anodic oxidation of xanthate ion on the surface of the pyrite coupled with cathodic reduction of adsorbed oxygen.

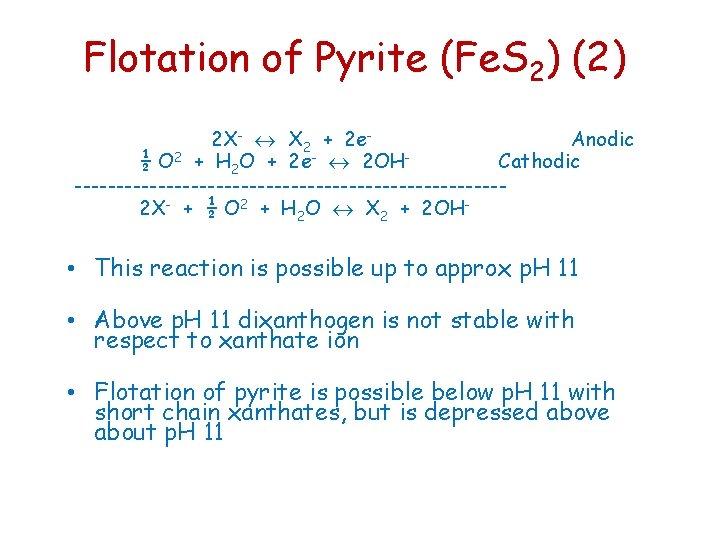
Flotation of Pyrite (Fe. S 2) (2) 2 X- X 2 + 2 e. Anodic ½ O 2 + H 2 O + 2 e- 2 OHCathodic --------------------------2 X- + ½ O 2 + H 2 O X 2 + 2 OH- • This reaction is possible up to approx p. H 11 • Above p. H 11 dixanthogen is not stable with respect to xanthate ion • Flotation of pyrite is possible below p. H 11 with short chain xanthates, but is depressed above about p. H 11

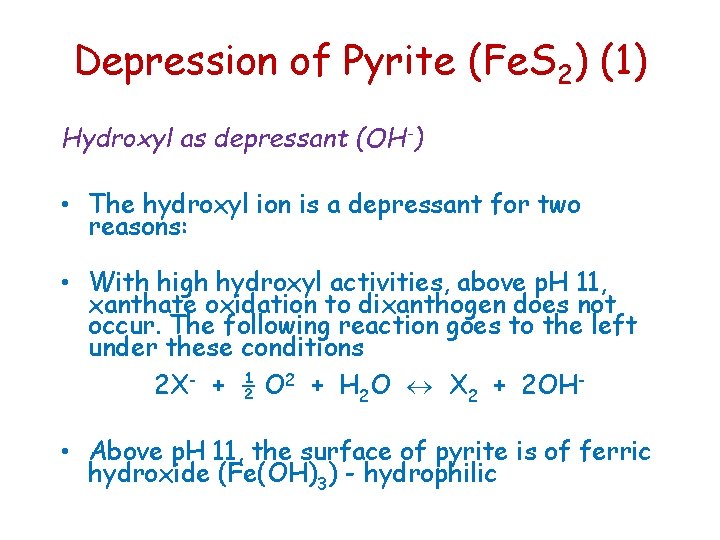
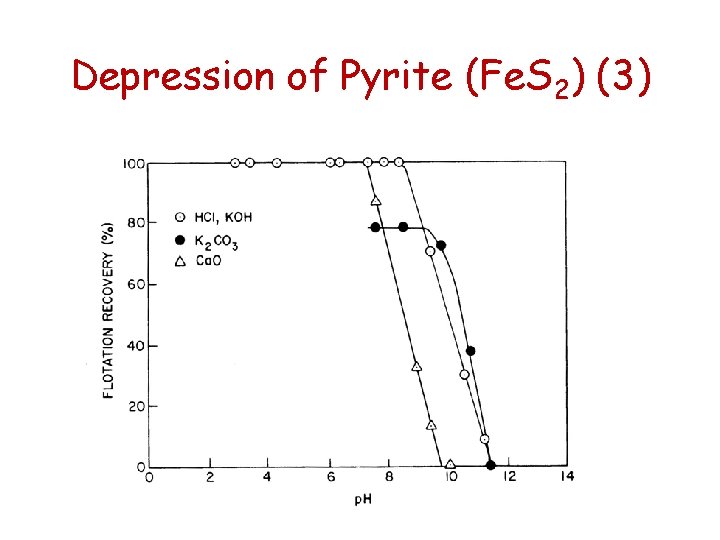
Depression of Pyrite (Fe. S 2) (1) Hydroxyl as depressant (OH-) • The hydroxyl ion is a depressant for two reasons: • With high hydroxyl activities, above p. H 11, xanthate oxidation to dixanthogen does not occur. The following reaction goes to the left under these conditions 2 X- + ½ O 2 + H 2 O X 2 + 2 OH • Above p. H 11, the surface of pyrite is of ferric hydroxide (Fe(OH)3) - hydrophilic

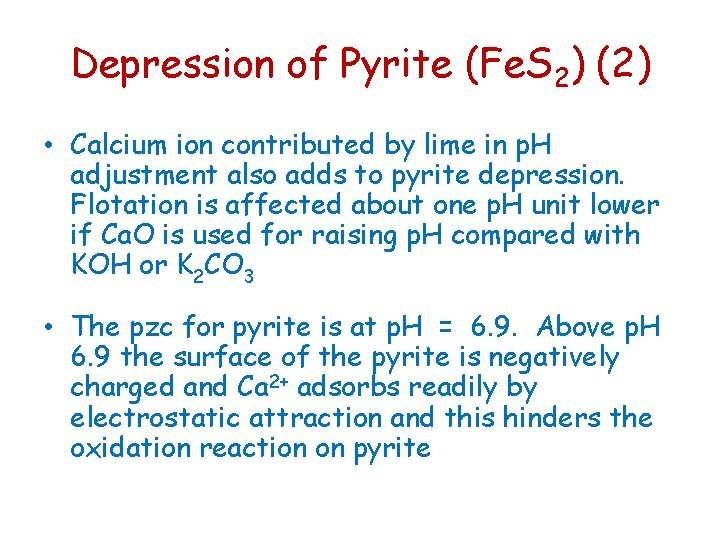
Depression of Pyrite (Fe. S 2) (2) • Calcium ion contributed by lime in p. H adjustment also adds to pyrite depression. Flotation is affected about one p. H unit lower if Ca. O is used for raising p. H compared with KOH or K 2 CO 3 • The pzc for pyrite is at p. H = 6. 9. Above p. H 6. 9 the surface of the pyrite is negatively charged and Ca 2+ adsorbs readily by electrostatic attraction and this hinders the oxidation reaction on pyrite

Depression of Pyrite (Fe. S 2) (3)

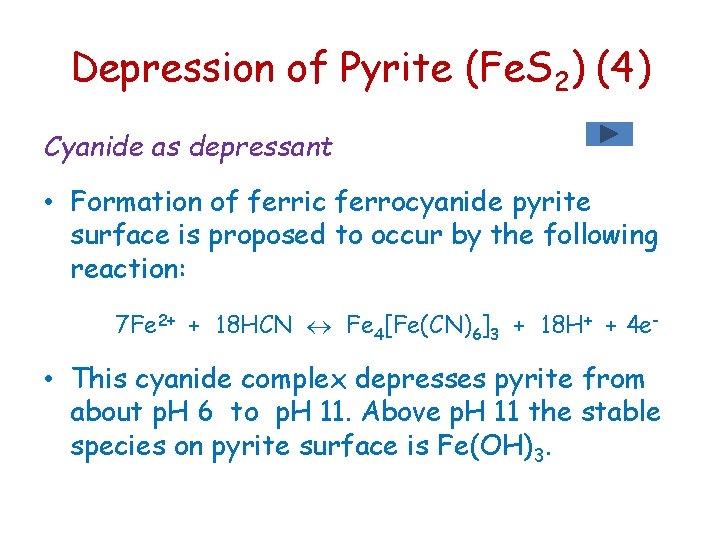
Depression of Pyrite (Fe. S 2) (4) Cyanide as depressant • Formation of ferric ferrocyanide pyrite surface is proposed to occur by the following reaction: 7 Fe 2+ + 18 HCN Fe 4[Fe(CN)6]3 + 18 H+ + 4 e- • This cyanide complex depresses pyrite from about p. H 6 to p. H 11. Above p. H 11 the stable species on pyrite surface is Fe(OH)3.

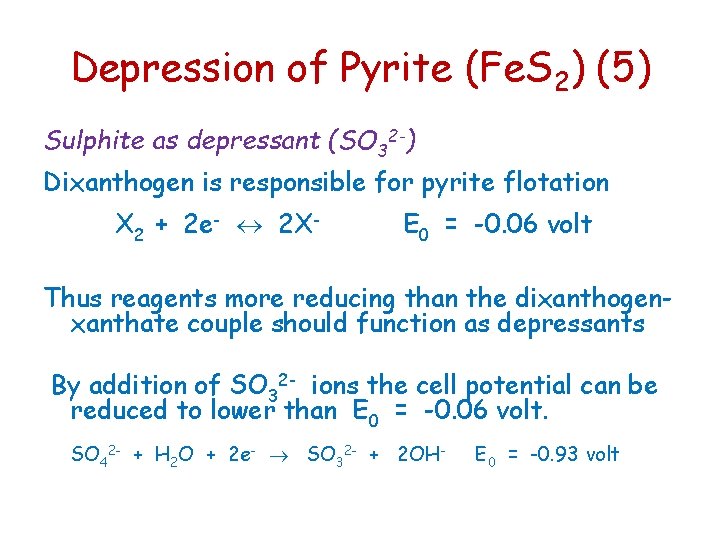
Depression of Pyrite (Fe. S 2) (5) Sulphite as depressant (SO 32 -) Dixanthogen is responsible for pyrite flotation X 2 + 2 e- 2 X- E 0 = -0. 06 volt Thus reagents more reducing than the dixanthogenxanthate couple should function as depressants By addition of SO 32 - ions the cell potential can be reduced to lower than E 0 = -0. 06 volt. SO 42 - + H 2 O + 2 e- SO 32 - + 2 OH- E 0 = -0. 93 volt

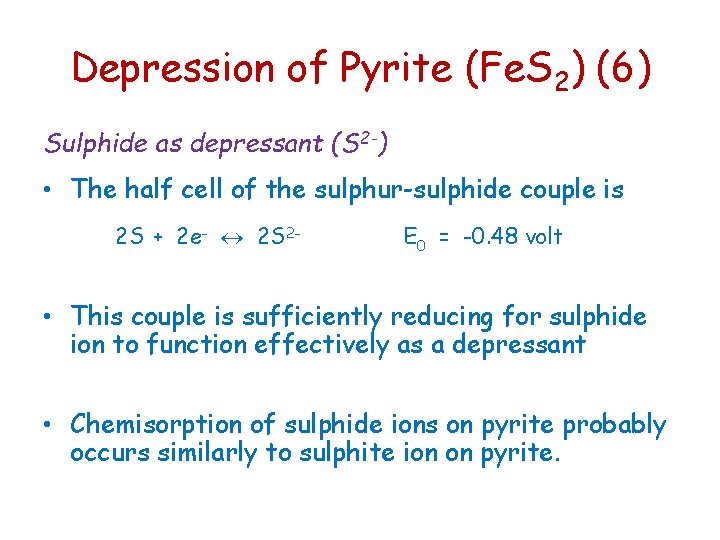
Depression of Pyrite (Fe. S 2) (6) Sulphide as depressant (S 2 -) • The half cell of the sulphur-sulphide couple is 2 S + 2 e- 2 S 2 - E 0 = -0. 48 volt • This couple is sufficiently reducing for sulphide ion to function effectively as a depressant • Chemisorption of sulphide ions on pyrite probably occurs similarly to sulphite ion on pyrite.

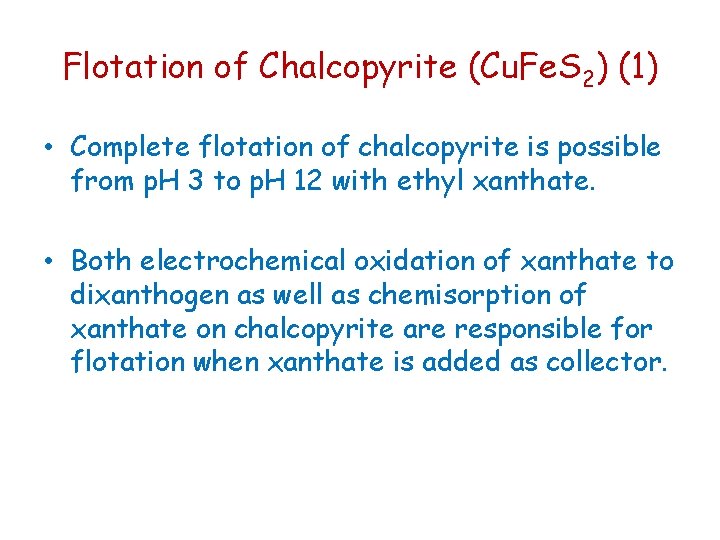
Flotation of Chalcopyrite (Cu. Fe. S 2) (1) • Complete flotation of chalcopyrite is possible from p. H 3 to p. H 12 with ethyl xanthate. • Both electrochemical oxidation of xanthate to dixanthogen as well as chemisorption of xanthate on chalcopyrite are responsible for flotation when xanthate is added as collector.

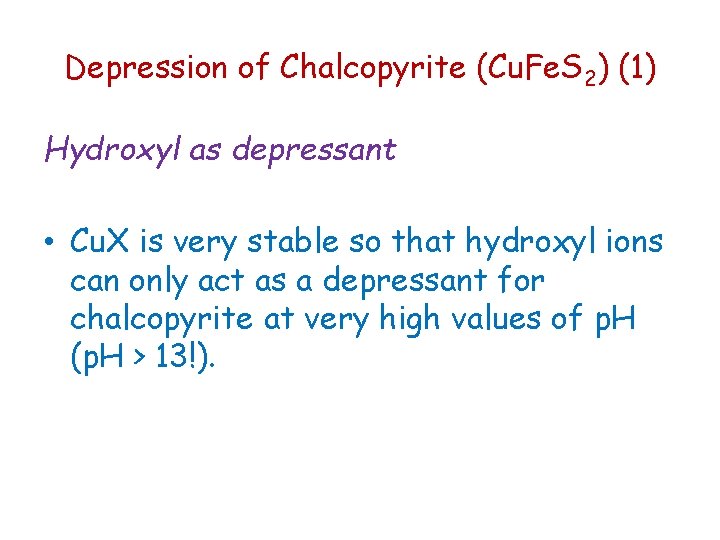
Depression of Chalcopyrite (Cu. Fe. S 2) (1) Hydroxyl as depressant • Cu. X is very stable so that hydroxyl ions can only act as a depressant for chalcopyrite at very high values of p. H (p. H > 13!).

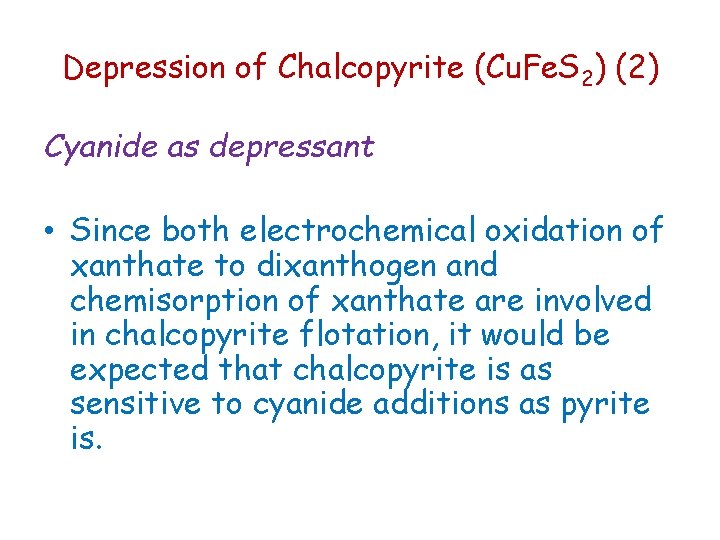
Depression of Chalcopyrite (Cu. Fe. S 2) (2) Cyanide as depressant • Since both electrochemical oxidation of xanthate to dixanthogen and chemisorption of xanthate are involved in chalcopyrite flotation, it would be expected that chalcopyrite is as sensitive to cyanide additions as pyrite is.

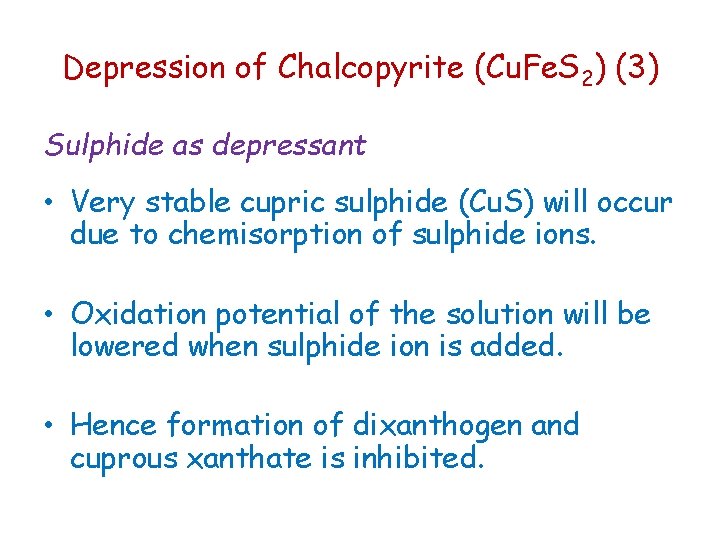
Depression of Chalcopyrite (Cu. Fe. S 2) (3) Sulphide as depressant • Very stable cupric sulphide (Cu. S) will occur due to chemisorption of sulphide ions. • Oxidation potential of the solution will be lowered when sulphide ion is added. • Hence formation of dixanthogen and cuprous xanthate is inhibited.

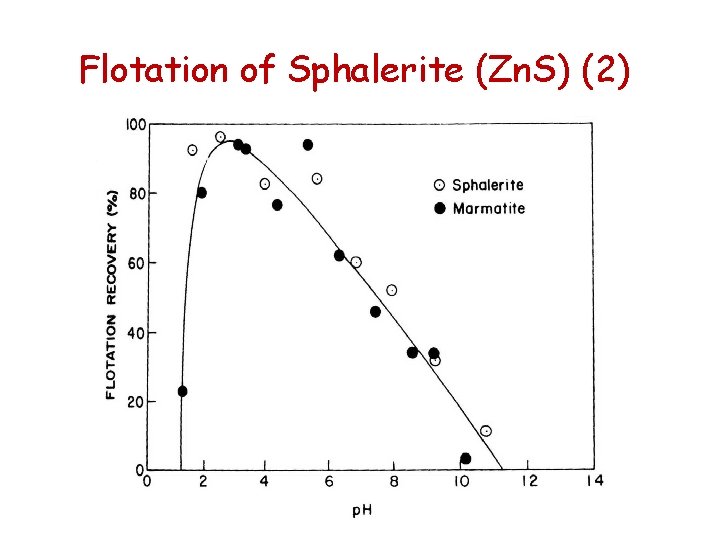
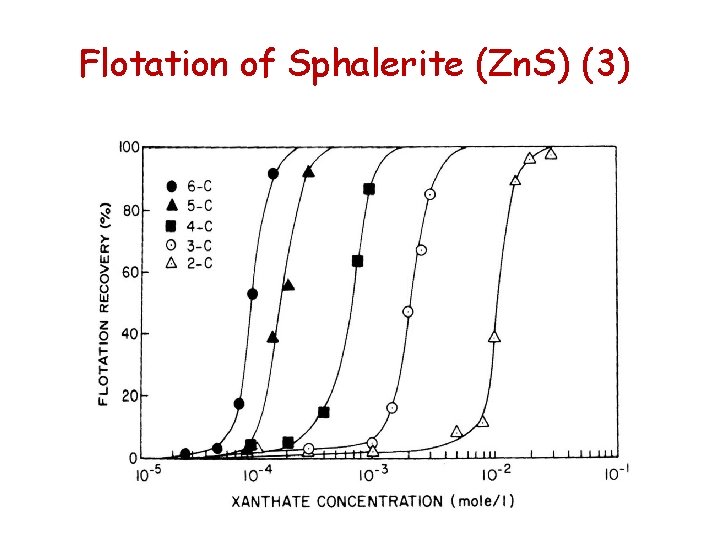
Flotation of Sphalerite (Zn. S) (1) • Flotation of sphalerite with amyl xanthate is possible at p. H 3. 5 and decreases as the p. H is increased • At higher concentrations of longer chain xanthates sphalerite responds quite readily to flotation

Flotation of Sphalerite (Zn. S) (2)

Flotation of Sphalerite (Zn. S) (3)

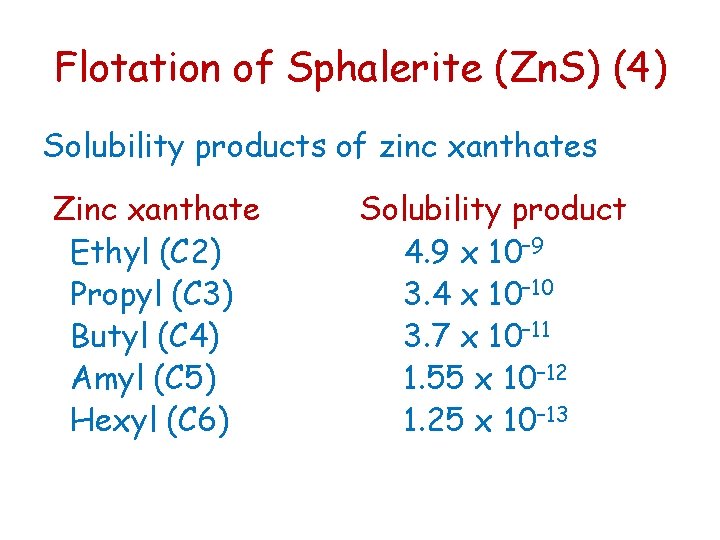
Flotation of Sphalerite (Zn. S) (4) Solubility products of zinc xanthates Zinc xanthate Ethyl (C 2) Propyl (C 3) Butyl (C 4) Amyl (C 5) Hexyl (C 6) Solubility product 4. 9 x 10– 9 3. 4 x 10– 10 3. 7 x 10– 11 1. 55 x 10– 12 1. 25 x 10– 13

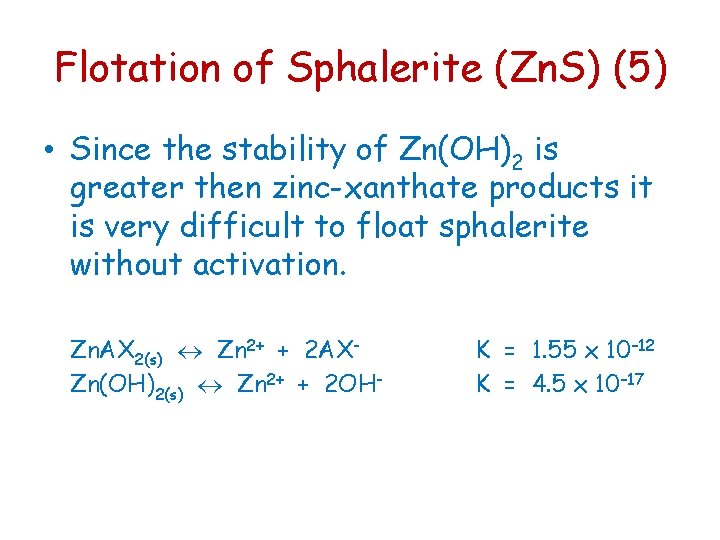
Flotation of Sphalerite (Zn. S) (5) • Since the stability of Zn(OH)2 is greater then zinc-xanthate products it is very difficult to float sphalerite without activation. Zn. AX 2(s) Zn 2+ + 2 AXZn(OH)2(s) Zn 2+ + 2 OH- K = 1. 55 x 10– 12 K = 4. 5 x 10– 17

Flotation of Sphalerite (Zn. S) (6) Sphalerite is activated with copper sulphate Zn. S + Cu 2+ Cu. S + Zn 2+ Forming copper sulphide on the surface. The stability of Cu. S is greater than Zn. S and Zn(OH)2. Cu. S 4. 0 x 10 -36 Zn. S 2. 6 x 10– 26 Pb. AX 2 1. 0 x 10– 24 Cu. AX 5. 2 x 10– 20 Cu(OH)2 2. 0 x 10 -15 Sphalerite floated with xanthate after Cu activation

Flotation of Sphalerite (Zn. S) (7) Prevention of activation • Unintentional activation of sphalerite in ores is most commonly due to Cu 2+ and Pb 2+ • In the case of Cu 2+, cyanide is most commonly used to prevent activation. Cu 2+ tends to form a complex with cyanide Cu(CN)2 - • In the case of Pb 2+, Zn. SO 4 is added as a source of Zn 2+. When activity of Zn 2+ is 1000 times greater that Pb 2+ in solution, the activation by Pb 2+ will be prevented.

Selective Flotation • Aim to separate concentrates in a complex ore • Driven by smelting practice and concentrate contracts which penalise impurities in cons • For example in a lead/zinc sulphide ore – galena can be floated first at higher p. H using cyanide to prevent sphalerite and pyrite from floating – then activation of sphalerite with copper sulphate and high p. H to depress pyrite gives sphalerite concentrate – finally a lowering of p. H can enable a pyrite concentrate to be made if required.

Bulk Flotation For new smelting practices, eg ISF furnace, and for precious metal recovery in sulphides, it is sometimes preferable to make a bulk concentrate of all sulphides together. In this case no depressants are needed, sphalerite is activated if present, and p. H is kept around neutral.

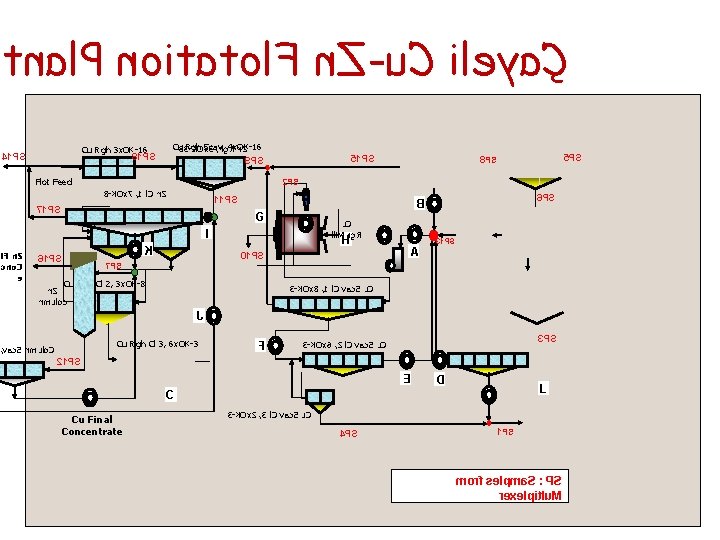
tnal. P noitatol. F n. Z-u. C ileyaÇ Cu Rgh 3 x. OK-16 41 PS 81 PS Cu 83 Rgh -KOx. Scav, 3 , hg 4 x. OK-16 R n. Z Flot Feed 11 PS Cu Rgh Cl 1, 3 x. OK-8 + 2 x. OK-16 61 PS K G u. C lli. M rg. R H A 01 PS 7 PS 6 PS B I i. F n. Z cno. C e 5 PS 8 PS 2 PS 8 -KOx 7 , 1 l. C n. Z 71 PS 51 PS 9 PS Cu Rgh Cl 2, 3 x. OK-8 n. Z nmuloc 31 PS 3 -KOx 8 , 1 l. C vac. S u. C J Cu Rgh Cl 3, 6 x. OK-3 , vac. S nmulo. C F 3 PS 3 -KOx 6 , 2 l. C vac. S u. C 21 PS E D L C Cu Final Concentrate 3 -KOx 2 , 3 l. C vac. S u. C 4 PS 1 PS morf selpma. S : PS rexelpitlu. M

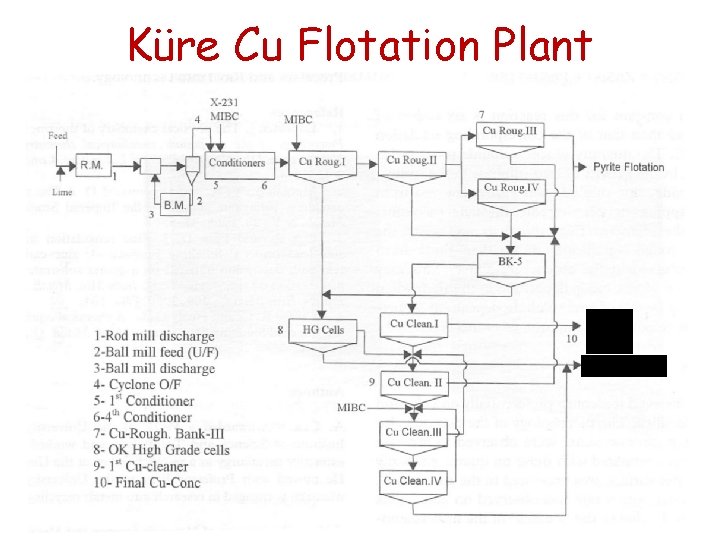
Küre Cu Flotation Plant Tail Concentrate